611d049b28a82b965592dea8e2736f99.ppt
- Количество слайдов: 46

Chapter 1 Basic Principles and Practice of Clinical Chemistry, part 2 1
n C. REAGENTS; Chemical Grades q q q Reagent preparation in the clinical lab is decreasing most reagents are obtained from commercial manufacturers Objective: Identify and differentiate the different degrees of chemical purity. Common terms that relate to reagent purity: n n Analytical Grade (purest), also called reagent grade or ACS grades - best choice for lab work. National Formulary (NF) or US Pharmacopeia (USP) – used for drugs, may be OK for lab work Chemical Pure (least pure) – not recommended for lab Technical or commercial grade – never for lab use 2
n REAGENTS; Chemical Grades q Primary Standard : Highly purified solution of known concentration. These standards are used in the clinical lab to “calibrate” / “standardize” instruments in order to measure other solutions of unknown concentration n q q Primary Standards must be 99. 98% pure Secondary Standard : Less pure substance whose concentration was determined by comparison to a Primary Standard Reference Material / Calibrator n n The name for biological substances used as ‘standards’ Most biological standards cannot be 99. 98% pure because the chemical processes to achieve this level of purity would destroy the substances. 3

n Controls q For our purposes: n q A control should have the same appearance and consistency as does the patient samples: n n q Defined: substance, whose physical and chemical properties resemble the unknown specimen … If patient sample is ‘serum’; the control should look like and have the same consistency as serum. If the patient sample is ‘urine’, the control is urine, etc. - Controls are used to verify the accuracy and acceptability of a run. 4

n Control Solutions vs. Standard Solutions q q A Control specimen is used to monitor Quality Control (QC) A Control has known acceptable ranges, established either by the manufacturer (assayed) or the hospital lab itself (un-assayed) It is usually a serum/plasma based solution that is treated just as if it were a patient specimen Control specimens must produce results within established ranges in order for the ‘run’ to be acceptable. 5

n Control Solutions vs. Standard Solutions q q q A Standard solution is a highly purified solution that is usually not serum / plasma based Standard solutions have set, listed values that are established by the manufacturer Standard solutions are used to “calibrate” instruments, that is to “set” instruments to measure correctly at known concentration 6
n Control Solutions vs. Standard Solutions q Standard solutions are also called “ Calibrators” – if they are biological in nature n Consider for example, analytes such as bilirubin. n These substances do not come in the ‘highly purified state, as calcium, glucose, etc. n A bilirubin standard is biological based, and technically a calibrator rather than the purely defined standard. n What about the ‘hematology standards’? Standard or calibrator? 7
n Water Specifications q Tap water is unsuitable for lab use (too many impurities) q Types of water purification techniques n n q Reagent Grades of water n n n q Distillation – removes most organic matter Reverse osmosis Filtration Deionization – ions removed Type I Purest – Required for sensitive tests Type II Acceptable for most uses Type III OK for washing glassware CAP - QC of water : p. H, electrical resistance, bacterial culture 8

Water filtration system for Automated chemistry analyzer. 9

n Use of Blanks q q Review: Blanks used to eliminate or subtract the effects of reagent or specimen colors that would interfere with accurately measuring an analyte. Water Blank – DI water, used to ‘zero’ the spectrophotometer. Seen mostly in UV procedures. Reagent Blank – contains all the reagents used in the ‘tests’. DI water sometimes used in the place of the amount of patient specimen. Colorimetric procedures. Patient Blank – required by some procedures if patient sample has deep color that would affect results. Name 3 situations that would warrant use of ‘patient blank’. 10
n Labware q Types of glass q High thermal borosilicate § Can take long periods of high temperatures § Scratches easily § Acceptable for chemistry work § Examples: Pyrex, Kimax 11
n Labware q Types of glass q Aluminosilicate § Can withstand heat as long as not in contact with acids or alkalis § Resists scratching § Acceptable for chemistry work § Examples: centrifuge tubes, thermometers 12

n Labware q Types of glass q Soda lime – not suitable for lab use 13

q Types of plastic resins n Polystyrene q q q Clear, rigid Can withstand temperatures to 70 C Examples: many disposables 14

q Types of plastic resins Polyethylene q q Translucent in appearance Two types § One type can withstand temperatures up to 80 C, and is flexible, i. e. , reagent wash bottles § Other can withstand temperatures up to 120 C and is rigid, i. e. , droppers 15
q Types of plastic resins n Polyvinyl chloride q q q Translucent in appearance, but rigid Withstands temperatures to 135 C Examples: screw cap enclosures 16
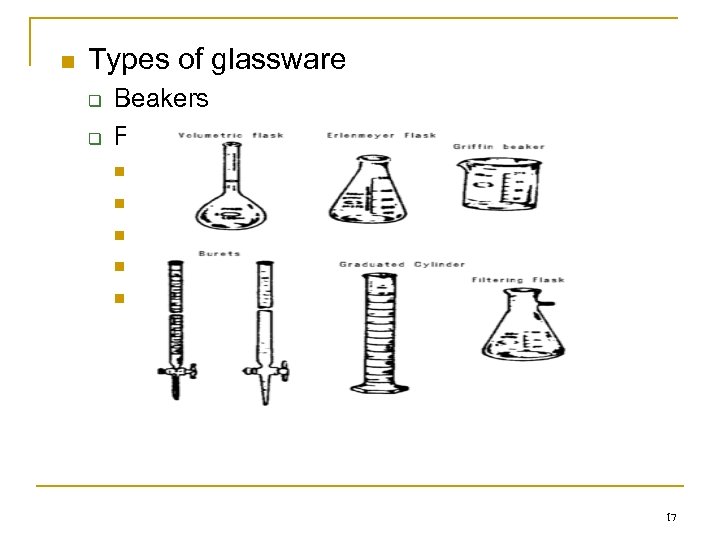
n Types of glassware q q Beakers Flasks n n n Volumetric Erlenmeyer Graduated cylinders Reagent bottles Test tubes 17
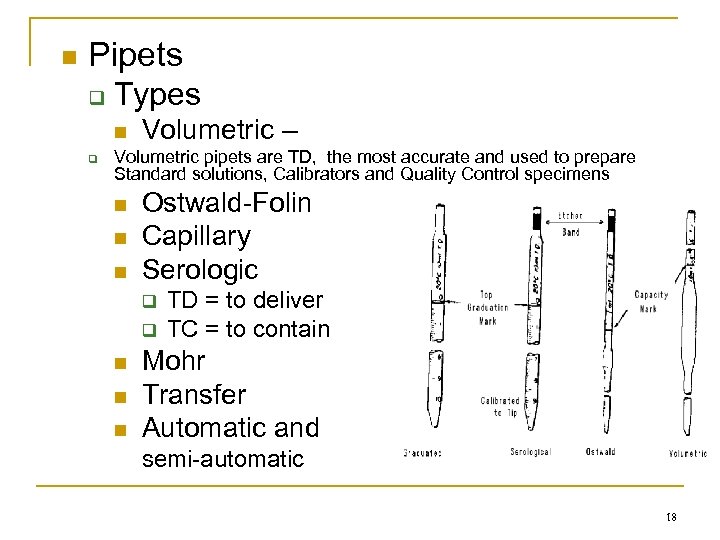
n Pipets q q Types n Volumetric – Volumetric pipets are TD, the most accurate and used to prepare Standard solutions, Calibrators and Quality Control specimens n n n Ostwald-Folin Capillary Serologic q q n n n TD = to deliver TC = to contain Mohr Transfer Automatic and semi-automatic 18

n Laboratory Vessels and Pipets q Volumetric flasks : The line indicates the level that contains an exact volume Erlenmeyer flasks : Hold variable volumes Graduated cylinders : Hold variable volumes q Pipet rules q q n TC = needs to be blown out TD = let drain along the side of the receiving vessel Read pipets from the bottom of the meniscus Hold pipets straight up and down Use suction bulbs to aspirate fluids into pipets NEVER MOUTH PIPET !!! n Place dirty pipets in soapy water with tips up n n n 19
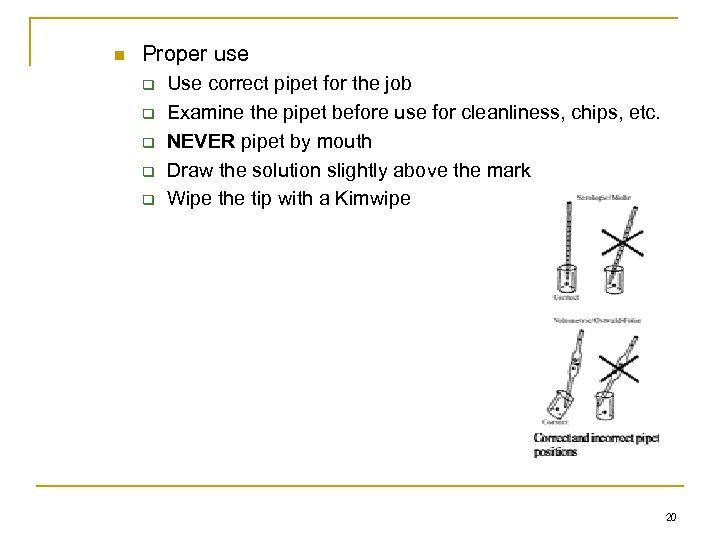
n Proper use q q q Use correct pipet for the job Examine the pipet before use for cleanliness, chips, etc. NEVER pipet by mouth Draw the solution slightly above the mark Wipe the tip with a Kimwipe 20
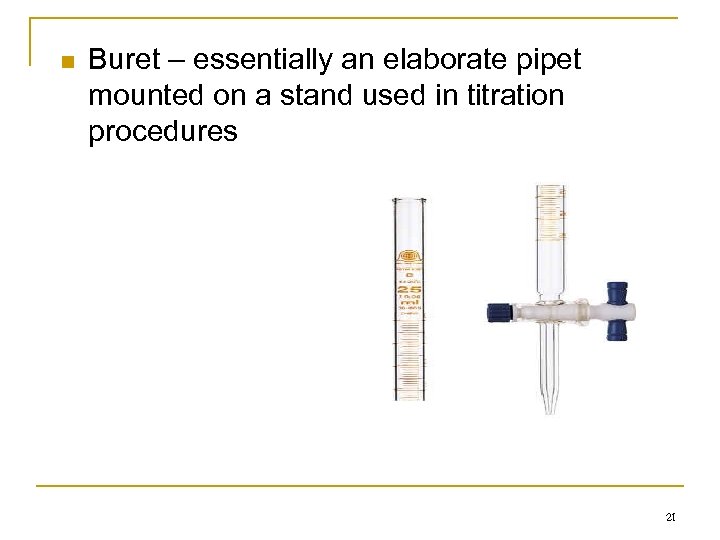
n Buret – essentially an elaborate pipet mounted on a stand used in titration procedures 21
q Cleaning of Lab Glassware n Majority of time can simply presoak, dishwash, and thoroughly rinse with tap and finally distilled/deionized water n Chemically clean glassware is required for certain chemistry procedures (enzymes, iron, heavy metals, etc. ) ‘dichromate acid’ or ‘acid dichromate’ n 22

n General Laboratory Equipment q q Balances – type chosen dependent on volume/weight needed and degree of accuracy required. What is the best choice for clinical work? 23
n General Laboratory Equipment q Centrifuge n n n Purpose Types Characteristics q q Fixed rotor head / swinging bucket Closing – locked closed lid now required 24
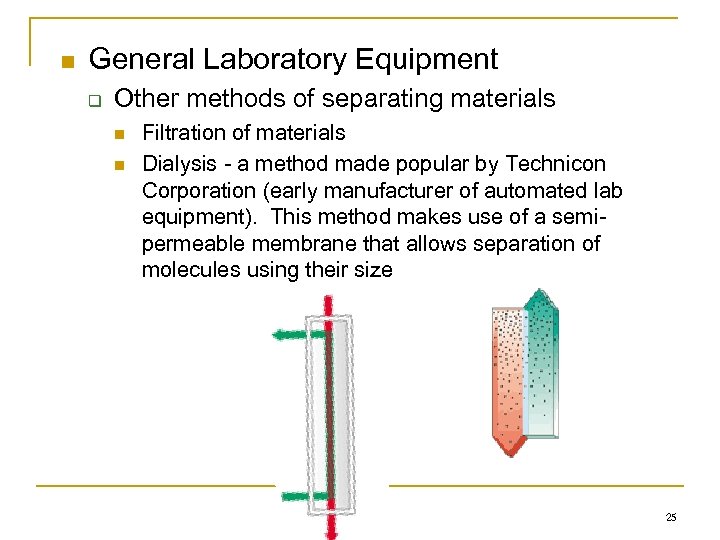
n General Laboratory Equipment q Other methods of separating materials n n Filtration of materials Dialysis - a method made popular by Technicon Corporation (early manufacturer of automated lab equipment). This method makes use of a semipermeable membrane that allows separation of molecules using their size 25

n Specimen Collection and Processing q Medical ethics in specimen collection – professionalism and confidentiality at all times n Special collection procedures q q Fasting specimens: overnight for most tests, 12 hours for lipid studies Timed interval specimens § Examples include glucose tolerance, therapeutic drug monitoring, and hormone stimulation testing § In some cases urine collection also required Legal chain of evidence Other special collection procedures 26
n Specimen processing q Determining specimen acceptability n Other than improper timing, identify things that can affect chemical analysis of clinical specimens. q Specimen accessioning 27
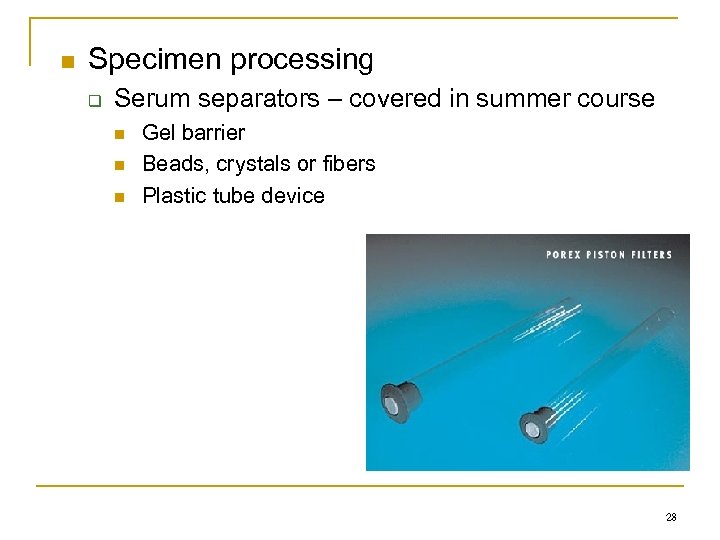
n Specimen processing q Serum separators – covered in summer course n n n Gel barrier Beads, crystals or fibers Plastic tube device 28
n Other / SPECIMEN CONSIDERATIONS q Specimen collection and processing are critical A poor specimen = poor specimen results Most lab errors are pre-analytical !!! q Common sources of error q q n n n Contamination with IV fluids Hemolysis of RBCs contaminates plasma and serum Labeling errors Collection with improper anticoagulants and preservatives Analyzers clogged by clotted specimens 29

n The slides that follow are from another information source and remain here only for general use, at this time. 30
q Collection tubes / Additives n n n n q Red / Black Lavender Orange Blue Gray Green None – Gel separator EDTA anticoagulant Thrombin promotes clotting Sodium citrate anticoagulant Sodium fluoride / Potassium oxalate Heparin anticoagulant Collection order ( to avoid contamination / interference ) n n n 1 2 3 4 5 6 Sterile specimens – Blood Cultures (yellow) Blue Gold / Red / Orange Green Lavender Gray 31
n Colligative Properties q Properties of solutions that are based only on the numbers of particles that are dissolved in the solvent q It doesn’t matter what the particles are or how big they are q Examples of colligative properties Freezing Point Boiling Point Vapor Pressure Osmotic Pressure 32
n Redox Potential ( Oxidation-Reduction Potential) q q If a substance Loses Electrons , it is Oxidized (LEO) It may also be called a Reducing Agent ( donates electrons) q If a substance Gains Electrons , it is Reduced (GER) It may also be called a Oxidizing Agent ( accepts electrons) q Remember … The lion ( LEO ) says “gerr” ( GER ) q n n Conductivity: Measure of electrical current Resistance: Measure of resistance to current 33
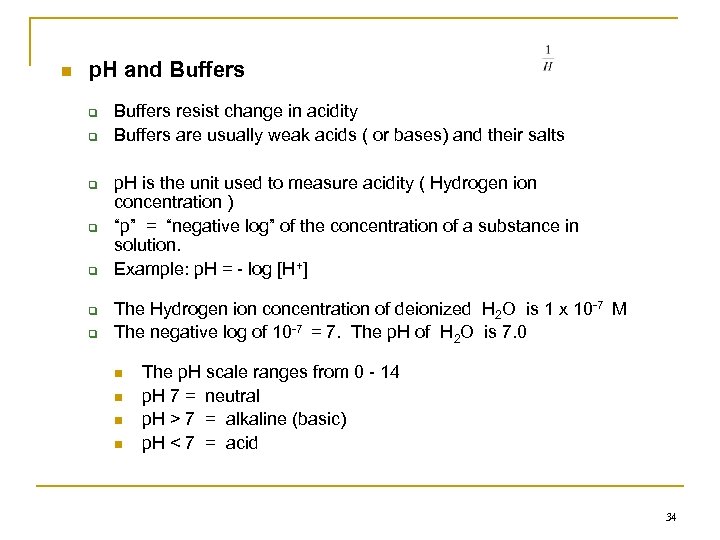
n p. H and Buffers q q q q Buffers resist change in acidity Buffers are usually weak acids ( or bases) and their salts p. H is the unit used to measure acidity ( Hydrogen ion concentration ) “p” = “negative log” of the concentration of a substance in solution. Example: p. H = - log [H+] The Hydrogen ion concentration of deionized H 2 O is 1 x 10 -7 M The negative log of 10 -7 = 7. The p. H of H 2 O is 7. 0 n n The p. H scale ranges from 0 - 14 p. H 7 = neutral p. H > 7 = alkaline (basic) p. H < 7 = acid 34
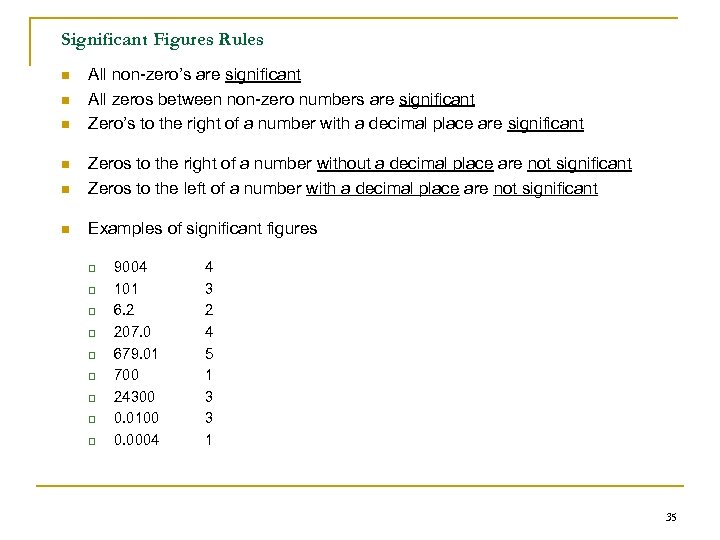
Significant Figures Rules n n n All non-zero’s are significant All zeros between non-zero numbers are significant Zero’s to the right of a number with a decimal place are significant n Zeros to the right of a number without a decimal place are not significant Zeros to the left of a number with a decimal place are not significant n Examples of significant figures n q q q q q 9004 101 6. 2 207. 0 679. 01 700 24300 0. 0100 0. 0004 4 3 2 4 5 1 3 3 1 35

n Conversions q q You must remember this, conversions do NOT change the value of the concentration … Conversions only change the UNITS the value is being expressed in. Whatever we are converting is just as big or small as before we did the conversion. 36
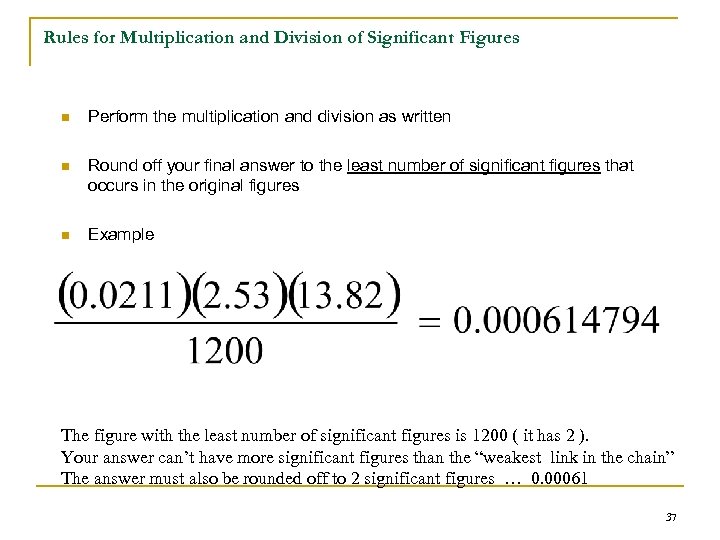
Rules for Multiplication and Division of Significant Figures n Perform the multiplication and division as written n Round off your final answer to the least number of significant figures that occurs in the original figures n Example The figure with the least number of significant figures is 1200 ( it has 2 ). Your answer can’t have more significant figures than the “weakest link in the chain” The answer must also be rounded off to 2 significant figures … 0. 00061 37

n Example of a conversion q How many mls are there in 2. 5 liters? ( this is an easy one ) The question you have to ask yourself is, what is the relationship between liters and mls? The answer : 1 liter = 1000 ml … This is a true statement … But now what? We want to get rid of the “liters’ units and end up with “mls” … Right ? So all you need to do is put in a truthful mathematical statement that gets rid of the stuff you want to lose and adds the stuff you want to pick up … So THIS IS THE SECRET !!! The fraction I created equals 1. 0 … It doesn’t change the value! I wrote it with the Liter on the bottom so it would cancel out the Liter on the top … and I also picked up the mls I need. All conversions use this strategy 38
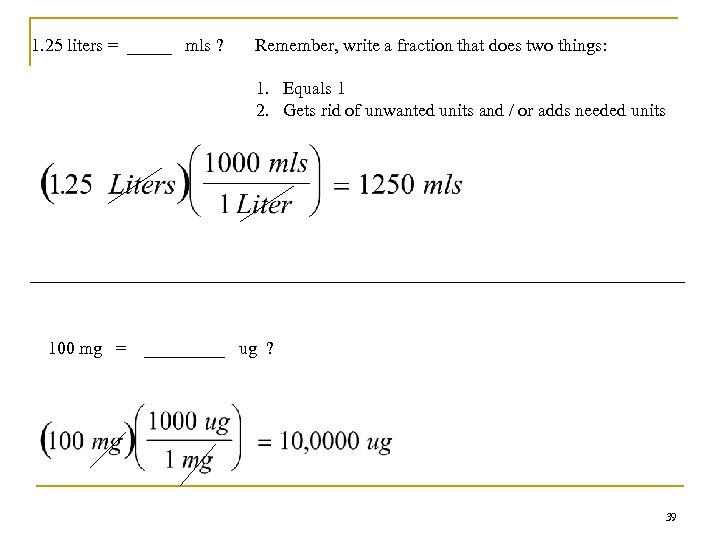
1. 25 liters = _____ mls ? Remember, write a fraction that does two things: 1. Equals 1 2. Gets rid of unwanted units and / or adds needed units 100 mg = _____ ug ? 39
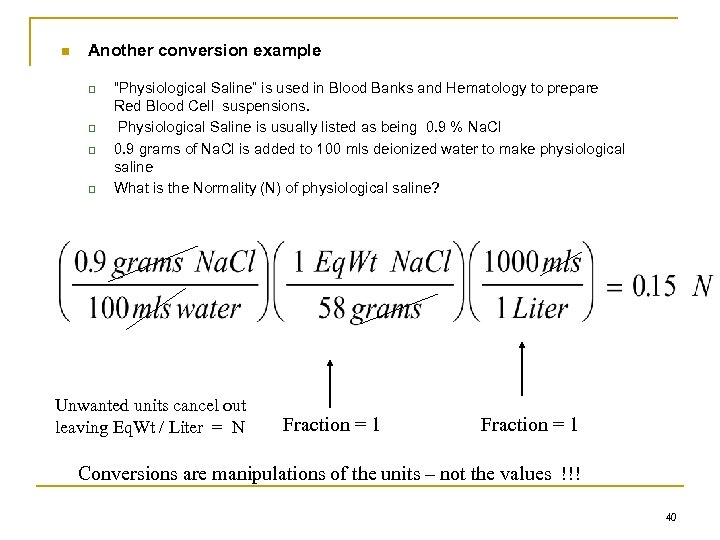
n Another conversion example q q “Physiological Saline” is used in Blood Banks and Hematology to prepare Red Blood Cell suspensions. Physiological Saline is usually listed as being 0. 9 % Na. Cl 0. 9 grams of Na. Cl is added to 100 mls deionized water to make physiological saline What is the Normality (N) of physiological saline? Unwanted units cancel out leaving Eq. Wt / Liter = N Fraction = 1 Conversions are manipulations of the units – not the values !!! 40
n Dilutions q q q A dilution is a numerical ratio of the original material to the final volume ( after the addition of a diluent ) Dilutions of serum or plasma are required when the concentration of a chemical substance being measured exceeds the linearity of the test methodology Example n A plasma glucose concentration exceeds the analyzer’s ability to accurately measure it. The automated analyzer is programmed to dilute the specimen 1: 2. n The concentration of the diluted specimen must be multiplied by 2 , the dilution factor ( the reciprocal of the dilution ) to correct for the dilution of the specimen. 41
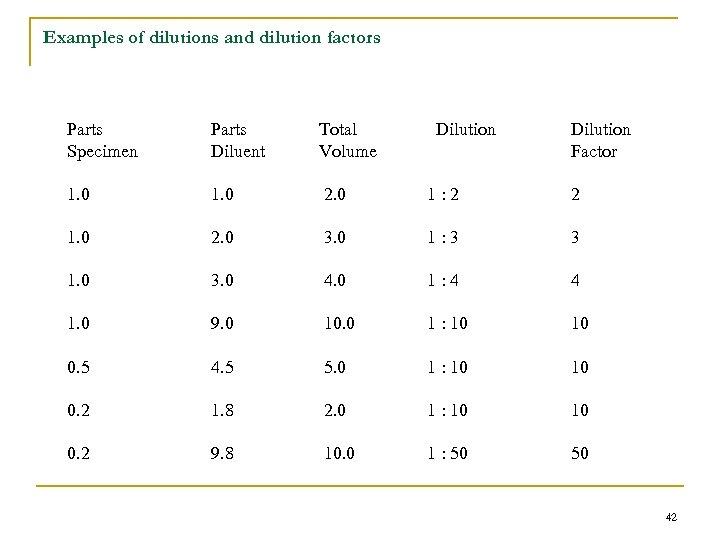
Examples of dilutions and dilution factors Parts Specimen Parts Diluent Total Volume Dilution Factor 1. 0 2. 0 1: 2 2 1. 0 2. 0 3. 0 1: 3 3 1. 0 3. 0 4. 0 1: 4 4 1. 0 9. 0 10. 0 1 : 10 10 0. 5 4. 5 5. 0 1 : 10 10 0. 2 1. 8 2. 0 1 : 10 10 0. 2 9. 8 10. 0 1 : 50 50 42
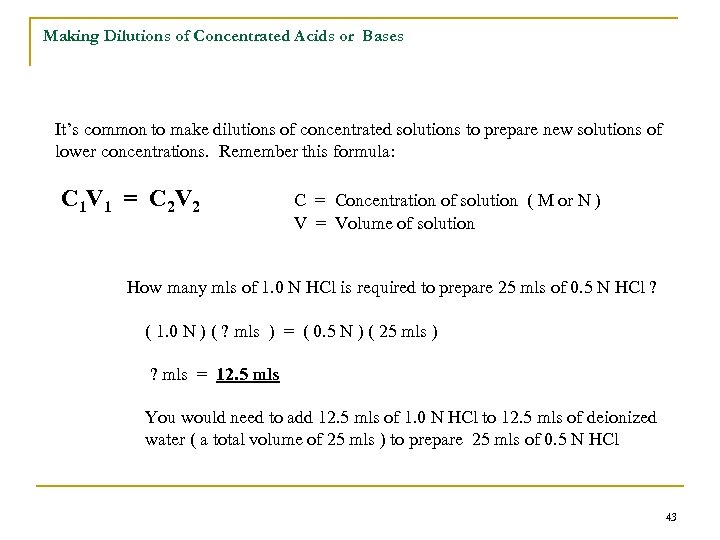
Making Dilutions of Concentrated Acids or Bases It’s common to make dilutions of concentrated solutions to prepare new solutions of lower concentrations. Remember this formula: C 1 V 1 = C 2 V 2 C = Concentration of solution ( M or N ) V = Volume of solution How many mls of 1. 0 N HCl is required to prepare 25 mls of 0. 5 N HCl ? ( 1. 0 N ) ( ? mls ) = ( 0. 5 N ) ( 25 mls ) ? mls = 12. 5 mls You would need to add 12. 5 mls of 1. 0 N HCl to 12. 5 mls of deionized water ( a total volume of 25 mls ) to prepare 25 mls of 0. 5 N HCl 43

TOP 10 n Know those prefixes !!! Molarity = Moles / Liter Molality = Moles / 1000 grams solvent n Normality = Eq Wt / Liter n Per Cent Solutions = parts / 100 … ( Be careful if your dealing with liquids and solid materials ) n Do some simple conversions n TD pipet ( don’t blow out ) … TC ( blow out ) n Buffers resist changes in p. H ( p = - log ) n A dilution is a ratio of original material to the final total volume n “The lion says gerr” 44

45

46
611d049b28a82b965592dea8e2736f99.ppt