64cd7ad31f2d9adfd3186286ad072af7.ppt
- Количество слайдов: 42
 Challenging Cases in Cancer: Integration of Findings from ASCO 2007 Gastrointestinal Stromal Tumors Charles D. Blanke, MD, FACP Associate Professor of Medicine Director of the Oregon Cancer Center’s Gastrointestinal Malignancies Focus Group
Challenging Cases in Cancer: Integration of Findings from ASCO 2007 Gastrointestinal Stromal Tumors Charles D. Blanke, MD, FACP Associate Professor of Medicine Director of the Oregon Cancer Center’s Gastrointestinal Malignancies Focus Group
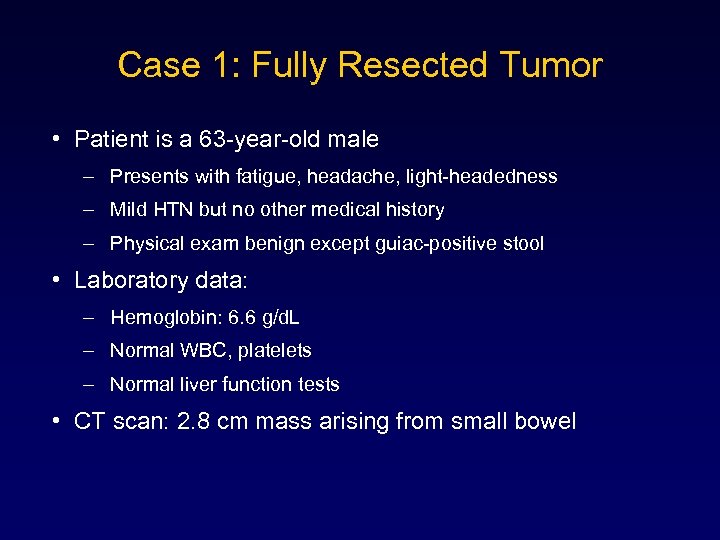 Case 1: Fully Resected Tumor • Patient is a 63 -year-old male – Presents with fatigue, headache, light-headedness – Mild HTN but no other medical history – Physical exam benign except guiac-positive stool • Laboratory data: – Hemoglobin: 6. 6 g/d. L – Normal WBC, platelets – Normal liver function tests • CT scan: 2. 8 cm mass arising from small bowel
Case 1: Fully Resected Tumor • Patient is a 63 -year-old male – Presents with fatigue, headache, light-headedness – Mild HTN but no other medical history – Physical exam benign except guiac-positive stool • Laboratory data: – Hemoglobin: 6. 6 g/d. L – Normal WBC, platelets – Normal liver function tests • CT scan: 2. 8 cm mass arising from small bowel
 Decision Point • What is your major differential diagnosis? • Are there any other tests you would like to do? • Do you want a biopsy?
Decision Point • What is your major differential diagnosis? • Are there any other tests you would like to do? • Do you want a biopsy?
 Case 1: Fully Resected Tumor (cont. ) • Patient goes directly to surgery • Small mass arising from the small bowel is resected – No metastases are seen • Recovery is uneventful • Pathology: 2. 0 cm GIST, CD-117+, arising from small bowel – Ulcerated – negative margins – <1 mitosis/10 HPFs
Case 1: Fully Resected Tumor (cont. ) • Patient goes directly to surgery • Small mass arising from the small bowel is resected – No metastases are seen • Recovery is uneventful • Pathology: 2. 0 cm GIST, CD-117+, arising from small bowel – Ulcerated – negative margins – <1 mitosis/10 HPFs
 Case 1: Additional Decisions • Do you want additional testing on the specimen? • Do you tell the patient his tumor is benign or malignant? • Do you treat the patient adjuvantly? • How do you monitor him?
Case 1: Additional Decisions • Do you want additional testing on the specimen? • Do you tell the patient his tumor is benign or malignant? • Do you treat the patient adjuvantly? • How do you monitor him?
 Case 1: Therapeutic Options • Observe only • Imatinib mesylate 400 mg/day for 1 -year • Imatinib mesylate 800 mg/day for 1 -year • Sunitinib malate 50 mg/day weeks 4/6 for 1 -year • Clinical trial
Case 1: Therapeutic Options • Observe only • Imatinib mesylate 400 mg/day for 1 -year • Imatinib mesylate 800 mg/day for 1 -year • Sunitinib malate 50 mg/day weeks 4/6 for 1 -year • Clinical trial
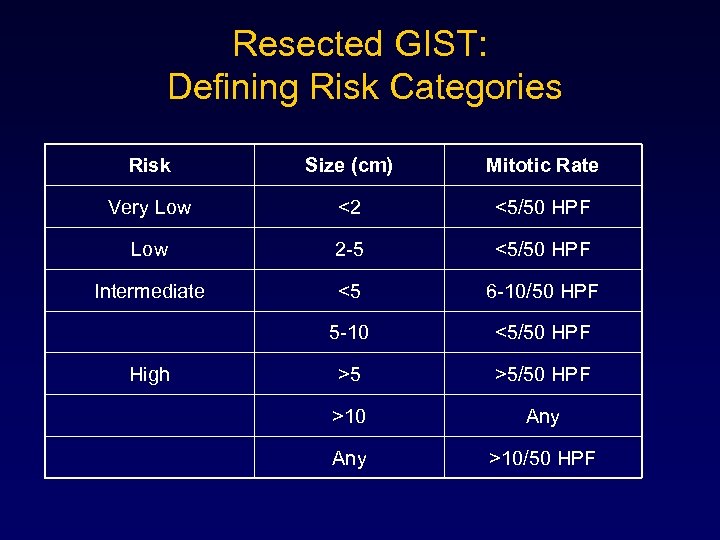 Resected GIST: Defining Risk Categories Risk Size (cm) Mitotic Rate Very Low <2 <5/50 HPF Low 2 -5 <5/50 HPF Intermediate <5 6 -10/50 HPF 5 -10 <5/50 HPF >5 >5/50 HPF >10 Any >10/50 HPF High
Resected GIST: Defining Risk Categories Risk Size (cm) Mitotic Rate Very Low <2 <5/50 HPF Low 2 -5 <5/50 HPF Intermediate <5 6 -10/50 HPF 5 -10 <5/50 HPF >5 >5/50 HPF >10 Any >10/50 HPF High
 Adjuvant Imatinib Mesylate Increases Recurrence Free Survival (RFS) in Patients with Completely Resected Localized Primary Gastrointestinal Stromal Tumor (GIST): North American Intergroup Phase III Trial ACOSOG Z 9001 R. De. Matteo, K. Owzar, R. Maki, P. Pisters, M. Blackstein, C. Antonescu, C. Blanke, G. Demetri, M. von Mehren, K. Ballman, and the American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team R. De. Matteo et al. ASCO 2007 Abstract: 10079
Adjuvant Imatinib Mesylate Increases Recurrence Free Survival (RFS) in Patients with Completely Resected Localized Primary Gastrointestinal Stromal Tumor (GIST): North American Intergroup Phase III Trial ACOSOG Z 9001 R. De. Matteo, K. Owzar, R. Maki, P. Pisters, M. Blackstein, C. Antonescu, C. Blanke, G. Demetri, M. von Mehren, K. Ballman, and the American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team R. De. Matteo et al. ASCO 2007 Abstract: 10079
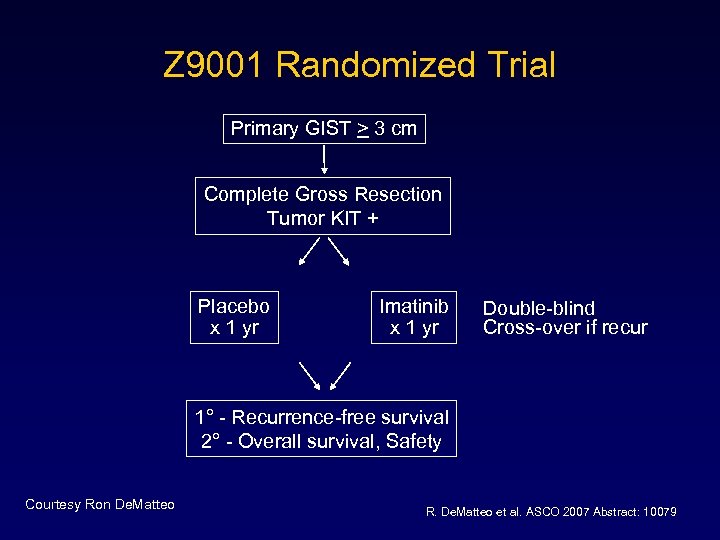 Z 9001 Randomized Trial Primary GIST > 3 cm Complete Gross Resection Tumor KIT + Placebo x 1 yr Imatinib x 1 yr Double-blind Cross-over if recur 1° - Recurrence-free survival 2° - Overall survival, Safety Courtesy Ron De. Matteo R. De. Matteo et al. ASCO 2007 Abstract: 10079
Z 9001 Randomized Trial Primary GIST > 3 cm Complete Gross Resection Tumor KIT + Placebo x 1 yr Imatinib x 1 yr Double-blind Cross-over if recur 1° - Recurrence-free survival 2° - Overall survival, Safety Courtesy Ron De. Matteo R. De. Matteo et al. ASCO 2007 Abstract: 10079
 Z 9001: Results • One-year RFS 83% (placebo) versus 97% imatinib – HR 0. 33, P <0. 001 • No difference in overall survival seen • 33% of patients on imatinib arm could not complete one year of therapy R. De. Matteo et al. ASCO 2007 Abstract: 10079
Z 9001: Results • One-year RFS 83% (placebo) versus 97% imatinib – HR 0. 33, P <0. 001 • No difference in overall survival seen • 33% of patients on imatinib arm could not complete one year of therapy R. De. Matteo et al. ASCO 2007 Abstract: 10079
 Case 1: Therapeutic Options Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day for 1 -year q Imatinib mesylate 800 mg/day for 1 -year q Sunitinib malate 50 mg/day weeks 4/6 for 1 -year q Clinical trial
Case 1: Therapeutic Options Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day for 1 -year q Imatinib mesylate 800 mg/day for 1 -year q Sunitinib malate 50 mg/day weeks 4/6 for 1 -year q Clinical trial
 Case 1: Therapeutic Recommendation Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day for 1 -year q Imatinib mesylate 800 mg/day for 1 -year q Sunitinib malate 50 mg/day weeks 4/6 for 1 -year q Clinical trial Recommended Approach: • Observe only
Case 1: Therapeutic Recommendation Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day for 1 -year q Imatinib mesylate 800 mg/day for 1 -year q Sunitinib malate 50 mg/day weeks 4/6 for 1 -year q Clinical trial Recommended Approach: • Observe only
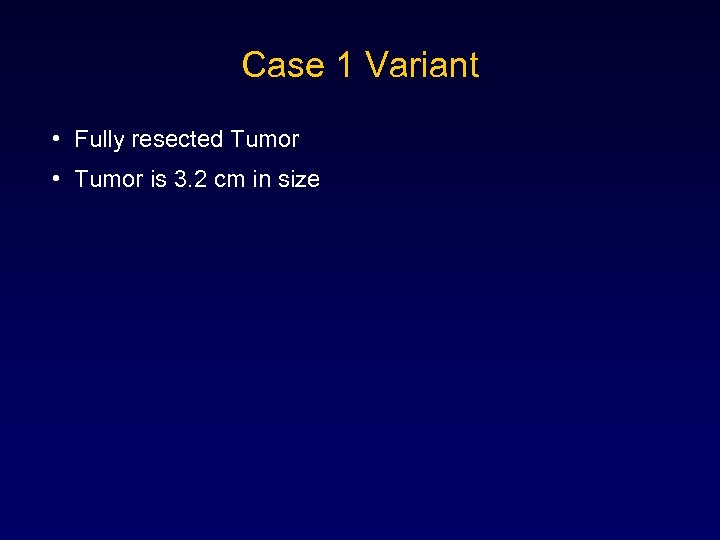 Case 1 Variant • Fully resected Tumor • Tumor is 3. 2 cm in size
Case 1 Variant • Fully resected Tumor • Tumor is 3. 2 cm in size
 Decisions Point • Do you want additional testing on the specimen? • Do you tell the patient his tumor is benign or malignant? • Do you treat the patient adjuvantly? • How do you monitor him?
Decisions Point • Do you want additional testing on the specimen? • Do you tell the patient his tumor is benign or malignant? • Do you treat the patient adjuvantly? • How do you monitor him?
 Case 1 Variant: Therapeutic Options Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day for 1 -year q Imatinib mesylate 800 mg/day for 1 -year q Sunitinib malate 50 mg/day weeks 4/6 for 1 -year q Clinical trial
Case 1 Variant: Therapeutic Options Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day for 1 -year q Imatinib mesylate 800 mg/day for 1 -year q Sunitinib malate 50 mg/day weeks 4/6 for 1 -year q Clinical trial
 Case 1 Variant: Therapeutic Recommendation Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day for 1 -year q Imatinib mesylate 800 mg/day for 1 -year q Sunitinib malate 50 mg/day weeks 4/6 for 1 -year q Clinical trial Recommended Approach: • Imatinib mesylate 400 mg/day for 1 -year
Case 1 Variant: Therapeutic Recommendation Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day for 1 -year q Imatinib mesylate 800 mg/day for 1 -year q Sunitinib malate 50 mg/day weeks 4/6 for 1 -year q Clinical trial Recommended Approach: • Imatinib mesylate 400 mg/day for 1 -year
 Monitoring Patients with GIST • Depends on risk of recurrence • Includes labs, CT; not PET • Different time-table if on imatinib
Monitoring Patients with GIST • Depends on risk of recurrence • Includes labs, CT; not PET • Different time-table if on imatinib
 Case 1 b: Treatment • Patient develops weight loss, abdominal pain, early satiety 3 years later • Physical exam shows hepatomegaly • Labs essentially normal • CT: multiple liver and peritoneal masses
Case 1 b: Treatment • Patient develops weight loss, abdominal pain, early satiety 3 years later • Physical exam shows hepatomegaly • Labs essentially normal • CT: multiple liver and peritoneal masses
 Decision Point • What is your major differential diagnosis? • Are there any other tests you would like to do? • Do you want a biopsy?
Decision Point • What is your major differential diagnosis? • Are there any other tests you would like to do? • Do you want a biopsy?
 Case 1 b: Therapeutic Options • Observe only • Imatinib mesylate 400 mg/day • Imatinib mesylate 800 mg/day • Sunitinib malate 50 mg/day weeks 4/6 • Clinical trial • Debulk
Case 1 b: Therapeutic Options • Observe only • Imatinib mesylate 400 mg/day • Imatinib mesylate 800 mg/day • Sunitinib malate 50 mg/day weeks 4/6 • Clinical trial • Debulk
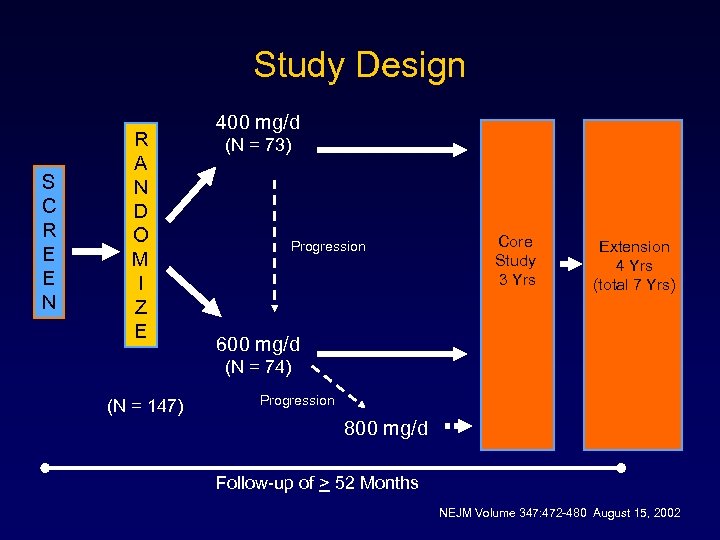 Study Design S C R E E N R A N D O M I Z E 400 mg/d (N = 73) Progression Core Study 3 Yrs Extension 4 Yrs (total 7 Yrs) 600 mg/d (N = 74) (N = 147) Progression 800 mg/d Follow-up of > 52 Months NEJM Volume 347: 472 -480 August 15, 2002
Study Design S C R E E N R A N D O M I Z E 400 mg/d (N = 73) Progression Core Study 3 Yrs Extension 4 Yrs (total 7 Yrs) 600 mg/d (N = 74) (N = 147) Progression 800 mg/d Follow-up of > 52 Months NEJM Volume 347: 472 -480 August 15, 2002
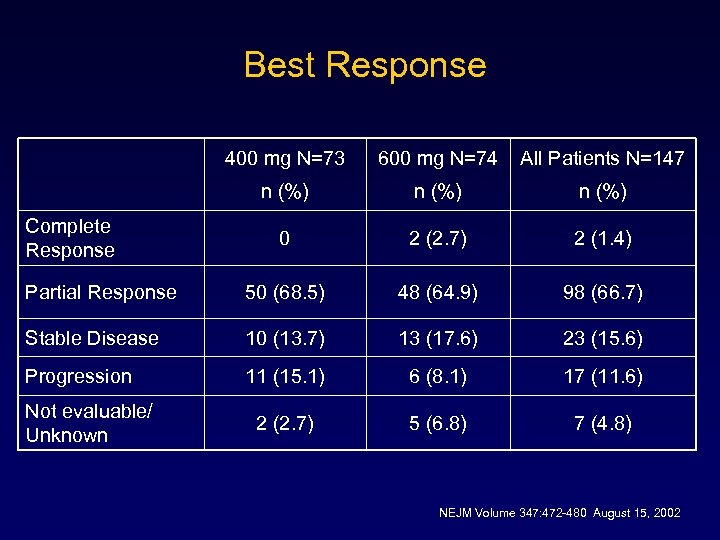 Best Response 400 mg N=73 600 mg N=74 All Patients N=147 n (%) 0 2 (2. 7) 2 (1. 4) Partial Response 50 (68. 5) 48 (64. 9) 98 (66. 7) Stable Disease 10 (13. 7) 13 (17. 6) 23 (15. 6) Progression 11 (15. 1) 6 (8. 1) 17 (11. 6) 2 (2. 7) 5 (6. 8) 7 (4. 8) Complete Response Not evaluable/ Unknown NEJM Volume 347: 472 -480 August 15, 2002
Best Response 400 mg N=73 600 mg N=74 All Patients N=147 n (%) 0 2 (2. 7) 2 (1. 4) Partial Response 50 (68. 5) 48 (64. 9) 98 (66. 7) Stable Disease 10 (13. 7) 13 (17. 6) 23 (15. 6) Progression 11 (15. 1) 6 (8. 1) 17 (11. 6) 2 (2. 7) 5 (6. 8) 7 (4. 8) Complete Response Not evaluable/ Unknown NEJM Volume 347: 472 -480 August 15, 2002
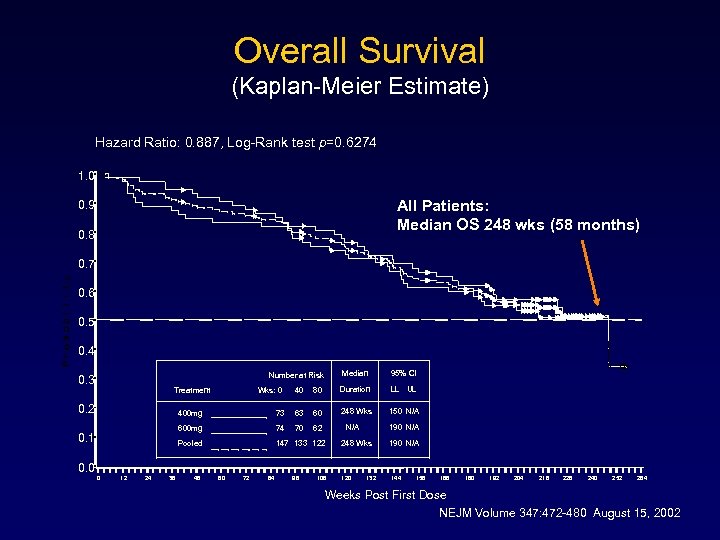 Overall Survival (Kaplan-Meier Estimate) Hazard Ratio: 0. 887, Log-Rank test p=0. 6274 1. 0 All Patients: Median OS 248 wks (58 months) 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 Median Number at Risk 0. 3 Treatment 0. 2 Wks: 0 40 80 Duration LL UL 248 Wks 150 N/A 400 mg 73 63 60 600 mg 74 70 62 Pooled 0. 1 95% CI 147 133 122 N/A 190 N/A 248 Wks 190 N/A 0. 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 168 180 192 204 216 228 240 252 264 Weeks Post First Dose NEJM Volume 347: 472 -480 August 15, 2002
Overall Survival (Kaplan-Meier Estimate) Hazard Ratio: 0. 887, Log-Rank test p=0. 6274 1. 0 All Patients: Median OS 248 wks (58 months) 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 Median Number at Risk 0. 3 Treatment 0. 2 Wks: 0 40 80 Duration LL UL 248 Wks 150 N/A 400 mg 73 63 60 600 mg 74 70 62 Pooled 0. 1 95% CI 147 133 122 N/A 190 N/A 248 Wks 190 N/A 0. 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 168 180 192 204 216 228 240 252 264 Weeks Post First Dose NEJM Volume 347: 472 -480 August 15, 2002
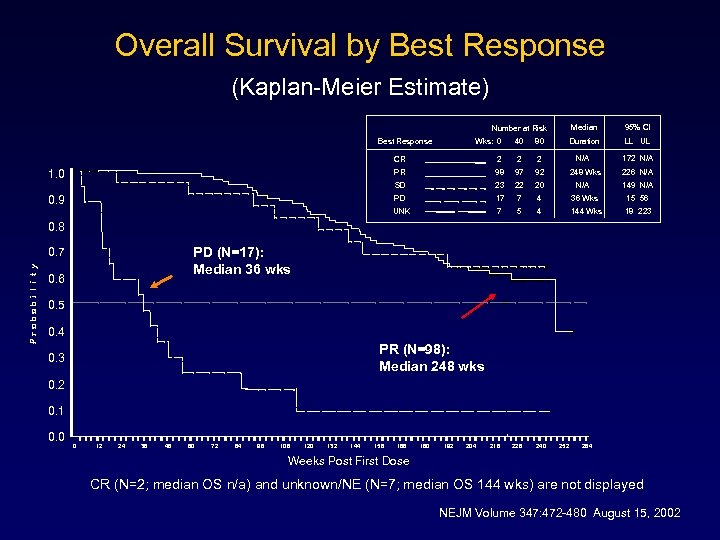 Overall Survival by Best Response (Kaplan-Meier Estimate) Median Best Response Wks: 0 40 80 95% CI Duration Number at Risk LL UL N/A 172 N/A CR 2 97 92 23 22 20 PD 17 7 4 36 Wks 15 56 UNK 0. 9 2 98 SD 1. 0 2 PR 7 5 4 144 Wks 18 223 248 Wks N/A 226 N/A 149 N/A 0. 8 PD (N=17): Median 36 wks 0. 7 0. 6 0. 5 0. 4 PR (N=98): Median 248 wks 0. 3 0. 2 0. 1 0. 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 168 180 192 204 216 228 240 252 264 Weeks Post First Dose CR (N=2; median OS n/a) and unknown/NE (N=7; median OS 144 wks) are not displayed NEJM Volume 347: 472 -480 August 15, 2002
Overall Survival by Best Response (Kaplan-Meier Estimate) Median Best Response Wks: 0 40 80 95% CI Duration Number at Risk LL UL N/A 172 N/A CR 2 97 92 23 22 20 PD 17 7 4 36 Wks 15 56 UNK 0. 9 2 98 SD 1. 0 2 PR 7 5 4 144 Wks 18 223 248 Wks N/A 226 N/A 149 N/A 0. 8 PD (N=17): Median 36 wks 0. 7 0. 6 0. 5 0. 4 PR (N=98): Median 248 wks 0. 3 0. 2 0. 1 0. 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 168 180 192 204 216 228 240 252 264 Weeks Post First Dose CR (N=2; median OS n/a) and unknown/NE (N=7; median OS 144 wks) are not displayed NEJM Volume 347: 472 -480 August 15, 2002
 Comparison of Two Doses of Imatinib for the Treatment of Gastrointestinal Stromal Tumors (GIST): A Meta-analysis Based on 1640 Patients M. M. Van Glabbeke, K. Owzar, C. Rankin, J. Simes, J. Crowley, GIST Meta-analysis Group (Meta. GIST) M. M. Van Glabbeke et al. ASCO 2007: 10004
Comparison of Two Doses of Imatinib for the Treatment of Gastrointestinal Stromal Tumors (GIST): A Meta-analysis Based on 1640 Patients M. M. Van Glabbeke, K. Owzar, C. Rankin, J. Simes, J. Crowley, GIST Meta-analysis Group (Meta. GIST) M. M. Van Glabbeke et al. ASCO 2007: 10004
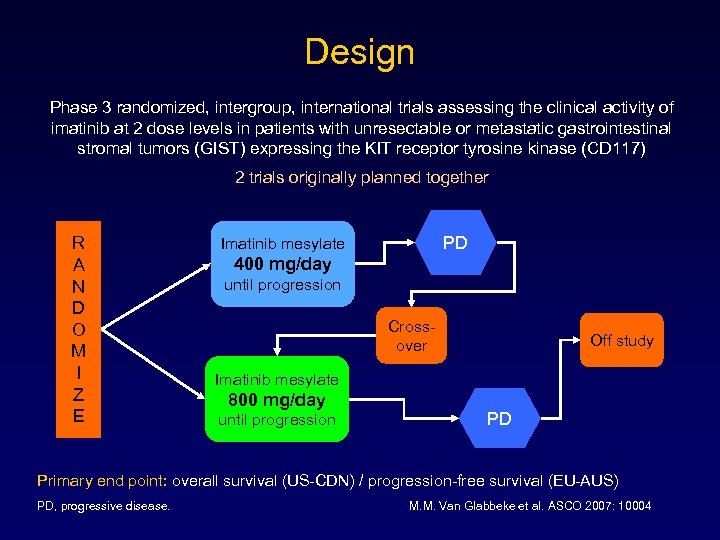 Design Phase 3 randomized, intergroup, international trials assessing the clinical activity of imatinib at 2 dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors (GIST) expressing the KIT receptor tyrosine kinase (CD 117) 2 trials originally planned together R A N D O M I Z E PD Imatinib mesylate 400 mg/day until progression Crossover Off study Imatinib mesylate 800 mg/day until progression PD Primary end point: overall survival (US-CDN) / progression-free survival (EU-AUS) PD, progressive disease. M. M. Van Glabbeke et al. ASCO 2007: 10004
Design Phase 3 randomized, intergroup, international trials assessing the clinical activity of imatinib at 2 dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors (GIST) expressing the KIT receptor tyrosine kinase (CD 117) 2 trials originally planned together R A N D O M I Z E PD Imatinib mesylate 400 mg/day until progression Crossover Off study Imatinib mesylate 800 mg/day until progression PD Primary end point: overall survival (US-CDN) / progression-free survival (EU-AUS) PD, progressive disease. M. M. Van Glabbeke et al. ASCO 2007: 10004
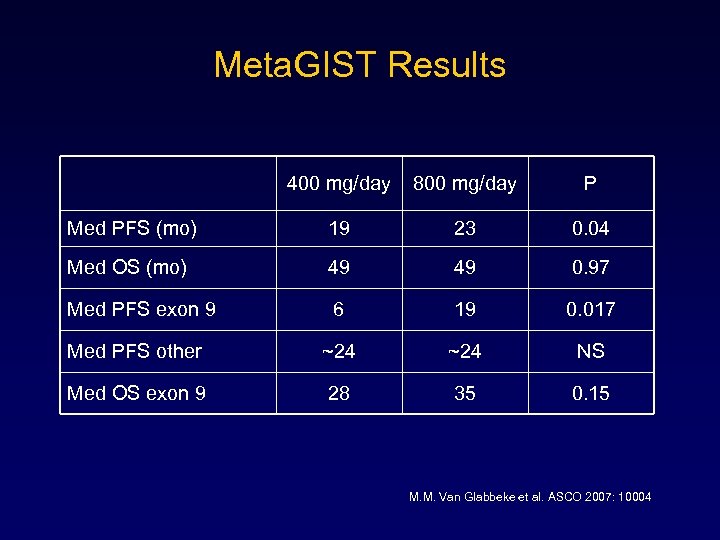 Meta. GIST Results 400 mg/day 800 mg/day P Med PFS (mo) 19 23 0. 04 Med OS (mo) 49 49 0. 97 Med PFS exon 9 6 19 0. 017 Med PFS other ~24 NS Med OS exon 9 28 35 0. 15 M. M. Van Glabbeke et al. ASCO 2007: 10004
Meta. GIST Results 400 mg/day 800 mg/day P Med PFS (mo) 19 23 0. 04 Med OS (mo) 49 49 0. 97 Med PFS exon 9 6 19 0. 017 Med PFS other ~24 NS Med OS exon 9 28 35 0. 15 M. M. Van Glabbeke et al. ASCO 2007: 10004
 Case 1 b: Therapeutic Recommendation Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day q Imatinib mesylate 800 mg/day q Sunitinib malate 50 mg/day weeks 4/6 q Clinical trial q Debulk Recommended Approach: • Imatinib mesylate 400 mg/day or 800 mg/day
Case 1 b: Therapeutic Recommendation Which treatment option would you recommend? q Observe only q Imatinib mesylate 400 mg/day q Imatinib mesylate 800 mg/day q Sunitinib malate 50 mg/day weeks 4/6 q Clinical trial q Debulk Recommended Approach: • Imatinib mesylate 400 mg/day or 800 mg/day
 Case 2: Advanced GIST • 54 -year-old female presents for “screening” CT – 6 cm peritoneal mass found – Biopsy: CD-117+ GIST, exon 11 mutant – No other disease – No major PMH
Case 2: Advanced GIST • 54 -year-old female presents for “screening” CT – 6 cm peritoneal mass found – Biopsy: CD-117+ GIST, exon 11 mutant – No other disease – No major PMH
 Case 2: Therapeutic Options Which treatment option would you recommend? q Observation q Imatinib mesylate 400 mg/day indefinitely q Imatinib followed by resection q Sunitinib malate 50 mg/day weeks 4/6 q Immediate resection
Case 2: Therapeutic Options Which treatment option would you recommend? q Observation q Imatinib mesylate 400 mg/day indefinitely q Imatinib followed by resection q Sunitinib malate 50 mg/day weeks 4/6 q Immediate resection
 Case 2: Therapeutic Recommendation Which treatment option would you recommend? q Observation q Imatinib mesylate 400 mg/day indefinitely q Imatinib followed by resection q Sunitinib malate 50 mg/day weeks 4/6 q Immediate resection Recommended Approach: • Imatinib followed by resection
Case 2: Therapeutic Recommendation Which treatment option would you recommend? q Observation q Imatinib mesylate 400 mg/day indefinitely q Imatinib followed by resection q Sunitinib malate 50 mg/day weeks 4/6 q Immediate resection Recommended Approach: • Imatinib followed by resection
 Case 2 Variant • Everything identical EXCEPT: – PMH Small bowel leiomyosarcoma resected 6 years ago
Case 2 Variant • Everything identical EXCEPT: – PMH Small bowel leiomyosarcoma resected 6 years ago
 Case 2 Variant: Therapeutic Options Which treatment option would you recommend? q Observation q Imatinib mesylate 400 mg/day indefinitely q Imatinib followed by resection q Sunitinib malate 50 mg/day weeks 4/6 q Immediate resection
Case 2 Variant: Therapeutic Options Which treatment option would you recommend? q Observation q Imatinib mesylate 400 mg/day indefinitely q Imatinib followed by resection q Sunitinib malate 50 mg/day weeks 4/6 q Immediate resection
 Case 2 Variant: Therapeutic Recommendation Which treatment option would you recommend? q Observation q Imatinib mesylate 400 mg/day indefinitely q Imatinib followed by resection q Sunitinib malate 50 mg/day weeks 4/6 q Immediate resection Recommended Approach: • Imatinib followed by resection
Case 2 Variant: Therapeutic Recommendation Which treatment option would you recommend? q Observation q Imatinib mesylate 400 mg/day indefinitely q Imatinib followed by resection q Sunitinib malate 50 mg/day weeks 4/6 q Immediate resection Recommended Approach: • Imatinib followed by resection
 Case 2 Variant 2 • Patient receives imatinib mesylate, 400 mg/day, neoadjuvantly • Tumor rapidly progresses
Case 2 Variant 2 • Patient receives imatinib mesylate, 400 mg/day, neoadjuvantly • Tumor rapidly progresses
 Case 2 Variant 2: Therapeutic Options Which treatment option would you recommend? q Continue imatinib mesylate 400 mg/day q Increase imatinib to 800 mg/day q Sunitinib malate 50 mg/day weeks 4/6 q Sunitinib malate 37. 5 mg/day continuously
Case 2 Variant 2: Therapeutic Options Which treatment option would you recommend? q Continue imatinib mesylate 400 mg/day q Increase imatinib to 800 mg/day q Sunitinib malate 50 mg/day weeks 4/6 q Sunitinib malate 37. 5 mg/day continuously
 Case 2 Variant 2: Therapeutic Recommendation Which treatment option would you recommend? q Continue imatinib mesylate 400 mg/day q Increase imatinib to 800 mg/day q Sunitinib malate 50 mg/day weeks 4/6 q Sunitinib malate 37. 5 mg/day continuously Recommended Approach: • Increase imatinib to 800 mg/day • Sunitinib malate 50 mg/day weeks 4/6 • Sunitinib malate 37. 5 mg/day continuously
Case 2 Variant 2: Therapeutic Recommendation Which treatment option would you recommend? q Continue imatinib mesylate 400 mg/day q Increase imatinib to 800 mg/day q Sunitinib malate 50 mg/day weeks 4/6 q Sunitinib malate 37. 5 mg/day continuously Recommended Approach: • Increase imatinib to 800 mg/day • Sunitinib malate 50 mg/day weeks 4/6 • Sunitinib malate 37. 5 mg/day continuously
 Resistant GIST • 25 -33% of patients benefit from an imatinib doseincrease – However, actual responses are rare • Sunitinib malate has activity against multiple receptor tyrosine kinases + anti-angiogenic activity • A phase III trial showed marked survival benefits for salvage sunitinib versus placebo
Resistant GIST • 25 -33% of patients benefit from an imatinib doseincrease – However, actual responses are rare • Sunitinib malate has activity against multiple receptor tyrosine kinases + anti-angiogenic activity • A phase III trial showed marked survival benefits for salvage sunitinib versus placebo
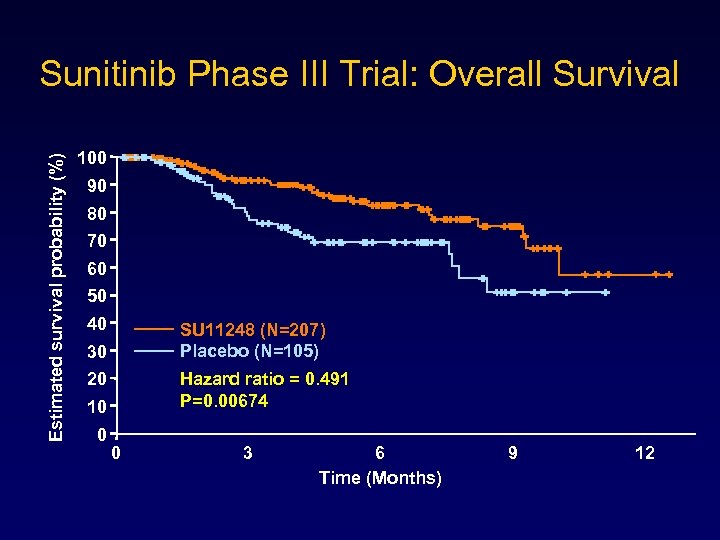 Estimated survival probability (%) Sunitinib Phase III Trial: Overall Survival 100 90 80 70 60 50 40 SU 11248 (N=207) Placebo (N=105) 30 Hazard ratio = 0. 491 P=0. 00674 20 10 0 0 3 6 Time (Months) 9 12
Estimated survival probability (%) Sunitinib Phase III Trial: Overall Survival 100 90 80 70 60 50 40 SU 11248 (N=207) Placebo (N=105) 30 Hazard ratio = 0. 491 P=0. 00674 20 10 0 0 3 6 Time (Months) 9 12
 Additional ASCO 2007 Findings • Continuous daily sunitinib dosing @ 37. 5 mg is probably as safe and effective as 50 mg intermittently – George et al. PASCO 2007, abstr #10015 • Nilotinib (TKR-inhibitor of KIT, PDGFR) has promising activity in GIST pts resistant to imatinib – Von Mehren et al. PASCO 2007, abstr #10023 • IPI-504 (inhibitor of heat shock protein 90 chaperone) has potential activity in pts resistant to imatinib and sunitinib – Demetri et al. PASCO 2007, abstr #10024
Additional ASCO 2007 Findings • Continuous daily sunitinib dosing @ 37. 5 mg is probably as safe and effective as 50 mg intermittently – George et al. PASCO 2007, abstr #10015 • Nilotinib (TKR-inhibitor of KIT, PDGFR) has promising activity in GIST pts resistant to imatinib – Von Mehren et al. PASCO 2007, abstr #10023 • IPI-504 (inhibitor of heat shock protein 90 chaperone) has potential activity in pts resistant to imatinib and sunitinib – Demetri et al. PASCO 2007, abstr #10024
 Other Drugs/Targets in GIST • SU 11248 -PDGFR, VEGFR, KIT, and FLT 3 • BAY 43 -9006 -Raf, KIT, VEGFR, PDGFRβ, FLT 3, RET • AMG 706 -VEGFR, PDGFR, KIT, Ret • BMS 354825 -Src, abl, KIT, PDGFR • PKC 412 -PKC • IPI-504 -Heat shock protein 90 • AMN 107 -KIT, PDGFRA, BCR/ABL • Genasense-bcl-2
Other Drugs/Targets in GIST • SU 11248 -PDGFR, VEGFR, KIT, and FLT 3 • BAY 43 -9006 -Raf, KIT, VEGFR, PDGFRβ, FLT 3, RET • AMG 706 -VEGFR, PDGFR, KIT, Ret • BMS 354825 -Src, abl, KIT, PDGFR • PKC 412 -PKC • IPI-504 -Heat shock protein 90 • AMN 107 -KIT, PDGFRA, BCR/ABL • Genasense-bcl-2
 ASCO 2007: GIST Conclusions • Treat patients with resected tumors ≥ 3 cm with at least 1 -year of imatinib • 400 mg/day remains the standard dose in metastatic disease – Exon 9 patients should probably get 800 mg/day • Sunitinib malate is the standard of care salvage drug for patients with imatinib resistance – Treat with 37. 5 mg/day continuously • New drugs are expected
ASCO 2007: GIST Conclusions • Treat patients with resected tumors ≥ 3 cm with at least 1 -year of imatinib • 400 mg/day remains the standard dose in metastatic disease – Exon 9 patients should probably get 800 mg/day • Sunitinib malate is the standard of care salvage drug for patients with imatinib resistance – Treat with 37. 5 mg/day continuously • New drugs are expected


