78b90382e45ccbb7c7a5f6909ef2fc84.ppt
- Количество слайдов: 33
 Challenges and Opportunities in Enhancement of the CMC Section of NDAs: Quality – by - Design Ajaz S. Hussain, Ph. D. Deputy Director Office of Pharmaceutical Science CDER, FDA DIA Annual Meeting, Washington D. C. , June 2004
Challenges and Opportunities in Enhancement of the CMC Section of NDAs: Quality – by - Design Ajaz S. Hussain, Ph. D. Deputy Director Office of Pharmaceutical Science CDER, FDA DIA Annual Meeting, Washington D. C. , June 2004
 Background: Yesterday at DIA ► Risk Based CMC Review - Moheb Nasr: § The 21 st Century Initiative and the new paradigm for Pharmaceutical Quality § The “Desired State” § New Quality Assessment Paradigm at ONDC (under construction) q Assessment conducted by interdisciplinary scientists (chemists, pharmacists, engineers and others as needed) q Focus on critical quality attributes and their relevance to safety and efficacy (chemistry, formulations, manufacturing processes, dosage forms, product performance, etc. ) q Reliance on knowledge provided by firms q Utilization of Risk-based analysis
Background: Yesterday at DIA ► Risk Based CMC Review - Moheb Nasr: § The 21 st Century Initiative and the new paradigm for Pharmaceutical Quality § The “Desired State” § New Quality Assessment Paradigm at ONDC (under construction) q Assessment conducted by interdisciplinary scientists (chemists, pharmacists, engineers and others as needed) q Focus on critical quality attributes and their relevance to safety and efficacy (chemistry, formulations, manufacturing processes, dosage forms, product performance, etc. ) q Reliance on knowledge provided by firms q Utilization of Risk-based analysis
 Opportunity ► Over the last two decades we have improved our ability to solve complex multi-factorial problems § A systems approach to development – pre-formulation, formulation development, and clinical relevance § Multivariate empirical methods (e. g. , Response Surface Methods) ► New measurement and information technologies § Measurements that can predict performance ► Such information is often filtered out of regulatory submissions § “fear” or “regulatory uncertinty” ► ICH Q 8 can open the door for sharing and utilizing this information
Opportunity ► Over the last two decades we have improved our ability to solve complex multi-factorial problems § A systems approach to development – pre-formulation, formulation development, and clinical relevance § Multivariate empirical methods (e. g. , Response Surface Methods) ► New measurement and information technologies § Measurements that can predict performance ► Such information is often filtered out of regulatory submissions § “fear” or “regulatory uncertinty” ► ICH Q 8 can open the door for sharing and utilizing this information
 Opportunity ► For companies that acquire extensive understanding about their product and manufacturing process and share this with the regulators § Enhanced science and risk-based regulatory quality assessment will be possible ►Setting specifications ►Reduction in the volume of data to be submitted – replaced by more knowledge based submissions ►Flexible post approval continuous improvement
Opportunity ► For companies that acquire extensive understanding about their product and manufacturing process and share this with the regulators § Enhanced science and risk-based regulatory quality assessment will be possible ►Setting specifications ►Reduction in the volume of data to be submitted – replaced by more knowledge based submissions ►Flexible post approval continuous improvement
 Janet Woodcock, M. D. May 19, 2004
Janet Woodcock, M. D. May 19, 2004
 http: //www. fda. gov/cder/gmp/21 stcenturysummary. htm Desired State · Product quality and performance achieved and assured by design of effective and efficient manufacturing processes · Product specifications based on mechanistic understanding of how formulation and process factors impact product performance · Continuous "real time" assurance of quality ICH Q 8 agreed “Desired State”
http: //www. fda. gov/cder/gmp/21 stcenturysummary. htm Desired State · Product quality and performance achieved and assured by design of effective and efficient manufacturing processes · Product specifications based on mechanistic understanding of how formulation and process factors impact product performance · Continuous "real time" assurance of quality ICH Q 8 agreed “Desired State”
 What do we wish to accomplish with ICH Q 8 Ensure Q 8 facilitates movement towards the “desired state” we have articulated ► This will ► § Help us better understand the proposed product and process design and its relation to the intended use ► improve process of establishing regulatory specifications § Improve our ability to identify and understand critical product and process factors ► improve our understanding and confidence in risk mitigation strategies § Allow us to utilize risk based approaches and recognize good science and facilitate continuous improvement § Improve communication and systems thinking ► More efficient review and inspection process § Be a “win win” for public health, regulators and industry
What do we wish to accomplish with ICH Q 8 Ensure Q 8 facilitates movement towards the “desired state” we have articulated ► This will ► § Help us better understand the proposed product and process design and its relation to the intended use ► improve process of establishing regulatory specifications § Improve our ability to identify and understand critical product and process factors ► improve our understanding and confidence in risk mitigation strategies § Allow us to utilize risk based approaches and recognize good science and facilitate continuous improvement § Improve communication and systems thinking ► More efficient review and inspection process § Be a “win win” for public health, regulators and industry
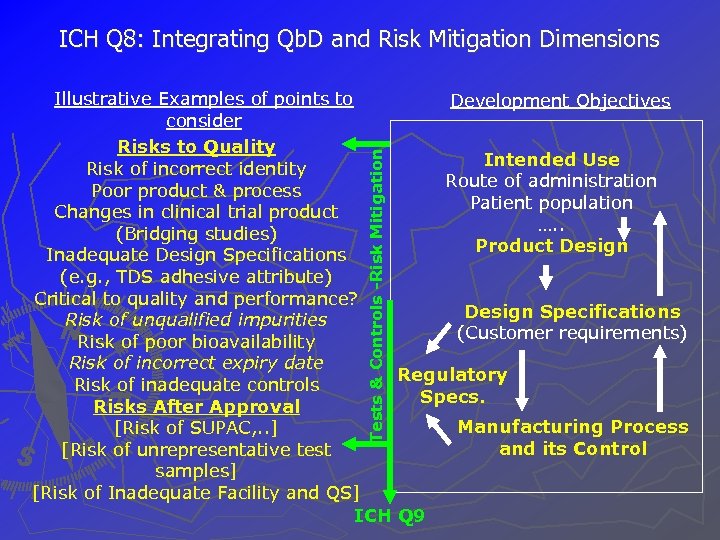 ICH Q 8: Integrating Qb. D and Risk Mitigation Dimensions Tests & Controls -Risk Mitigation Illustrative Examples of points to Development Objectives consider Risks to Quality Intended Use Risk of incorrect identity Route of administration Poor product & process Patient population Changes in clinical trial product …. . (Bridging studies) Product Design Inadequate Design Specifications (e. g. , TDS adhesive attribute) Critical to quality and performance? Design Specifications Risk of unqualified impurities (Customer requirements) Risk of poor bioavailability Risk of incorrect expiry date Regulatory Risk of inadequate controls Specs. Risks After Approval Manufacturing Process [Risk of SUPAC, . . ] and its Control [Risk of unrepresentative test samples] [Risk of Inadequate Facility and QS] ICH Q 9
ICH Q 8: Integrating Qb. D and Risk Mitigation Dimensions Tests & Controls -Risk Mitigation Illustrative Examples of points to Development Objectives consider Risks to Quality Intended Use Risk of incorrect identity Route of administration Poor product & process Patient population Changes in clinical trial product …. . (Bridging studies) Product Design Inadequate Design Specifications (e. g. , TDS adhesive attribute) Critical to quality and performance? Design Specifications Risk of unqualified impurities (Customer requirements) Risk of poor bioavailability Risk of incorrect expiry date Regulatory Risk of inadequate controls Specs. Risks After Approval Manufacturing Process [Risk of SUPAC, . . ] and its Control [Risk of unrepresentative test samples] [Risk of Inadequate Facility and QS] ICH Q 9
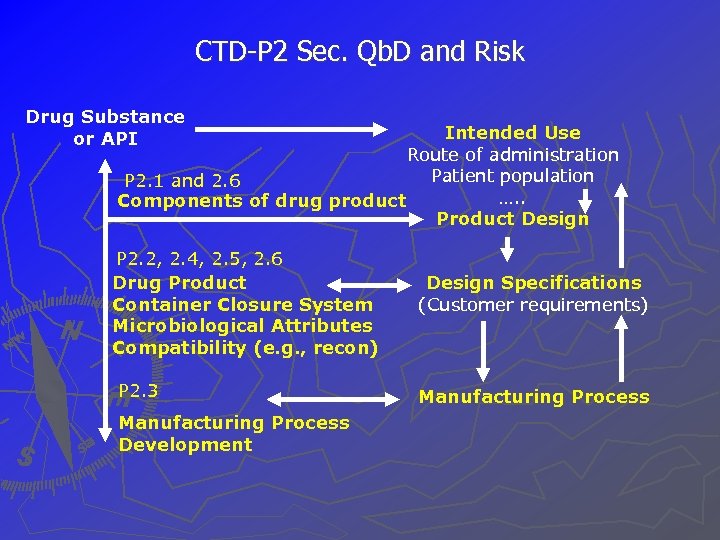 CTD-P 2 Sec. Qb. D and Risk Drug Substance or API Intended Use Route of administration Patient population P 2. 1 and 2. 6 …. . Components of drug product Product Design P 2. 2, 2. 4, 2. 5, 2. 6 Drug Product Container Closure System Microbiological Attributes Compatibility (e. g. , recon) P 2. 3 Manufacturing Process Development Design Specifications (Customer requirements) Manufacturing Process
CTD-P 2 Sec. Qb. D and Risk Drug Substance or API Intended Use Route of administration Patient population P 2. 1 and 2. 6 …. . Components of drug product Product Design P 2. 2, 2. 4, 2. 5, 2. 6 Drug Product Container Closure System Microbiological Attributes Compatibility (e. g. , recon) P 2. 3 Manufacturing Process Development Design Specifications (Customer requirements) Manufacturing Process
 Continuous Improvement – Emerging ICH Q 8 “Design Space” Concept ► Multi-dimensional space defined by critical vectors of product quality and performance § Examples of critical vectors ►Robust manufacturing process – consistent, reproducible delivery of product meeting its specifications § Manufacturing options ►Stability (shelf-life) and ►Bioavailability
Continuous Improvement – Emerging ICH Q 8 “Design Space” Concept ► Multi-dimensional space defined by critical vectors of product quality and performance § Examples of critical vectors ►Robust manufacturing process – consistent, reproducible delivery of product meeting its specifications § Manufacturing options ►Stability (shelf-life) and ►Bioavailability
 What could/should be the ICH Q 8 “Design Space” Concept? § Within this space, available knowledge (derived from established scientific literature, in-house experiments from previous and current projects) provides a basis for reliable (degree of reliability can be linked to risk based decisions) estimation and/or prediction of: ►manufacturing ►stability and ►bioavailability process capability,
What could/should be the ICH Q 8 “Design Space” Concept? § Within this space, available knowledge (derived from established scientific literature, in-house experiments from previous and current projects) provides a basis for reliable (degree of reliability can be linked to risk based decisions) estimation and/or prediction of: ►manufacturing ►stability and ►bioavailability process capability,
 More on the “Design Space” Concept ► This knowledge (preferably – quantitative; e. g. , a valid multivariate mathematical model) is utilized to identify and define formulation and manufacturing factors ranges that provide acceptable product quality and performance § Regulatory assessment and utility for establishing specifications and controls (wider range than the current approach) § Manufacturing options (e. g. , process, equipment; scale, etc. ), parameters within this space should not require prior review/approval and should be addressed within a companies quality system and subject to CGMP inspections ► Change the “change” to Continuous Improvement
More on the “Design Space” Concept ► This knowledge (preferably – quantitative; e. g. , a valid multivariate mathematical model) is utilized to identify and define formulation and manufacturing factors ranges that provide acceptable product quality and performance § Regulatory assessment and utility for establishing specifications and controls (wider range than the current approach) § Manufacturing options (e. g. , process, equipment; scale, etc. ), parameters within this space should not require prior review/approval and should be addressed within a companies quality system and subject to CGMP inspections ► Change the “change” to Continuous Improvement
 Data based decisions: No Generalization Current CMC Submissions raw material properties process conditions environmental
Data based decisions: No Generalization Current CMC Submissions raw material properties process conditions environmental
 Knowledge based decisions: Improved Ability to Generalize Pharmaceutical Development Knowledge raw material properties process conditions environmental Robust process Stable and Bioavailable product
Knowledge based decisions: Improved Ability to Generalize Pharmaceutical Development Knowledge raw material properties process conditions environmental Robust process Stable and Bioavailable product
 ICH Q 8 Company’s Quality system Process Understanding Post approval change CMC regulatory oversight c. GMP regulatory oversight Risk ICH Q 8&9
ICH Q 8 Company’s Quality system Process Understanding Post approval change CMC regulatory oversight c. GMP regulatory oversight Risk ICH Q 8&9
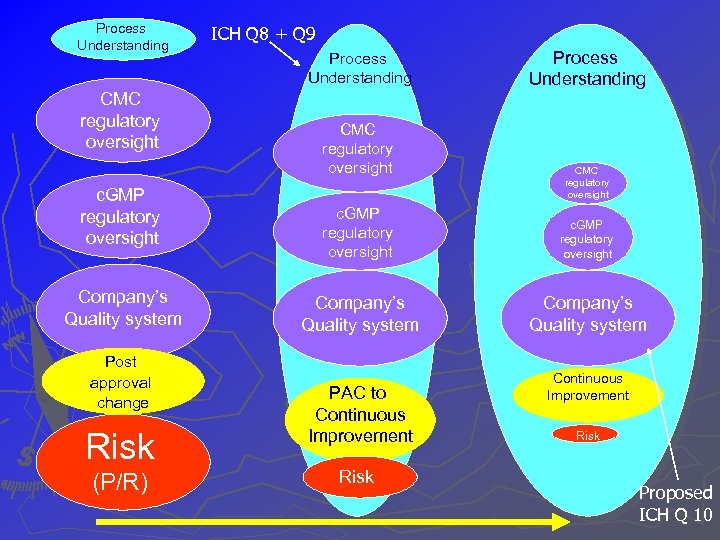 Process Understanding CMC regulatory oversight c. GMP regulatory oversight Company’s Quality system Post approval change Risk (P/R) ICH Q 8 + Q 9 Process Understanding CMC regulatory oversight c. GMP regulatory oversight Company’s Quality system PAC to Continuous Improvement Risk Proposed ICH Q 10
Process Understanding CMC regulatory oversight c. GMP regulatory oversight Company’s Quality system Post approval change Risk (P/R) ICH Q 8 + Q 9 Process Understanding CMC regulatory oversight c. GMP regulatory oversight Company’s Quality system PAC to Continuous Improvement Risk Proposed ICH Q 10
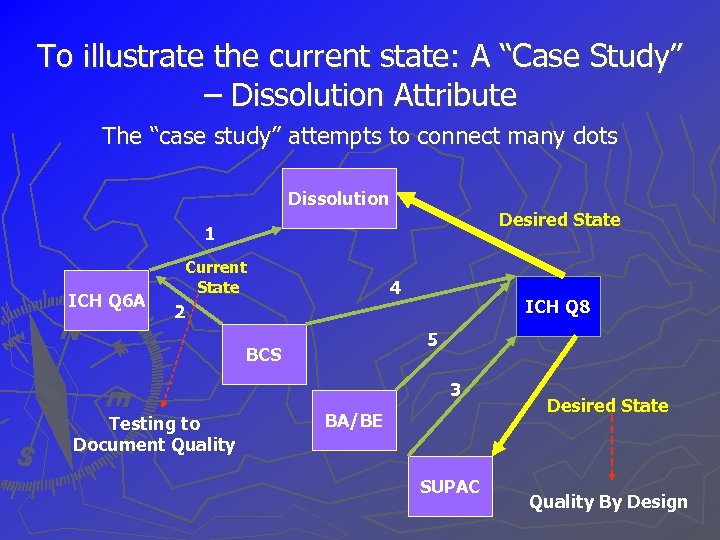 To illustrate the current state: A “Case Study” – Dissolution Attribute The “case study” attempts to connect many dots Dissolution Desired State 1 ICH Q 6 A Current State 4 ICH Q 8 2 5 BCS 3 Testing to Document Quality BA/BE SUPAC Desired State Quality By Design
To illustrate the current state: A “Case Study” – Dissolution Attribute The “case study” attempts to connect many dots Dissolution Desired State 1 ICH Q 6 A Current State 4 ICH Q 8 2 5 BCS 3 Testing to Document Quality BA/BE SUPAC Desired State Quality By Design
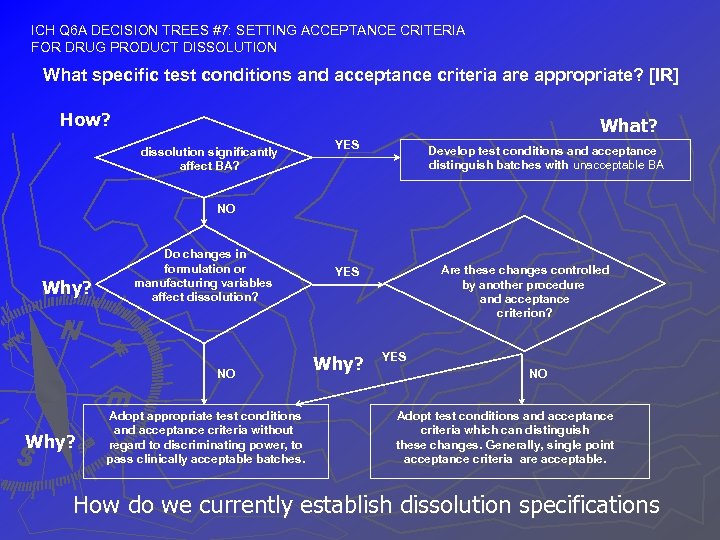 ICH Q 6 A DECISION TREES #7: SETTING ACCEPTANCE CRITERIA FOR DRUG PRODUCT DISSOLUTION What specific test conditions and acceptance criteria are appropriate? [IR] How? What? dissolution significantly affect BA? YES Develop test conditions and acceptance distinguish batches with unacceptable BA NO Why? Do changes in formulation or manufacturing variables affect dissolution? NO Why? Adopt appropriate test conditions and acceptance criteria without regard to discriminating power, to pass clinically acceptable batches. Are these changes controlled by another procedure and acceptance criterion? YES Why? YES NO Adopt test conditions and acceptance criteria which can distinguish these changes. Generally, single point acceptance criteria are acceptable. How do we currently establish dissolution specifications
ICH Q 6 A DECISION TREES #7: SETTING ACCEPTANCE CRITERIA FOR DRUG PRODUCT DISSOLUTION What specific test conditions and acceptance criteria are appropriate? [IR] How? What? dissolution significantly affect BA? YES Develop test conditions and acceptance distinguish batches with unacceptable BA NO Why? Do changes in formulation or manufacturing variables affect dissolution? NO Why? Adopt appropriate test conditions and acceptance criteria without regard to discriminating power, to pass clinically acceptable batches. Are these changes controlled by another procedure and acceptance criterion? YES Why? YES NO Adopt test conditions and acceptance criteria which can distinguish these changes. Generally, single point acceptance criteria are acceptable. How do we currently establish dissolution specifications
 Without adequate product and process development and/or knowledge sharing ► Without “design” consideration, high level of uncertainty with respect to critical attributes, “representative” test sample, and adequacy of risk coverage (e. g. , compendail tests) to assure batch quality [Regulatory Concern/Risk] ► Reduce concern/risk by covering all apparent attributes with acceptance criteria based on capability of test methods and/or manufacturing process plus very inflexible SOP’s [Current Regulatory Risk Mitigation Strategy]
Without adequate product and process development and/or knowledge sharing ► Without “design” consideration, high level of uncertainty with respect to critical attributes, “representative” test sample, and adequacy of risk coverage (e. g. , compendail tests) to assure batch quality [Regulatory Concern/Risk] ► Reduce concern/risk by covering all apparent attributes with acceptance criteria based on capability of test methods and/or manufacturing process plus very inflexible SOP’s [Current Regulatory Risk Mitigation Strategy]
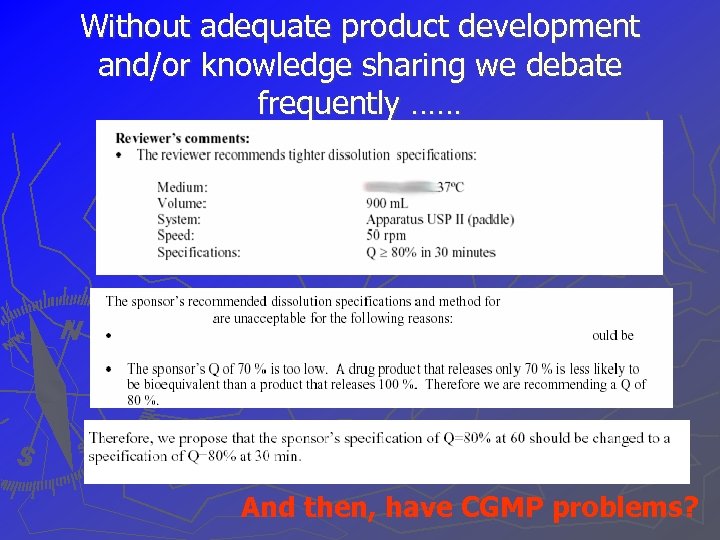 Without adequate product development and/or knowledge sharing we debate frequently …… And then, have CGMP problems?
Without adequate product development and/or knowledge sharing we debate frequently …… And then, have CGMP problems?
 A Warning Letter This can be catastrophic for the business and availability of Important drugs
A Warning Letter This can be catastrophic for the business and availability of Important drugs
 OOS or Exceptions Further Increase Cycle Times (Source: G. K. Raju, M. I. T. FDA Science Board Meeting, November 16, 2001) Dissolution
OOS or Exceptions Further Increase Cycle Times (Source: G. K. Raju, M. I. T. FDA Science Board Meeting, November 16, 2001) Dissolution
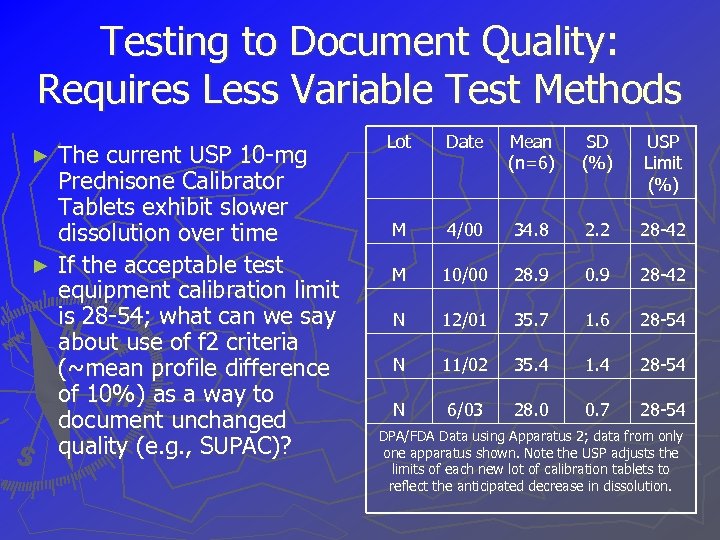 Testing to Document Quality: Requires Less Variable Test Methods The current USP 10 -mg Prednisone Calibrator Tablets exhibit slower dissolution over time ► If the acceptable test equipment calibration limit is 28 -54; what can we say about use of f 2 criteria (~mean profile difference of 10%) as a way to document unchanged quality (e. g. , SUPAC)? ► Lot Date Mean (n=6) SD (%) USP Limit (%) M 4/00 34. 8 2. 2 28 -42 M 10/00 28. 9 0. 9 28 -42 N 12/01 35. 7 1. 6 28 -54 N 11/02 35. 4 1. 4 28 -54 N 6/03 28. 0 0. 7 28 -54 DPA/FDA Data using Apparatus 2; data from only one apparatus shown. Note the USP adjusts the limits of each new lot of calibration tablets to reflect the anticipated decrease in dissolution.
Testing to Document Quality: Requires Less Variable Test Methods The current USP 10 -mg Prednisone Calibrator Tablets exhibit slower dissolution over time ► If the acceptable test equipment calibration limit is 28 -54; what can we say about use of f 2 criteria (~mean profile difference of 10%) as a way to document unchanged quality (e. g. , SUPAC)? ► Lot Date Mean (n=6) SD (%) USP Limit (%) M 4/00 34. 8 2. 2 28 -42 M 10/00 28. 9 0. 9 28 -42 N 12/01 35. 7 1. 6 28 -54 N 11/02 35. 4 1. 4 28 -54 N 6/03 28. 0 0. 7 28 -54 DPA/FDA Data using Apparatus 2; data from only one apparatus shown. Note the USP adjusts the limits of each new lot of calibration tablets to reflect the anticipated decrease in dissolution.
 Dissolution Experience at the FDA Division of Pharmaceutical Analysis ► Dissolution testing with USP Apparatus 1 and 2 requires diligent attention to details: mechanical and chemical ► Dosage forms can respond differently to small variations in apparatus set up or degassing ► Large differences in dissolution results are possible unless all parameters are carefully controlled ► The experience at DPA indicates that differences in reproducibility can often be traced to improper mechanical calibration and/or degassing
Dissolution Experience at the FDA Division of Pharmaceutical Analysis ► Dissolution testing with USP Apparatus 1 and 2 requires diligent attention to details: mechanical and chemical ► Dosage forms can respond differently to small variations in apparatus set up or degassing ► Large differences in dissolution results are possible unless all parameters are carefully controlled ► The experience at DPA indicates that differences in reproducibility can often be traced to improper mechanical calibration and/or degassing
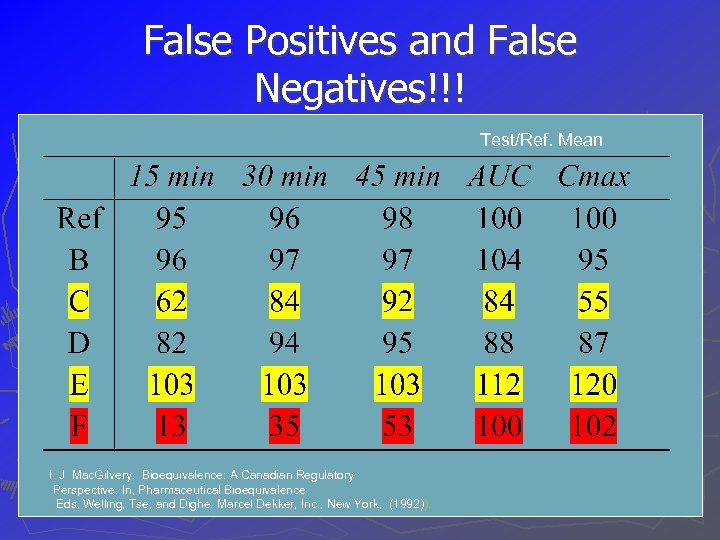 False Positives and False Negatives!!! Test/Ref. Mean I. J. Mac. Gilvery. Bioequivalence: A Canadian Regulatory Perspective. In, Pharmaceutical Bioequivalence. Eds. Welling, Tse, and Dighe. Marcel Dekker, Inc. , New York, (1992)).
False Positives and False Negatives!!! Test/Ref. Mean I. J. Mac. Gilvery. Bioequivalence: A Canadian Regulatory Perspective. In, Pharmaceutical Bioequivalence. Eds. Welling, Tse, and Dighe. Marcel Dekker, Inc. , New York, (1992)).
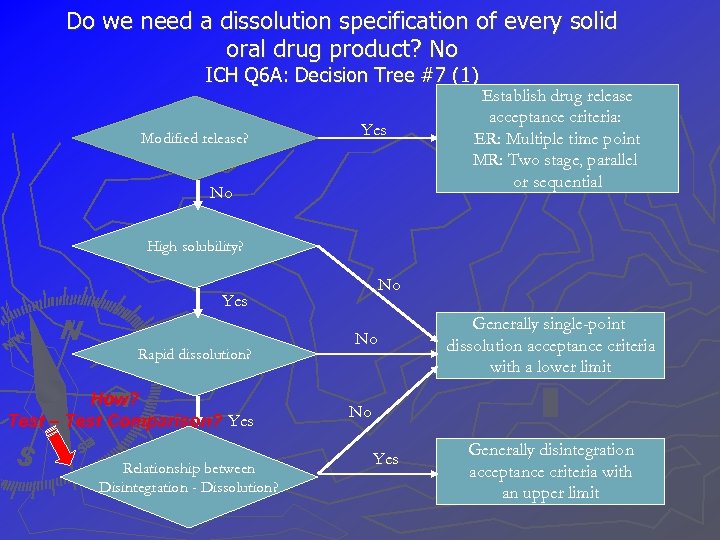 Do we need a dissolution specification of every solid oral drug product? No ICH Q 6 A: Decision Tree #7 (1) Modified release? Yes No Establish drug release acceptance criteria: ER: Multiple time point MR: Two stage, parallel or sequential High solubility? No Yes Rapid dissolution? How? Test – Test Comparison? Yes Relationship between Disintegration - Dissolution? No Generally single-point dissolution acceptance criteria with a lower limit No Yes Generally disintegration acceptance criteria with an upper limit
Do we need a dissolution specification of every solid oral drug product? No ICH Q 6 A: Decision Tree #7 (1) Modified release? Yes No Establish drug release acceptance criteria: ER: Multiple time point MR: Two stage, parallel or sequential High solubility? No Yes Rapid dissolution? How? Test – Test Comparison? Yes Relationship between Disintegration - Dissolution? No Generally single-point dissolution acceptance criteria with a lower limit No Yes Generally disintegration acceptance criteria with an upper limit
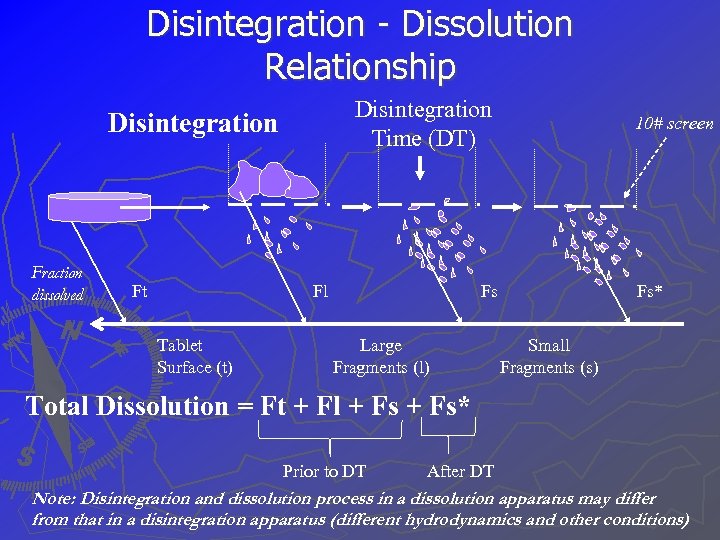 Disintegration - Dissolution Relationship Disintegration Time (DT) Disintegration Fraction dissolved Ft Fl Tablet Surface (t) 10# screen Fs Large Fragments (l) Fs* Small Fragments (s) Total Dissolution = Ft + Fl + Fs* Prior to DT After DT Note: Disintegration and dissolution process in a dissolution apparatus may differ from that in a disintegration apparatus (different hydrodynamics and other conditions)
Disintegration - Dissolution Relationship Disintegration Time (DT) Disintegration Fraction dissolved Ft Fl Tablet Surface (t) 10# screen Fs Large Fragments (l) Fs* Small Fragments (s) Total Dissolution = Ft + Fl + Fs* Prior to DT After DT Note: Disintegration and dissolution process in a dissolution apparatus may differ from that in a disintegration apparatus (different hydrodynamics and other conditions)
 “Testing to Document Quality” ► The phrase has many dimensions § In-process and end-product release and stability testing § Reliability of specifications (attribute, test method, and acceptance criteria) § Managing post approval changes/continuous improvement (e. g. , reduce variability, improve efficiency, . . ) § Product and process knowledge acquisition and generalization
“Testing to Document Quality” ► The phrase has many dimensions § In-process and end-product release and stability testing § Reliability of specifications (attribute, test method, and acceptance criteria) § Managing post approval changes/continuous improvement (e. g. , reduce variability, improve efficiency, . . ) § Product and process knowledge acquisition and generalization
 How can pharmaceutical development knowledge help? ► Demonstrate quality was designed in? ► Specifications based on mechanistic understanding? ► Continuous "real time" assurance of quality? ► Flexible continuous improvement?
How can pharmaceutical development knowledge help? ► Demonstrate quality was designed in? ► Specifications based on mechanistic understanding? ► Continuous "real time" assurance of quality? ► Flexible continuous improvement?
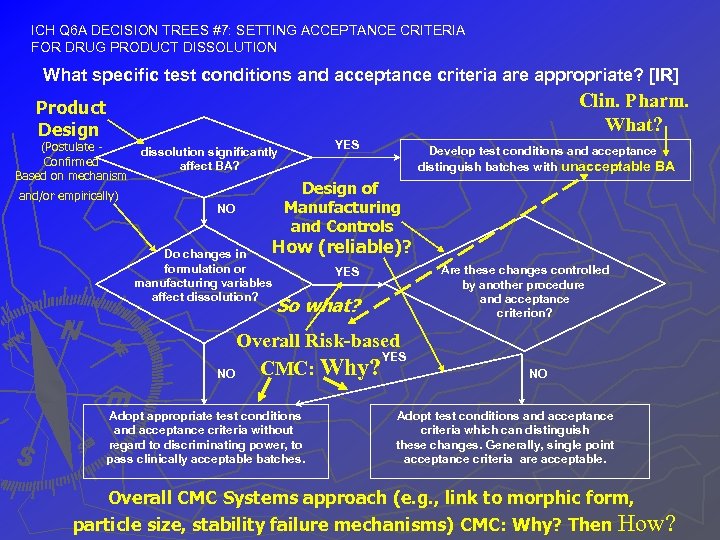 ICH Q 6 A DECISION TREES #7: SETTING ACCEPTANCE CRITERIA FOR DRUG PRODUCT DISSOLUTION What specific test conditions and acceptance criteria are appropriate? [IR] Clin. Pharm. What? Product Design (Postulate Confirmed Based on mechanism and/or empirically) YES dissolution significantly affect BA? NO Develop test conditions and acceptance distinguish batches with unacceptable BA Design of Manufacturing and Controls How Do changes in formulation or manufacturing variables affect dissolution? (reliable)? Are these changes controlled by another procedure and acceptance criterion? YES So what? Overall Risk-based YES CMC: Why? NO Adopt appropriate test conditions and acceptance criteria without regard to discriminating power, to pass clinically acceptable batches. NO Adopt test conditions and acceptance criteria which can distinguish these changes. Generally, single point acceptance criteria are acceptable. Overall CMC Systems approach (e. g. , link to morphic form, particle size, stability failure mechanisms) CMC: Why? Then How?
ICH Q 6 A DECISION TREES #7: SETTING ACCEPTANCE CRITERIA FOR DRUG PRODUCT DISSOLUTION What specific test conditions and acceptance criteria are appropriate? [IR] Clin. Pharm. What? Product Design (Postulate Confirmed Based on mechanism and/or empirically) YES dissolution significantly affect BA? NO Develop test conditions and acceptance distinguish batches with unacceptable BA Design of Manufacturing and Controls How Do changes in formulation or manufacturing variables affect dissolution? (reliable)? Are these changes controlled by another procedure and acceptance criterion? YES So what? Overall Risk-based YES CMC: Why? NO Adopt appropriate test conditions and acceptance criteria without regard to discriminating power, to pass clinically acceptable batches. NO Adopt test conditions and acceptance criteria which can distinguish these changes. Generally, single point acceptance criteria are acceptable. Overall CMC Systems approach (e. g. , link to morphic form, particle size, stability failure mechanisms) CMC: Why? Then How?
 Based on Quality of Pharmaceutical Development Knowledge can we not evaluate ► Overall CMC Systems approach (e. g. , link to morphic form, particle size, stability failure mechanisms) and address concerns and risks § Is a dissolution specification needed? § Instead of wet dissolution test, can we use disintegration test? § Real time release and stability based on process controls, and NIR test for capsules?
Based on Quality of Pharmaceutical Development Knowledge can we not evaluate ► Overall CMC Systems approach (e. g. , link to morphic form, particle size, stability failure mechanisms) and address concerns and risks § Is a dissolution specification needed? § Instead of wet dissolution test, can we use disintegration test? § Real time release and stability based on process controls, and NIR test for capsules?
 Not all information “mandatory” ► We are okay with this ► But we wish to avoid confusion and the potential vocabulary that may evolve from this – “two different systems” ► Instead we see this as one system with different levels of Qb. D § we will use the “process understanding – predictive ability” vocabulary as a means to create a continuous framework and avoid “two different systems” March 2004 ICH Q 8 Meeting: FDA’s Goals
Not all information “mandatory” ► We are okay with this ► But we wish to avoid confusion and the potential vocabulary that may evolve from this – “two different systems” ► Instead we see this as one system with different levels of Qb. D § we will use the “process understanding – predictive ability” vocabulary as a means to create a continuous framework and avoid “two different systems” March 2004 ICH Q 8 Meeting: FDA’s Goals
 Challenges we face ► Common approach to, and more clear articulation of § Not all information “mandatory” § Improved process understanding and control technologies may be afforded reductions in regulatory requirements § An inverse relation is expected between the effectiveness of the Quality by Design and the risk to a patient being exposed to product that is not fit for use § Ensuring continuous improvement and a process for continuous learning and updating of the knowledge base March 2004 ICH Q 8 Meeting: FDA’s Goals
Challenges we face ► Common approach to, and more clear articulation of § Not all information “mandatory” § Improved process understanding and control technologies may be afforded reductions in regulatory requirements § An inverse relation is expected between the effectiveness of the Quality by Design and the risk to a patient being exposed to product that is not fit for use § Ensuring continuous improvement and a process for continuous learning and updating of the knowledge base March 2004 ICH Q 8 Meeting: FDA’s Goals


