5a0c3e5ed4d3b3c53f40ea844b8f4dc1.ppt
- Количество слайдов: 43
 Ch. 6: Chemical Composition Dr. Namphol Sinkaset Chem 152: Introduction to General Chemistry
Ch. 6: Chemical Composition Dr. Namphol Sinkaset Chem 152: Introduction to General Chemistry
 I. Chapter Outline I. Introduction II. Counting by Weighing III. Chemical Formulas as Conversion Factors IV. Mass Percent Composition V. Finding Formulas from Mass Data
I. Chapter Outline I. Introduction II. Counting by Weighing III. Chemical Formulas as Conversion Factors IV. Mass Percent Composition V. Finding Formulas from Mass Data
 I. Introduction • The FDA RDA for Na = 2. 4 g. • Na usually consumed as Na. Cl. • But 2. 4 g Na. Cl ≠ 2. 4 g Na. • How do we figure out a problem like this?
I. Introduction • The FDA RDA for Na = 2. 4 g. • Na usually consumed as Na. Cl. • But 2. 4 g Na. Cl ≠ 2. 4 g Na. • How do we figure out a problem like this?
 II. Selling Nails • Nails can be sold two ways: as individual units or by the pound. • By the pound much more convenient if want to buy hundreds of nails. • Analogy extends to atoms/molecules.
II. Selling Nails • Nails can be sold two ways: as individual units or by the pound. • By the pound much more convenient if want to buy hundreds of nails. • Analogy extends to atoms/molecules.
 II. Sample Problem • If a dozen large nails weighs 0. 275 lb. , how many nails are contained in 5. 5 lb?
II. Sample Problem • If a dozen large nails weighs 0. 275 lb. , how many nails are contained in 5. 5 lb?
 II. Counting Atoms • Finding the number of atoms in a sample of matter is similar to selling nails by the pound. • We used a dozen as a unit of count, but 12 is way too small for atom. • In chemistry, the mole is used instead – it’s the chemist’s “dozen. ”
II. Counting Atoms • Finding the number of atoms in a sample of matter is similar to selling nails by the pound. • We used a dozen as a unit of count, but 12 is way too small for atom. • In chemistry, the mole is used instead – it’s the chemist’s “dozen. ”
 II. The Mole • The mole is the SI unit for amount and is defined as the amount of material containing 6. 0221421 x 1023 particles. • This # is known as Avogadro’s number. • This # is defined by the number of atoms in exactly 12 g of carbon-12. • One mole of atoms, ions, or molecules generally makes up a macroscopically meaningful size.
II. The Mole • The mole is the SI unit for amount and is defined as the amount of material containing 6. 0221421 x 1023 particles. • This # is known as Avogadro’s number. • This # is defined by the number of atoms in exactly 12 g of carbon-12. • One mole of atoms, ions, or molecules generally makes up a macroscopically meaningful size.
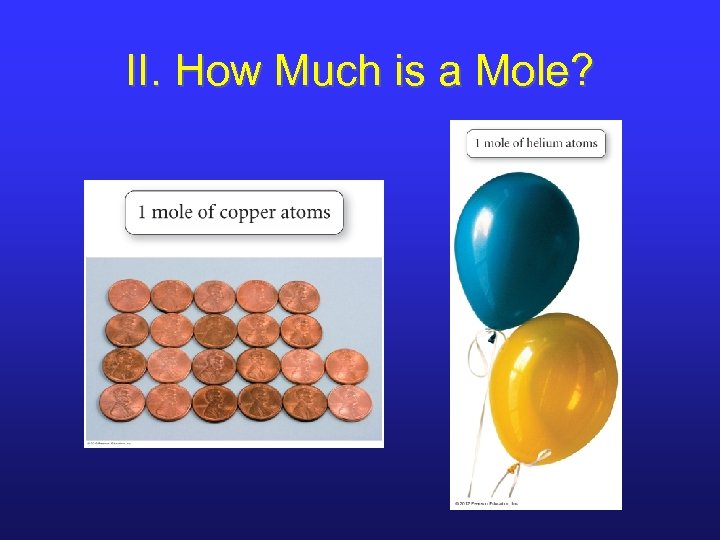 II. How Much is a Mole?
II. How Much is a Mole?
 II. Avogadro’s Number • Every time you hear “mole, ” you should think 6. 022 x 1023. • We can use Avogadro’s number as a conversion factor to calculate # of atoms. 6. 022 x 1023 atoms 1 mole atoms OR 1 mole atoms 6. 022 x 1023 atoms
II. Avogadro’s Number • Every time you hear “mole, ” you should think 6. 022 x 1023. • We can use Avogadro’s number as a conversion factor to calculate # of atoms. 6. 022 x 1023 atoms 1 mole atoms OR 1 mole atoms 6. 022 x 1023 atoms
 II. Sample Problem • How many atoms of gold are in a pure gold ring containing 8. 83 x 10 -2 moles of Au?
II. Sample Problem • How many atoms of gold are in a pure gold ring containing 8. 83 x 10 -2 moles of Au?
 II. Grams and Moles • In the nail example, we had a relationship between a dozen nails and a mass. • We need the same relationship for the mole. • The mass of 1 mole of atoms of an element is its molar mass. The value of an element’s molar mass in g/mole is numerically equal to the element’s atomic mass in amu (from periodic table).
II. Grams and Moles • In the nail example, we had a relationship between a dozen nails and a mass. • We need the same relationship for the mole. • The mass of 1 mole of atoms of an element is its molar mass. The value of an element’s molar mass in g/mole is numerically equal to the element’s atomic mass in amu (from periodic table).
 II. Mole of Atoms & Compounds • 1 Fe atom weighs 55. 85 amu, so 1 mole of Fe atoms weighs 55. 85 g. • 1 O atom weighs 16. 00 amu, so 1 mole of O atoms weighs 16. 00 g. • Same applies for compounds. § 1 molecule H 2 O weighs 18. 02 amu, so 1 mole H 2 O weighs 18. 02 g.
II. Mole of Atoms & Compounds • 1 Fe atom weighs 55. 85 amu, so 1 mole of Fe atoms weighs 55. 85 g. • 1 O atom weighs 16. 00 amu, so 1 mole of O atoms weighs 16. 00 g. • Same applies for compounds. § 1 molecule H 2 O weighs 18. 02 amu, so 1 mole H 2 O weighs 18. 02 g.
 II. Sample Problem • Calculate the molar mass of calcium sulfate.
II. Sample Problem • Calculate the molar mass of calcium sulfate.
 II. The Mass of a Mole Depends on Unit Size
II. The Mass of a Mole Depends on Unit Size
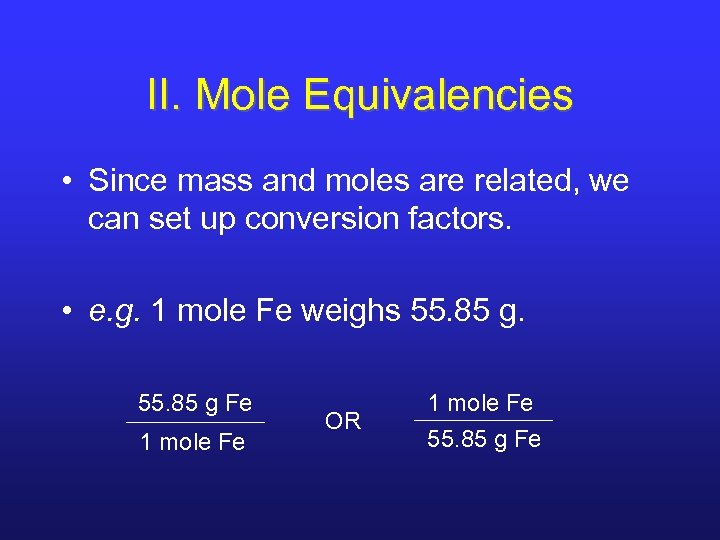 II. Mole Equivalencies • Since mass and moles are related, we can set up conversion factors. • e. g. 1 mole Fe weighs 55. 85 g Fe 1 mole Fe OR 1 mole Fe 55. 85 g Fe
II. Mole Equivalencies • Since mass and moles are related, we can set up conversion factors. • e. g. 1 mole Fe weighs 55. 85 g Fe 1 mole Fe OR 1 mole Fe 55. 85 g Fe
 II. Sample Problem • Graphite, a crystalline form of carbon, is used in pencils. How many moles of carbon are in 0. 315 g of graphite?
II. Sample Problem • Graphite, a crystalline form of carbon, is used in pencils. How many moles of carbon are in 0. 315 g of graphite?
 II. Sample Problem • How many grams of sulfur are in 2. 78 moles of sulfur?
II. Sample Problem • How many grams of sulfur are in 2. 78 moles of sulfur?
 II. Sample Problem • How many moles of NO 2 are in 1. 18 g of NO 2?
II. Sample Problem • How many moles of NO 2 are in 1. 18 g of NO 2?
 II. Sample Problem • How many molecules of H 2 O are in a sample of water with a mass of 3. 64 g?
II. Sample Problem • How many molecules of H 2 O are in a sample of water with a mass of 3. 64 g?
 II. Sample Problem • If a sample of molecular bromine weighs 2100 g, how many molecules of molecular bromine are in the sample?
II. Sample Problem • If a sample of molecular bromine weighs 2100 g, how many molecules of molecular bromine are in the sample?
 III. Inherent Conversion Factors • Any object that can be broken down into parts or pieces has an inherent conversion factor. • For example, a clover.
III. Inherent Conversion Factors • Any object that can be broken down into parts or pieces has an inherent conversion factor. • For example, a clover.
 III. 3 Leaves : 1 Clover • Since there are 3 leaves in one clover, we can write a conversion factor between them.
III. 3 Leaves : 1 Clover • Since there are 3 leaves in one clover, we can write a conversion factor between them.
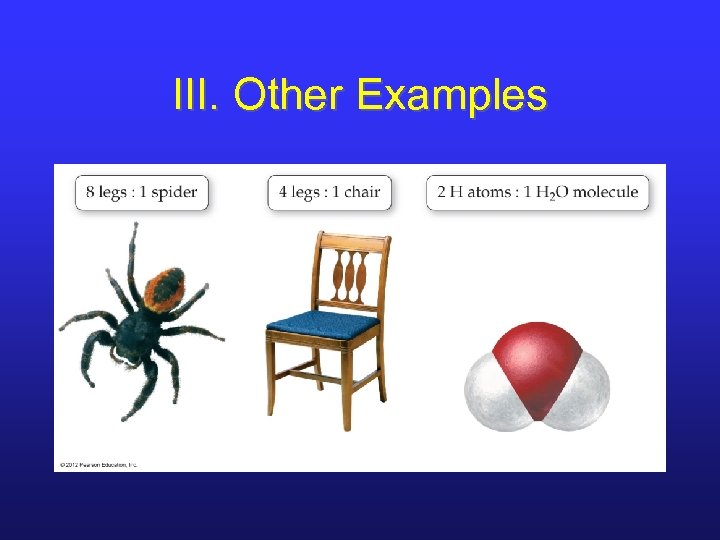 III. Other Examples
III. Other Examples
 III. Scaling Up • The ratios hold as long as we keep the unit the same for both the parts and the whole. • 3 leaves : 1 clover 3 dozen leaves : 1 dozen clovers. • In chemistry, we scale up to the mole. • 2 H atoms : 1 H 2 O molecule 2 moles H atoms : 1 mole H 2 O molecules.
III. Scaling Up • The ratios hold as long as we keep the unit the same for both the parts and the whole. • 3 leaves : 1 clover 3 dozen leaves : 1 dozen clovers. • In chemistry, we scale up to the mole. • 2 H atoms : 1 H 2 O molecule 2 moles H atoms : 1 mole H 2 O molecules.
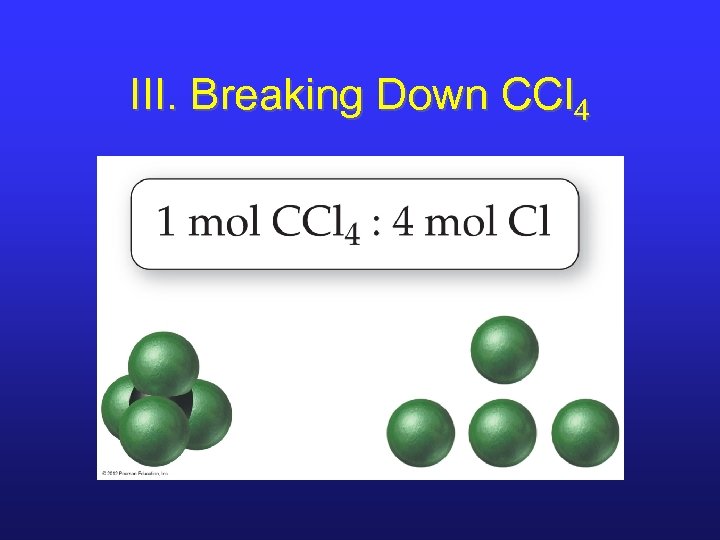 III. Breaking Down CCl 4
III. Breaking Down CCl 4
 III. Sample Problem • List all the possible atom : formula unit mole relationships in barium nitrate.
III. Sample Problem • List all the possible atom : formula unit mole relationships in barium nitrate.
 III. Mole Relationships • A chemical formula gives the relationships between moles of substances, NOT grams. • Always convert to moles then use a mole relationship to get to the substance of interest.
III. Mole Relationships • A chemical formula gives the relationships between moles of substances, NOT grams. • Always convert to moles then use a mole relationship to get to the substance of interest.
 III. Sample Problem • Determine the number of moles of oxygen in 2. 45 moles of nickel(II) phosphate.
III. Sample Problem • Determine the number of moles of oxygen in 2. 45 moles of nickel(II) phosphate.
 III. Sample Problem • Calculate the mass of carbon in 25. 0 g of C 6 H 12 O 6.
III. Sample Problem • Calculate the mass of carbon in 25. 0 g of C 6 H 12 O 6.
 III. Sample Problem • How many grams of sodium chloride contains 2. 00 grams of sodium?
III. Sample Problem • How many grams of sodium chloride contains 2. 00 grams of sodium?
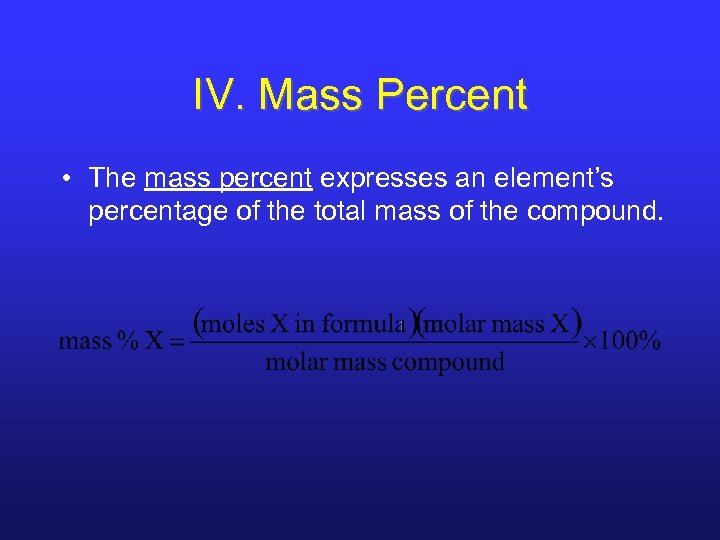 IV. Mass Percent • The mass percent expresses an element’s percentage of the total mass of the compound.
IV. Mass Percent • The mass percent expresses an element’s percentage of the total mass of the compound.
 IV. Sample Problem • Calculate the mass percent of nitrogen in ammonium nitrate.
IV. Sample Problem • Calculate the mass percent of nitrogen in ammonium nitrate.
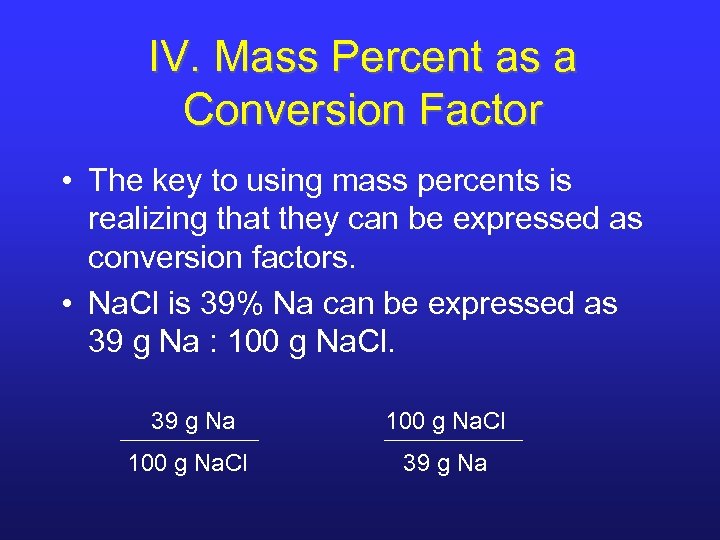 IV. Mass Percent as a Conversion Factor • The key to using mass percents is realizing that they can be expressed as conversion factors. • Na. Cl is 39% Na can be expressed as 39 g Na : 100 g Na. Cl. 39 g Na 100 g Na. Cl 39 g Na
IV. Mass Percent as a Conversion Factor • The key to using mass percents is realizing that they can be expressed as conversion factors. • Na. Cl is 39% Na can be expressed as 39 g Na : 100 g Na. Cl. 39 g Na 100 g Na. Cl 39 g Na
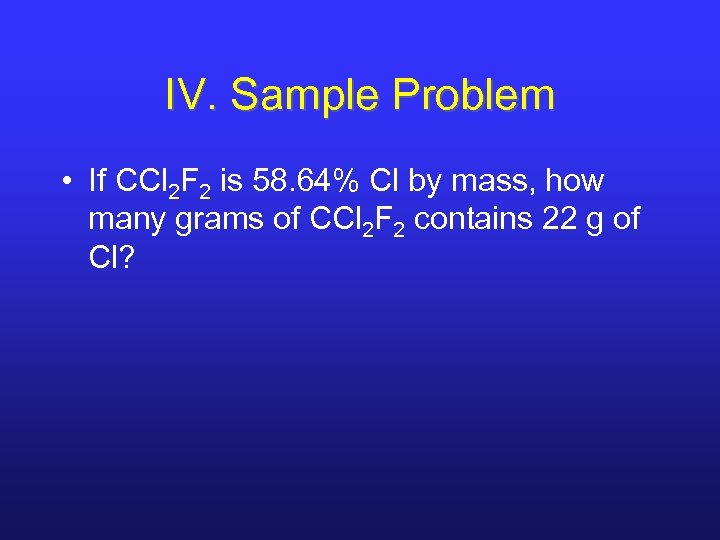 IV. Sample Problem • If CCl 2 F 2 is 58. 64% Cl by mass, how many grams of CCl 2 F 2 contains 22 g of Cl?
IV. Sample Problem • If CCl 2 F 2 is 58. 64% Cl by mass, how many grams of CCl 2 F 2 contains 22 g of Cl?
 V. Finding Formulas from Mass Data • Given elemental mass % data of a compound, it’s possible to find the formula of the compound. • Elemental analysis is a common test performed on newly synthesized compounds.
V. Finding Formulas from Mass Data • Given elemental mass % data of a compound, it’s possible to find the formula of the compound. • Elemental analysis is a common test performed on newly synthesized compounds.
 V. Empirical vs. Molecular • Often experiments will yield the relative masses of each element in a compound. • With just the relative masses, the best we can do is find the empirical formula. • Additional data is needed to go from an empirical formula to a molecular formula.
V. Empirical vs. Molecular • Often experiments will yield the relative masses of each element in a compound. • With just the relative masses, the best we can do is find the empirical formula. • Additional data is needed to go from an empirical formula to a molecular formula.
 V. The Key to Finding Empirical Formulas • In any formula, subscripts represent the number of atoms of a given element in that compound. • However, these ratios scale up! • Therefore, the subscripts can also be interpreted as mole ratios between atoms of a given element in the compound.
V. The Key to Finding Empirical Formulas • In any formula, subscripts represent the number of atoms of a given element in that compound. • However, these ratios scale up! • Therefore, the subscripts can also be interpreted as mole ratios between atoms of a given element in the compound.
 V. Finding Empirical Formulas • To find an empirical formula, you must calculate the number of moles of each atom present in a certain sample of the compound. • These moles become the temporary subscripts in the formula. • You then use math to convert to whole numbers.
V. Finding Empirical Formulas • To find an empirical formula, you must calculate the number of moles of each atom present in a certain sample of the compound. • These moles become the temporary subscripts in the formula. • You then use math to convert to whole numbers.
 V. Sample Problem • Calculate the empirical formula of ethyl butyrate (pineapple oil) if its mass composition is 62. 04% C, 10. 41% H, and 27. 55% O.
V. Sample Problem • Calculate the empirical formula of ethyl butyrate (pineapple oil) if its mass composition is 62. 04% C, 10. 41% H, and 27. 55% O.
 V. Sample Problem • A certain compound is 50. 66% C, 4. 25% H, and 45. 09% S by mass. Determine the empirical formula.
V. Sample Problem • A certain compound is 50. 66% C, 4. 25% H, and 45. 09% S by mass. Determine the empirical formula.
 V. Determining Molecular Formulas • To find a molecular formula from an empirical formula, you must know the molecular molar mass. • The molecular molar mass is always a multiple of the empirical molar mass. • To find the multiple, we divide the molecular molar mass by the empirical molar mass.
V. Determining Molecular Formulas • To find a molecular formula from an empirical formula, you must know the molecular molar mass. • The molecular molar mass is always a multiple of the empirical molar mass. • To find the multiple, we divide the molecular molar mass by the empirical molar mass.
 V. Sample Problem • Butane has an empirical formula of C 2 H 5. If its molar mass is 58. 12 g/mole, determine the molecular formula of butane.
V. Sample Problem • Butane has an empirical formula of C 2 H 5. If its molar mass is 58. 12 g/mole, determine the molecular formula of butane.
![V. Sample Problem • The carcinogen benzo[a]pyrene (MW = 252. 30 g/mole) is found V. Sample Problem • The carcinogen benzo[a]pyrene (MW = 252. 30 g/mole) is found](https://present5.com/presentation/5a0c3e5ed4d3b3c53f40ea844b8f4dc1/image-43.jpg) V. Sample Problem • The carcinogen benzo[a]pyrene (MW = 252. 30 g/mole) is found to be 95. 21% C and 4. 79% H by mass. What is its molecular formula?
V. Sample Problem • The carcinogen benzo[a]pyrene (MW = 252. 30 g/mole) is found to be 95. 21% C and 4. 79% H by mass. What is its molecular formula?


