42a98830837b61a9109d96207df1cb5a.ppt
- Количество слайдов: 60
CH 339 K Lecture 1

Textbook • Mathews, C. K. , van Holde, K. E. , Appling, D. R. , and Anthony-Cahill, S. J. (2012) Biochemistry, 4 th Ed. , Prentice Hall, New York • I believe there are copies in the Coop that will probably cost an arm and a leg. • It’s cheaper online. • If you already have a copy of Lehninger, you can probably get by.
Grades • 3 hourly exams • Final exam (cumulative) • Several problem sets assigned as homework throughout the semester • All weighted equally • Drop your lowest grade

Final Exam • Cumulative, but a little shallower than the hourly exams • No, you don’t have to take the final if you’re satisfied with your grade. • Don’t blame the instructor that the exam’s on a Saturday.

Other Stuff • • Since you’ve paid for this class, you can get a temporary library card by presenting your fee slip at the PCL Science Library is on the second floor of the tower. Chemistry (Mallet) library is on the second (ground) floor of Welch, in the old part of the building. Instructions for getting a UT EID are on your fee receipt Parking is catch-as-catch-can, and gets more and more removed every semester. Consider buying a “N” ($36, evening surface) or “N+” ($60, evening garage or surface) permit. Drop dates: – 09/06/2013 at NOON. - Last day to drop with 50% refund. – 09/13/2013. - Last day to drop with no signatures required. – 11/15/2013 - Last day a student may change registration in a class to or from the pass/fail or credit/no credit basis. – 11/15/2013 - Last day a student may drop a class except for urgent and substantiated, nonacademic reasons - that is, at the Dean’s discretion, which is pretty hard to come by – “compelling reason” is the key world • Your beloved professor’s salary is based on the number of students in the class a couple of weeks down the line. In the unlikely event that you decide to drop (after the refund date), please wait until I get paid for you.

Classroom Rules • Theoretically, you’re not supposed to eat or drink in here – This is an evening class – I don’t mind – Just no slurping, crunching, or overt drooling • No snoring • If anyone has a CHL: It is illegal to carry in the buildings on campus. Lock it in your car. • I will try to remember to post the lecture slides on the website before class so people can download if they wish.
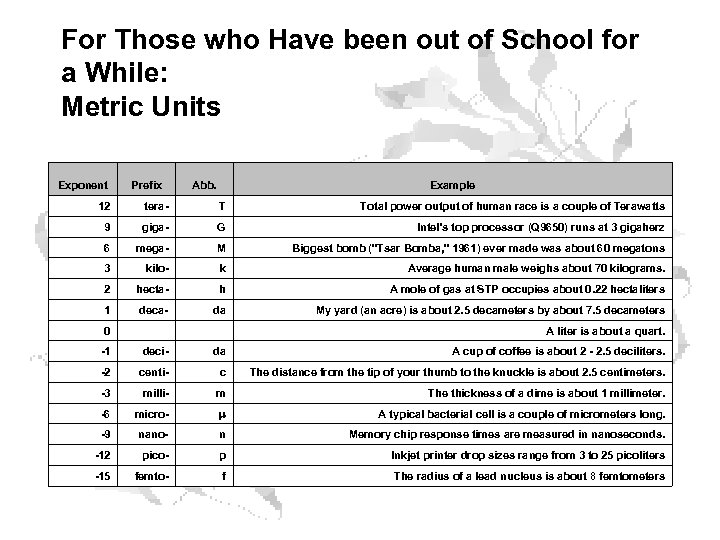
For Those who Have been out of School for a While: Metric Units Exponent Prefix Abb. Example 12 tera- T Total power output of human race is a couple of Terawatts 9 giga- G Intel's top processor (Q 9650) runs at 3 gigaherz 6 mega- M Biggest bomb ("Tsar Bomba, " 1961) ever made was about 60 megatons 3 kilo- k Average human male weighs about 70 kilograms. 2 hecta- h A mole of gas at STP occupies about 0. 22 hectaliters 1 deca- da My yard (an acre) is about 2. 5 decameters by about 7. 5 decameters 0 A liter is about a quart. -1 deci- da A cup of coffee is about 2 - 2. 5 deciliters. -2 centi- c The distance from the tip of your thumb to the knuckle is about 2. 5 centimeters. -3 milli- m The thickness of a dime is about 1 millimeter. -6 micro- m A typical bacterial cell is a couple of micrometers long. -9 nano- n Memory chip response times are measured in nanoseconds. -12 pico- p Inkjet printer drop sizes range from 3 to 25 picoliters -15 femto- f The radius of a lead nucleus is about 8 femtometers
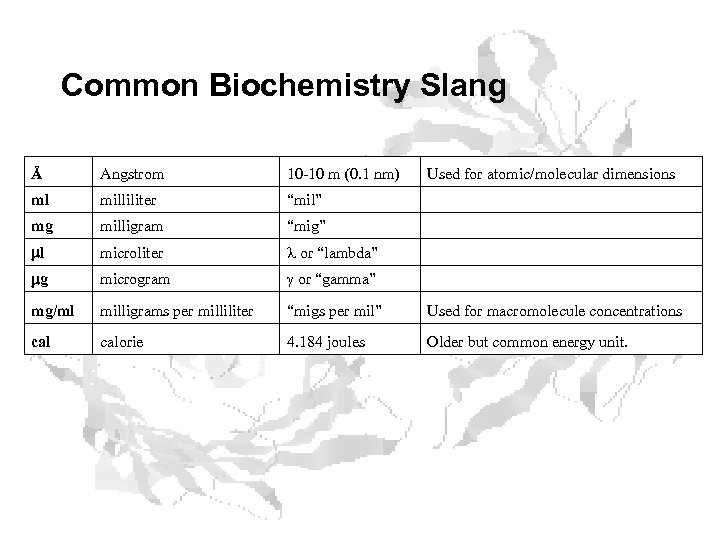
Common Biochemistry Slang Å Angstrom 10 -10 m (0. 1 nm) Used for atomic/molecular dimensions ml milliliter “mil” mg milligram “mig” ml microliter l or “lambda” mg microgram g or “gamma” mg/ml milligrams per milliliter “migs per mil” Used for macromolecule concentrations calorie 4. 184 joules Older but common energy unit.
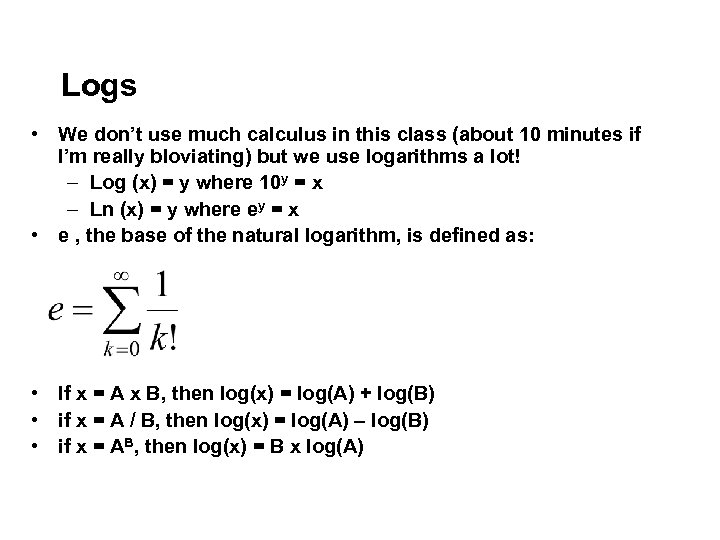
Logs • We don’t use much calculus in this class (about 10 minutes if I’m really bloviating) but we use logarithms a lot! – Log (x) = y where 10 y = x – Ln (x) = y where ey = x • e , the base of the natural logarithm, is defined as: • If x = A x B, then log(x) = log(A) + log(B) • if x = A / B, then log(x) = log(A) – log(B) • if x = AB, then log(x) = B x log(A)
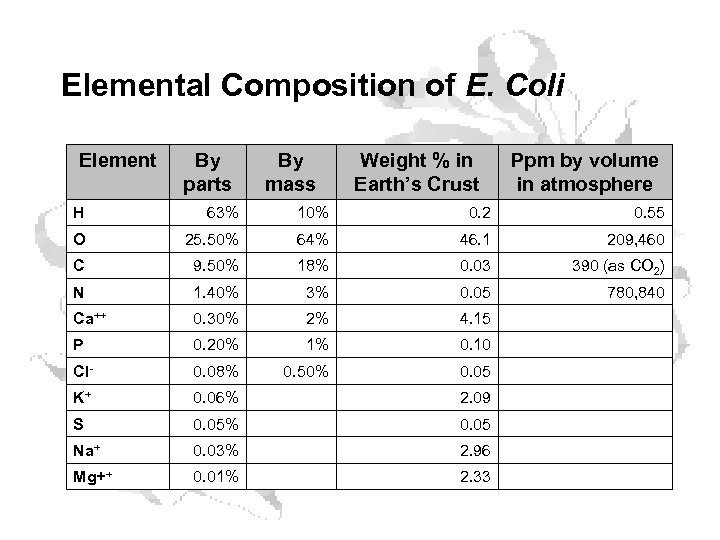
Elemental Composition of E. Coli Element By parts By mass Weight % in Earth’s Crust Ppm by volume in atmosphere H 63% 10% 0. 2 0. 55 O 25. 50% 64% 46. 1 209, 460 C 9. 50% 18% 0. 03 390 (as CO 2) N 1. 40% 3% 0. 05 780, 840 Ca++ 0. 30% 2% 4. 15 P 0. 20% 1% 0. 10 Cl- 0. 08% 0. 50% 0. 05 K+ 0. 06% 2. 09 S 0. 05% 0. 05 Na+ 0. 03% 2. 96 Mg++ 0. 01% 2. 33
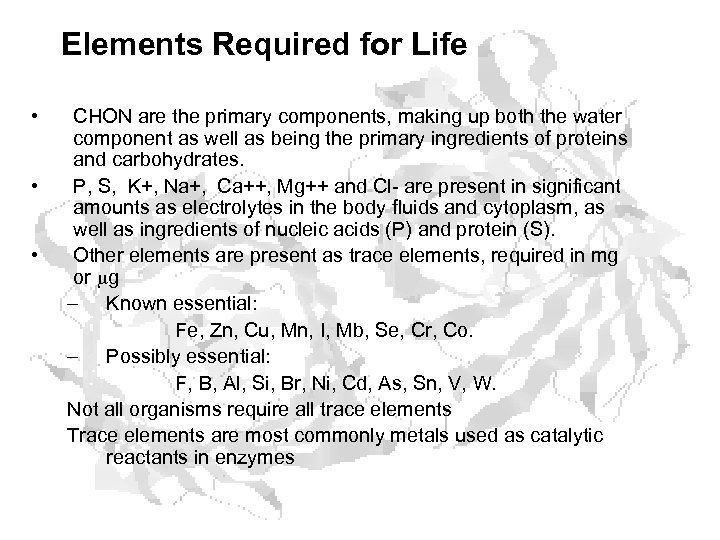
Elements Required for Life • • • CHON are the primary components, making up both the water component as well as being the primary ingredients of proteins and carbohydrates. P, S, K+, Na+, Ca++, Mg++ and Cl- are present in significant amounts as electrolytes in the body fluids and cytoplasm, as well as ingredients of nucleic acids (P) and protein (S). Other elements are present as trace elements, required in mg or mg – Known essential: Fe, Zn, Cu, Mn, I, Mb, Se, Cr, Co. – Possibly essential: F, B, Al, Si, Br, Ni, Cd, As, Sn, V, W. Not all organisms require all trace elements Trace elements are most commonly metals used as catalytic reactants in enzymes

Why Carbon? In order to make big, functional molecules like proteins, nucleic acids, and carbohydrates, you need: • Atoms that make several bonds • Atoms that make strong bonds • Atoms that aren’t too hard to come by Only a few elements pull that off…
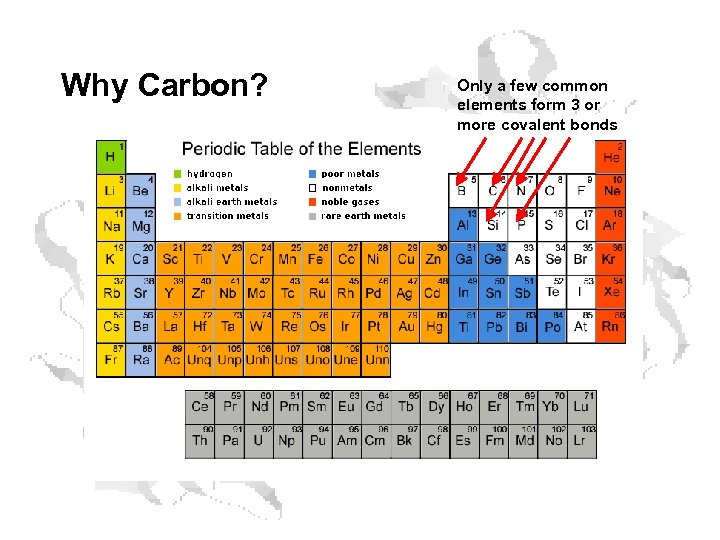
Why Carbon? Only a few common elements form 3 or more covalent bonds

• B: Electron deficient element forms few stable compounds, must be charged to reach octet. • N: Lone pairs of electrons in adjacent nitrogen atoms repel each other, resulting in low bond energy. • Si, P: Relatively large atom size destabilizes chains, and P has the same problem as N. • Si. O: The silicon-oxygen bond is stable, but interesting compounds don’t form at earthly temperatures, and those that do are frequently of low solubility.
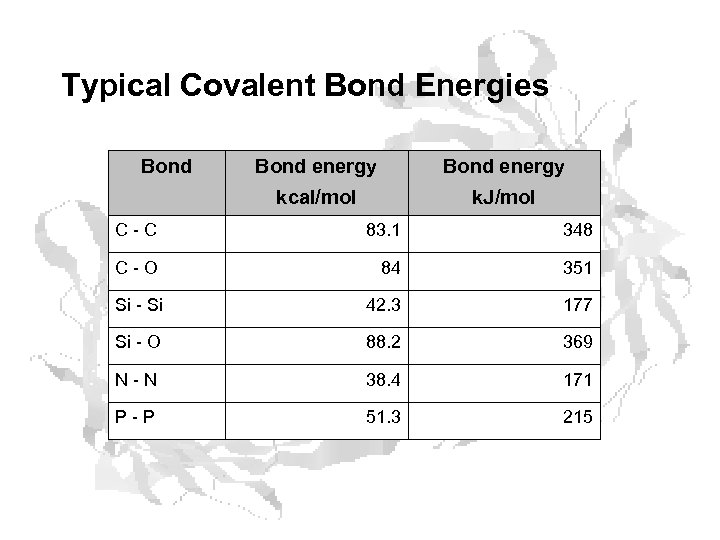
Typical Covalent Bond Energies Bond energy kcal/mol k. J/mol C - C 83. 1 348 C - O 84 351 Si - Si 42. 3 177 Si - O 88. 2 369 N - N 38. 4 171 P - P 51. 3 215
Silicon-Based Life Form Species Location Habitat Discovered Horta Janus VI Subsurface chemotroph Stardate 3196 “It’s life, Jim – but not as we know it…”
A Little O-Chem Review Bleagghhh…. .
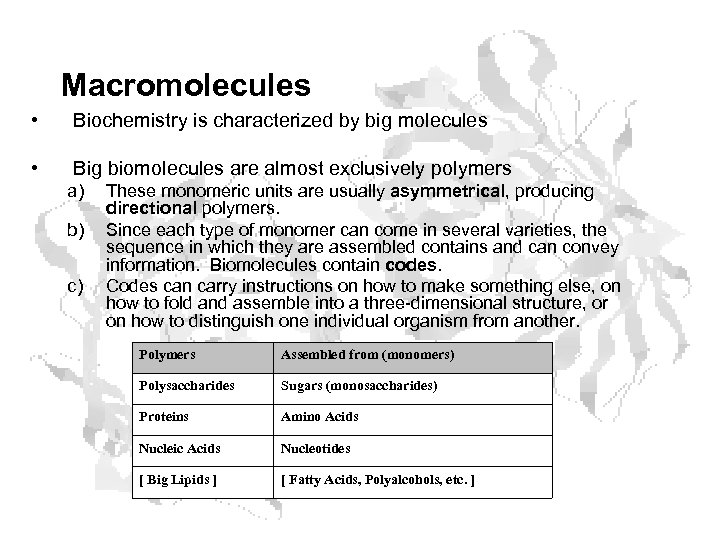
Macromolecules • Biochemistry is characterized by big molecules • Big biomolecules are almost exclusively polymers a) b) c) These monomeric units are usually asymmetrical, producing directional polymers. Since each type of monomer can come in several varieties, the sequence in which they are assembled contains and can convey information. Biomolecules contain codes. Codes can carry instructions on how to make something else, on how to fold and assemble into a three-dimensional structure, or on how to distinguish one individual organism from another. Polymers Assembled from (monomers) Polysaccharides Sugars (monosaccharides) Proteins Amino Acids Nucleic Acids Nucleotides [ Big Lipids ] [ Fatty Acids, Polyalcohols, etc. ]
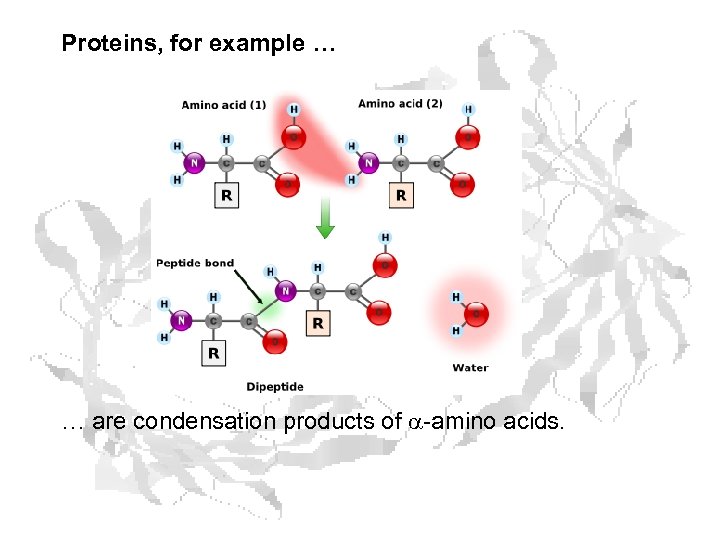
Proteins, for example … … are condensation products of a-amino acids.
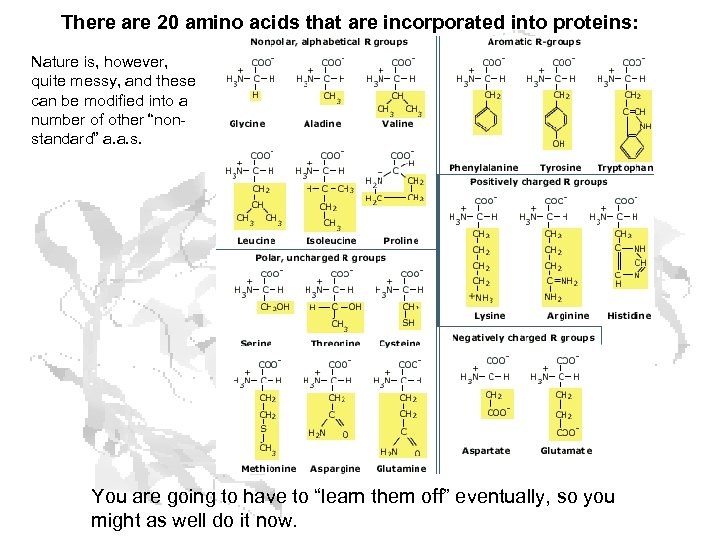
There are 20 amino acids that are incorporated into proteins: Nature is, however, quite messy, and these can be modified into a number of other “nonstandard” a. a. s. You are going to have to “learn them off” eventually, so you might as well do it now.

Cells • All living organisms (except viruses ) are composed of cells - self-contained, more or less self-sufficient units, which are the fundamental entities of life. • The largest cells are 5 orders of magnitude larger in diameter, translating to 15 orders of magnitude greater in volume, than the smallest. • Size is limited at the lower end by the minimum volume needed to contain and solvate the genome and the macromolecules necessary for metabolism and DNA replication. • At the upper end, size is limited by the decreasing surface to volume ratio and the increasing distance from the center to the periphery. Smallest Mycoplasmas (PPLOs) 0. 1 - 0. 2 um Largest Thiomargarita (prokaryote) . 75 mm Ostrich egg 17 cm (cheating!!!!!) Gromia sphaerica 38 mm Xenophyophores 20 cm (cheating as well)
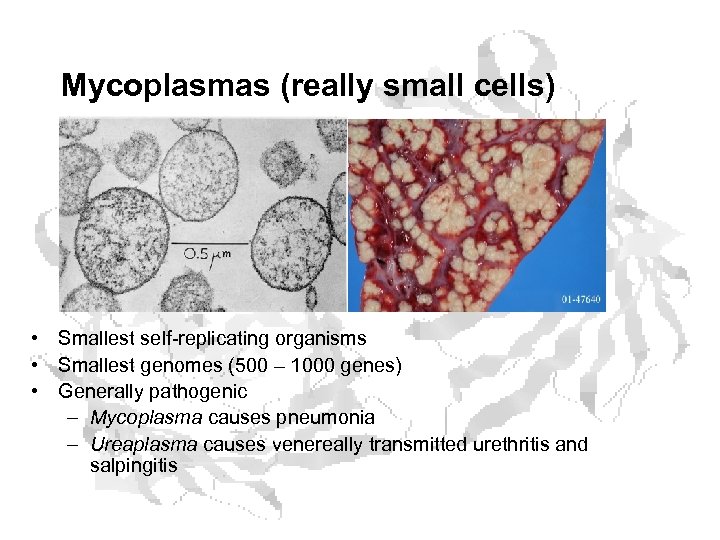
Mycoplasmas (really small cells) • Smallest self-replicating organisms • Smallest genomes (500 – 1000 genes) • Generally pathogenic – Mycoplasma causes pneumonia – Ureaplasma causes venereally transmitted urethritis and salpingitis
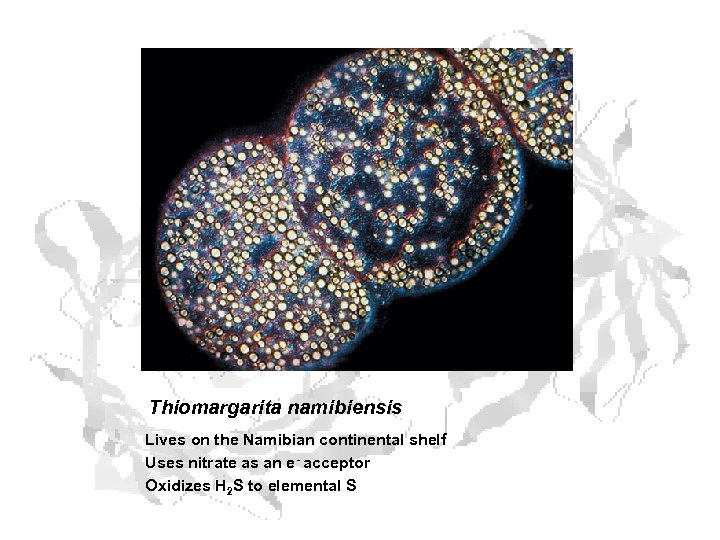
Thiomargarita namibiensis Lives on the Namibian continental shelf Uses nitrate as an e- acceptor Oxidizes H 2 S to elemental S

Modern Gromia sphaerica 565 MYA Fossil
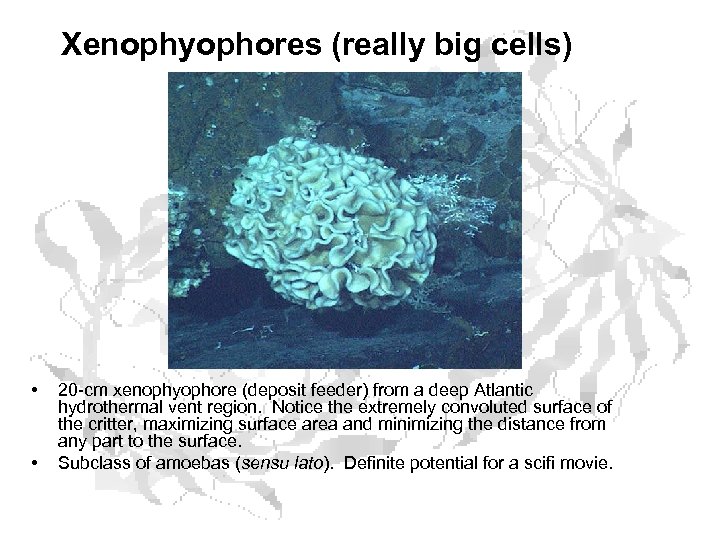
Xenophyophores (really big cells) • • 20 -cm xenophyophore (deposit feeder) from a deep Atlantic hydrothermal vent region. Notice the extremely convoluted surface of the critter, maximizing surface area and minimizing the distance from any part to the surface. Subclass of amoebas (sensu lato). Definite potential for a scifi movie.

There are, of course, even larger cells…
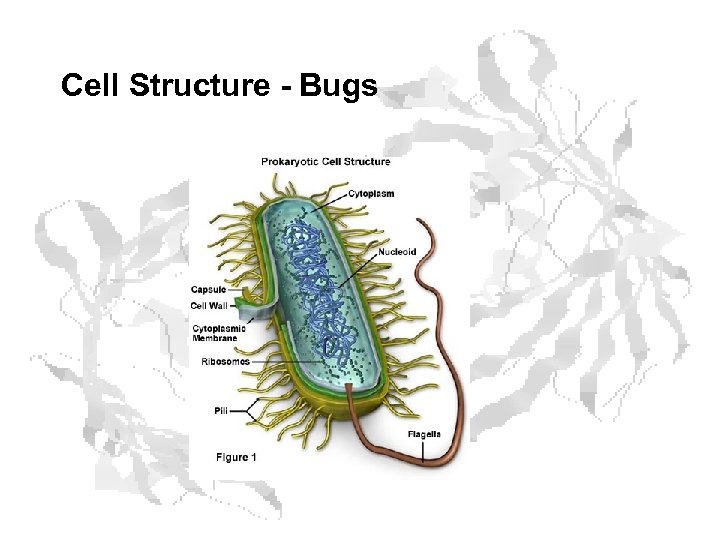
Cell Structure - Bugs
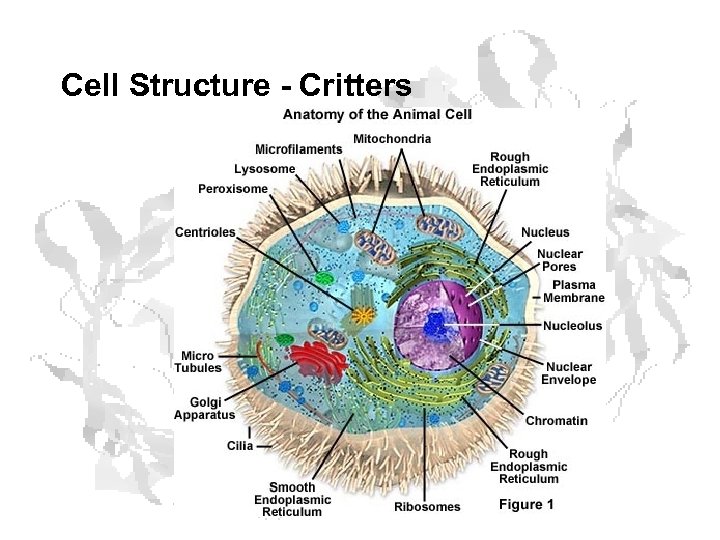
Cell Structure - Critters
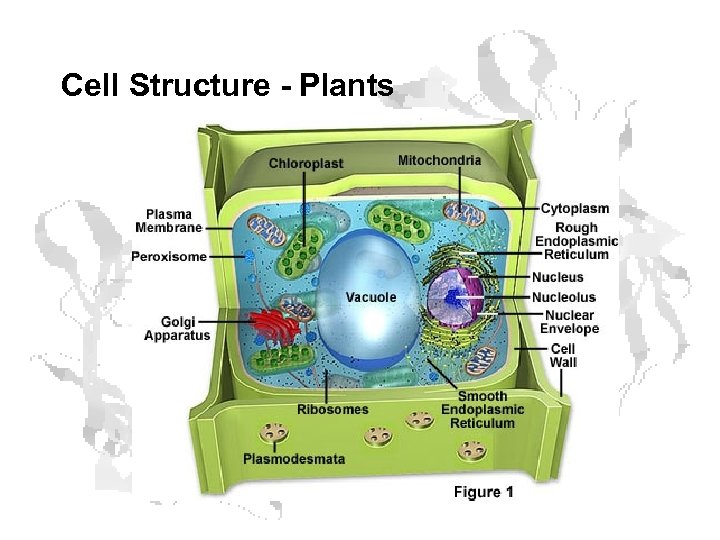
Cell Structure - Plants
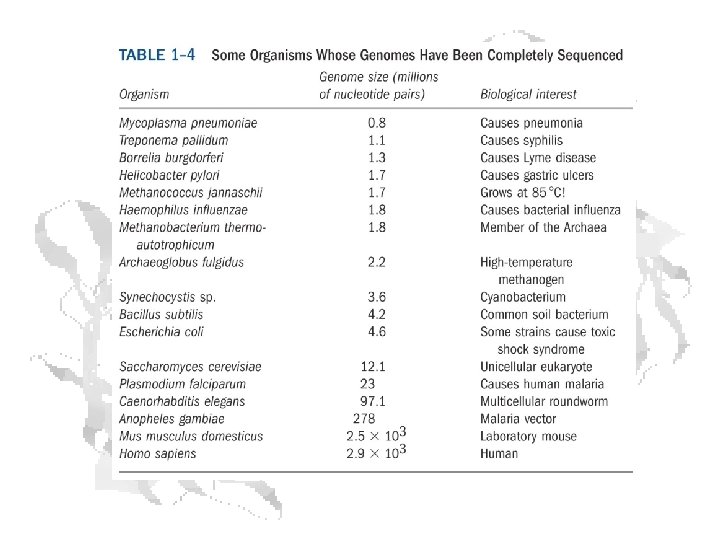
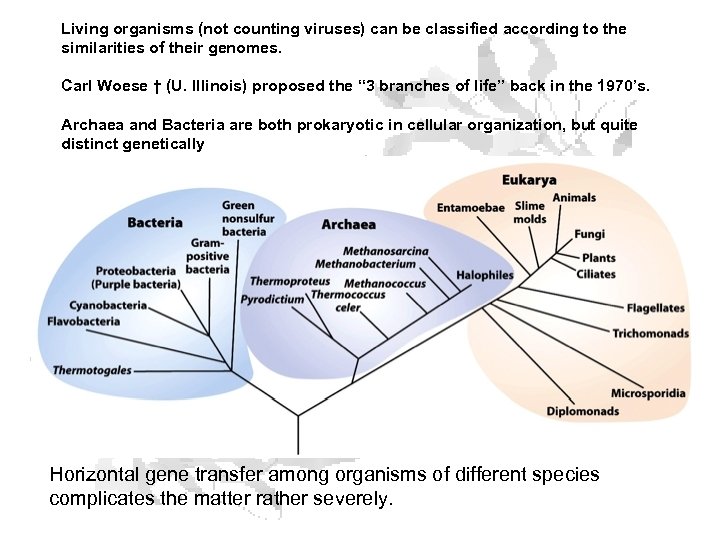
Living organisms (not counting viruses) can be classified according to the similarities of their genomes. Carl Woese † (U. Illinois) proposed the “ 3 branches of life” back in the 1970’s. Archaea and Bacteria are both prokaryotic in cellular organization, but quite distinct genetically Horizontal gene transfer among organisms of different species complicates the matter rather severely.

A LITTLE THERMODYNAMICS (which is probably more than anybody wants)
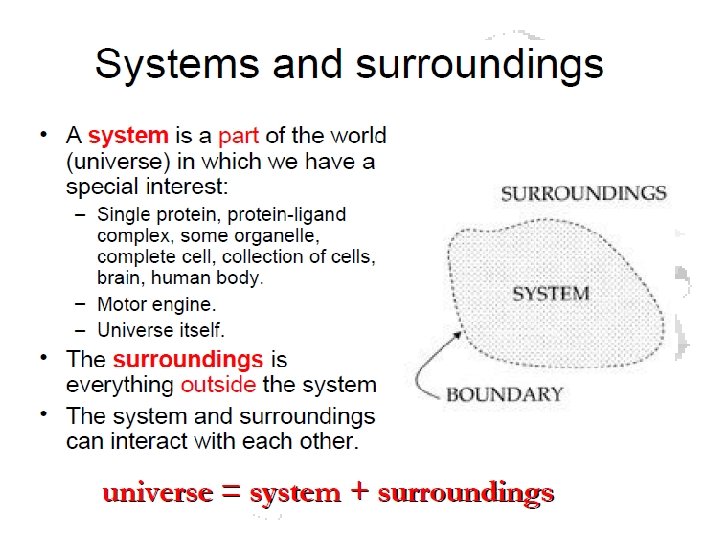
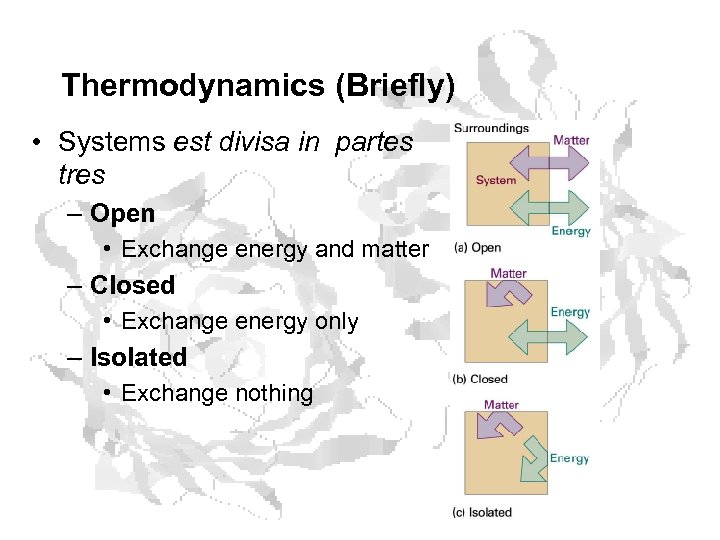
Thermodynamics (Briefly) • Systems est divisa in partes tres – Open • Exchange energy and matter – Closed • Exchange energy only – Isolated • Exchange nothing
More Thermodynamics • Energy can be exchanged as heat (q) or work (w) • By convention: – q > 0: heat has been gained by the system from the surroundings – q < 0: heat has been lost by the system to the surroundings – w > 0: work has been done by the system on the surroundings – w < 0: work has been done on the system by the surroundings
First Law of Thermo • E = q – w or, alternatively, q = E + w SYSTEM
First law of Thermo (cont. ) Example: Oxidation of a Fatty Acid (Palmitic): C 16 H 32 O 2 + 23 O 2 (g) 16 CO 2 (g) + 16 H 2 O (l) • Under Constant Volume: q = -9941. 4 k. J/mol. • Under Constant Pressure: q = -9958. 7 k. J/mol
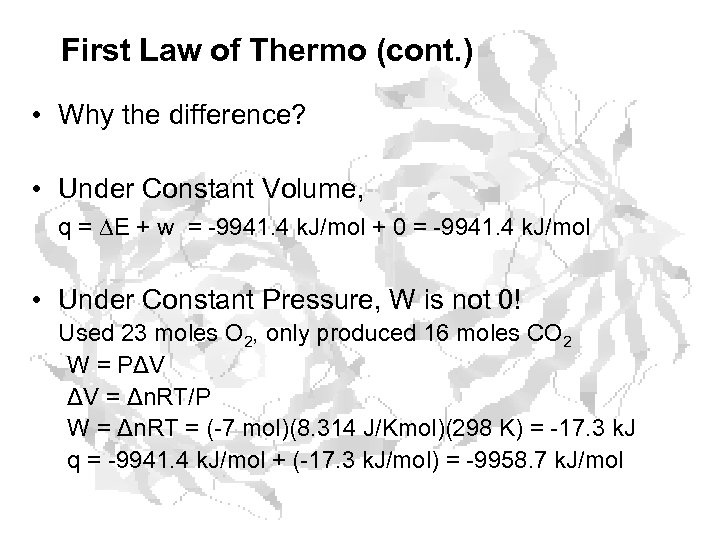
First Law of Thermo (cont. ) • Why the difference? • Under Constant Volume, q = E + w = -9941. 4 k. J/mol + 0 = -9941. 4 k. J/mol • Under Constant Pressure, W is not 0! Used 23 moles O 2, only produced 16 moles CO 2 W = PΔV ΔV = Δn. RT/P W = Δn. RT = (-7 mol)(8. 314 J/Kmol)(298 K) = -17. 3 k. J q = -9941. 4 k. J/mol + (-17. 3 k. J/mol) = -9958. 7 k. J/mol
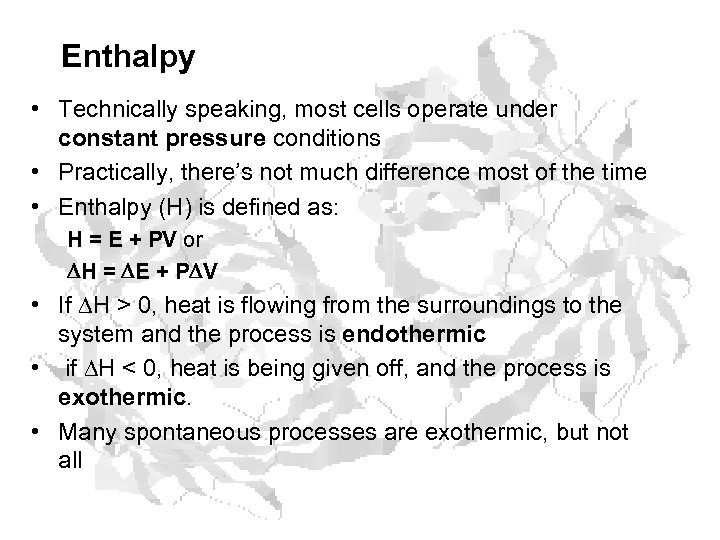
Enthalpy • Technically speaking, most cells operate under constant pressure conditions • Practically, there’s not much difference most of the time • Enthalpy (H) is defined as: H = E + PV or H = E + P V • If H > 0, heat is flowing from the surroundings to the system and the process is endothermic • if H < 0, heat is being given off, and the process is exothermic. • Many spontaneous processes are exothermic, but not all
Endothermic but spontaneous • Ammonium Nitrate spontaneously dissolves in water to the tune of about 2 kg/liter • Ammonium nitrate has a Hsolution of +25. 7 k. J/mol • Remember positive enthalpy = endothermic • This is the basis of instant cold packs
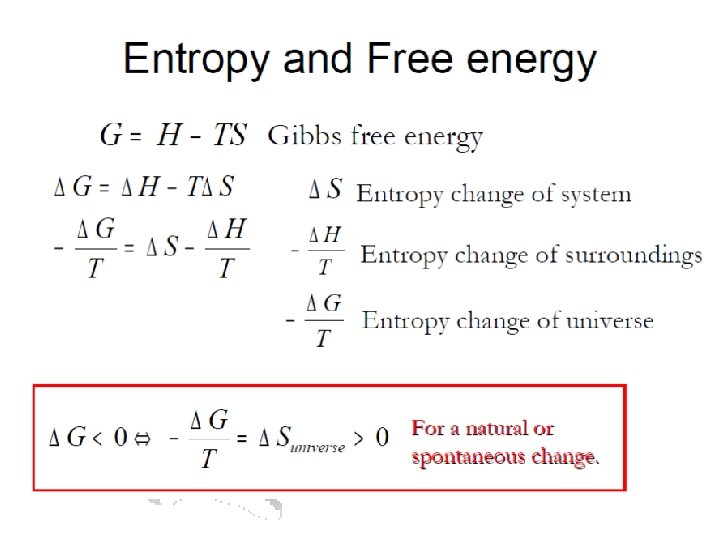
Second law of Thermo • Any spontaneous process must be accompanied by a net increase in entropy (S). • What the heck is entropy? • Entropy is a measure of the “disorderliness” of a system (and/or the surroundings). • What the heck does that mean? • Better, it is a measure of the number of states that a system can occupy. • Huh? . . . let me explain
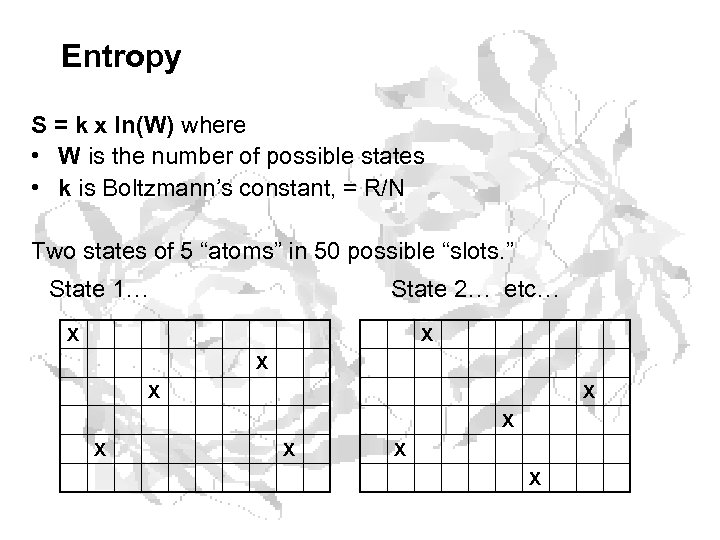
Entropy S = k x ln(W) where • W is the number of possible states • k is Boltzmann’s constant, = R/N Two states of 5 “atoms” in 50 possible “slots. ” State 1… State 2… etc… X X X X X
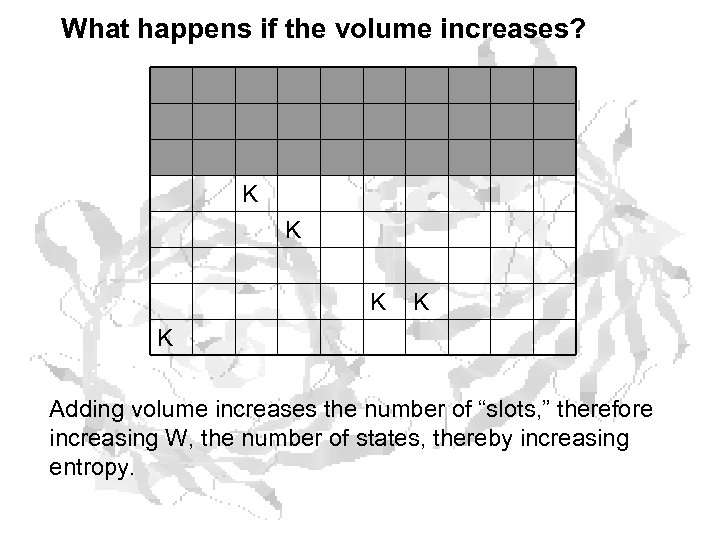
What happens if the volume increases? K K K Adding volume increases the number of “slots, ” therefore increasing W, the number of states, thereby increasing entropy.
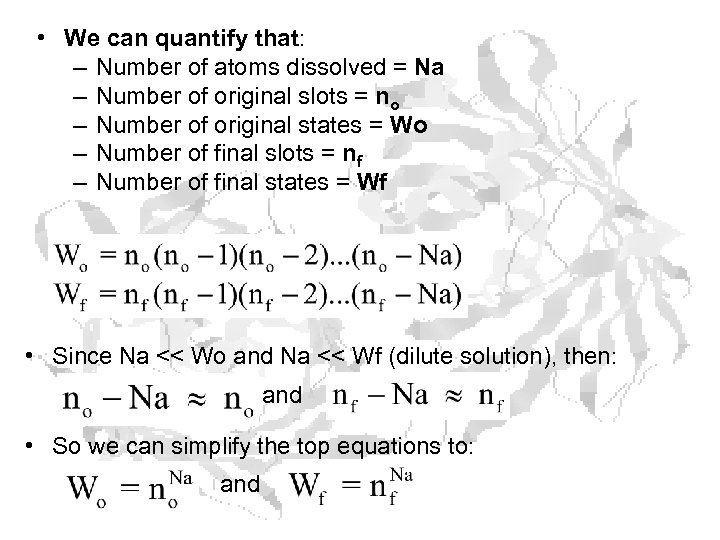
• We can quantify that: – Number of atoms dissolved = Na – Number of original slots = no – Number of original states = Wo – Number of final slots = nf – Number of final states = Wf • Since Na << Wo and Na << Wf (dilute solution), then: and • So we can simplify the top equations to: and
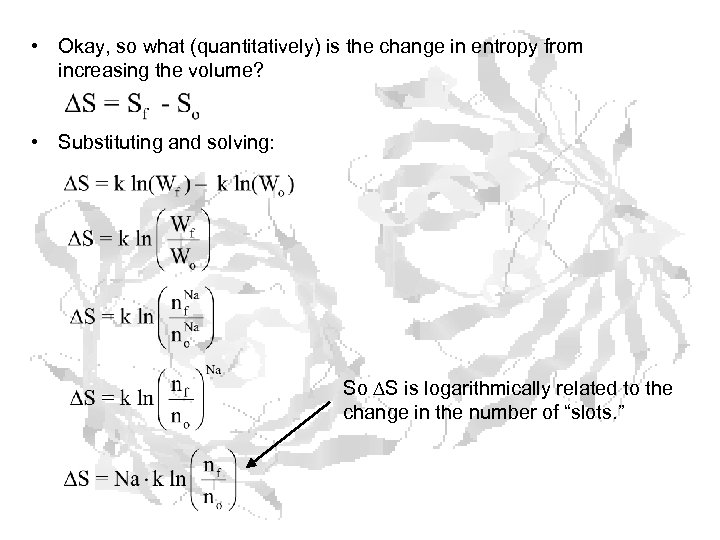
• Okay, so what (quantitatively) is the change in entropy from increasing the volume? • Substituting and solving: So S is logarithmically related to the change in the number of “slots. ”
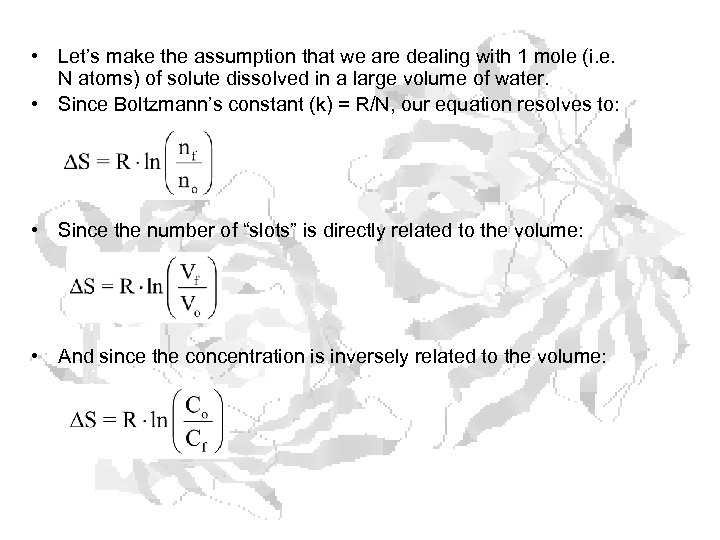
• Let’s make the assumption that we are dealing with 1 mole (i. e. N atoms) of solute dissolved in a large volume of water. • Since Boltzmann’s constant (k) = R/N, our equation resolves to: • Since the number of “slots” is directly related to the volume: • And since the concentration is inversely related to the volume:
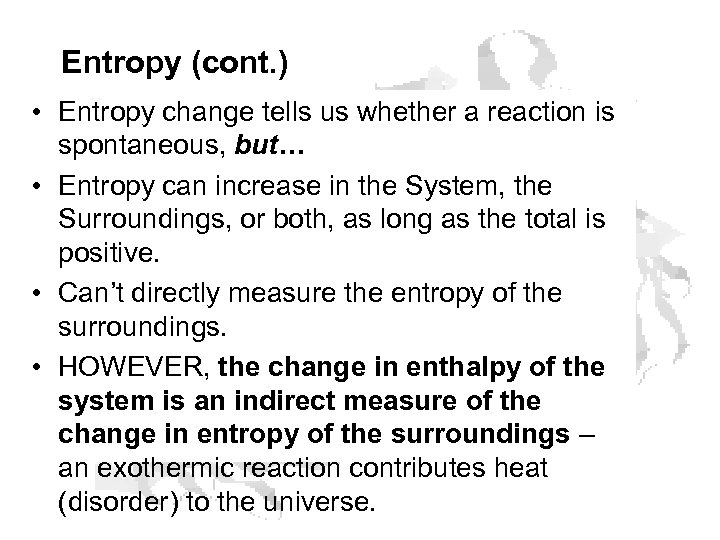
Entropy (cont. ) • Entropy change tells us whether a reaction is spontaneous, but… • Entropy can increase in the System, the Surroundings, or both, as long as the total is positive. • Can’t directly measure the entropy of the surroundings. • HOWEVER, the change in enthalpy of the system is an indirect measure of the change in entropy of the surroundings – an exothermic reaction contributes heat (disorder) to the universe.
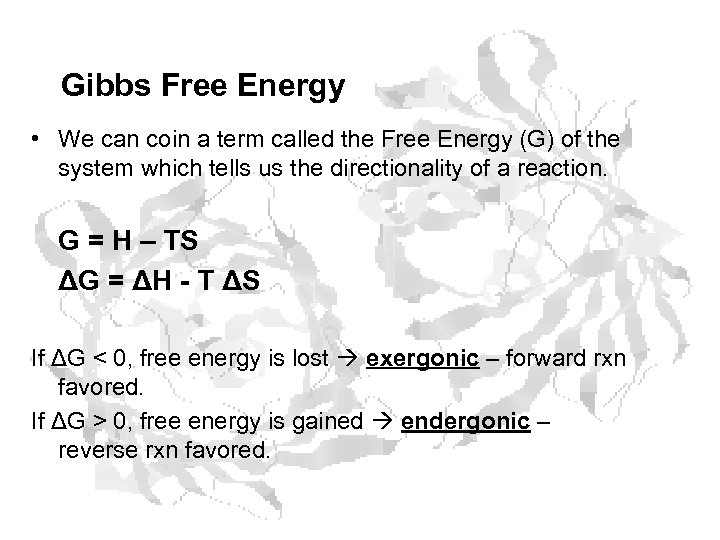
Gibbs Free Energy • We can coin a term called the Free Energy (G) of the system which tells us the directionality of a reaction. G = H – TS ΔG = ΔH - T ΔS If ΔG < 0, free energy is lost exergonic – forward rxn favored. If ΔG > 0, free energy is gained endergonic – reverse rxn favored.

Different ΔG’s • ΔG is the change in free energy for a reaction under some set of real conditions. • ΔGo is the change in free energy for a reaction under standard conditions (all reactants 1 M) • ΔGo’ is the change of free energy for a reaction with all reactants at 1 M and p. H 7.
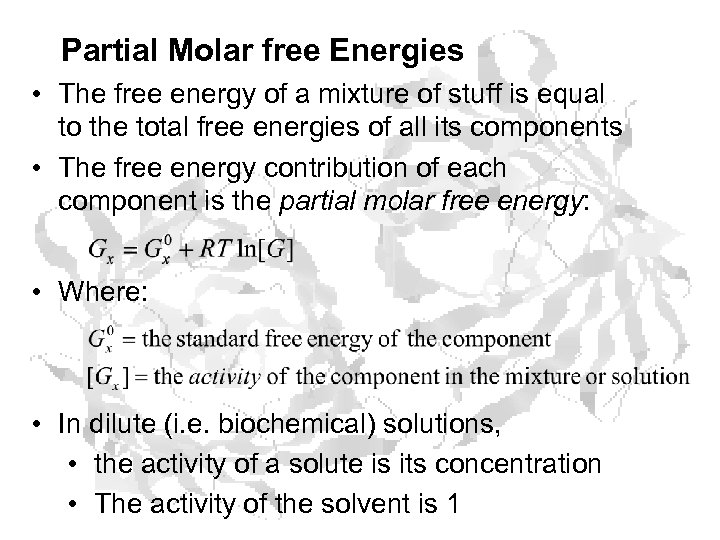
Partial Molar free Energies • The free energy of a mixture of stuff is equal to the total free energies of all its components • The free energy contribution of each component is the partial molar free energy: • Where: • In dilute (i. e. biochemical) solutions, • the activity of a solute is its concentration • The activity of the solvent is 1
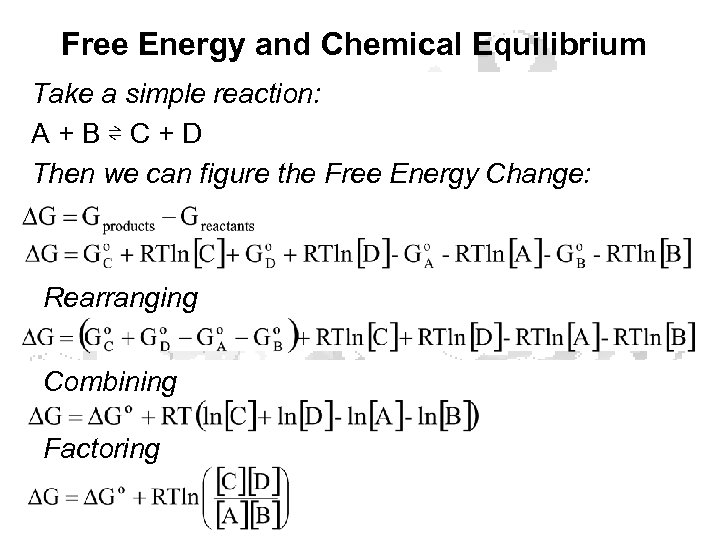
Free Energy and Chemical Equilibrium Take a simple reaction: A + B ⇌ C + D Then we can figure the Free Energy Change: Rearranging Combining Factoring
![Freee Energy and Equilibrium (cont. ) Hang on a second! [A][B] is the product Freee Energy and Equilibrium (cont. ) Hang on a second! [A][B] is the product](https://present5.com/presentation/42a98830837b61a9109d96207df1cb5a/image-54.jpg)
Freee Energy and Equilibrium (cont. ) Hang on a second! [A][B] is the product of the reactant concentrations [C][D] is the product of the product concentrations Remembering Freshman Chem, we have a word for that ratio.
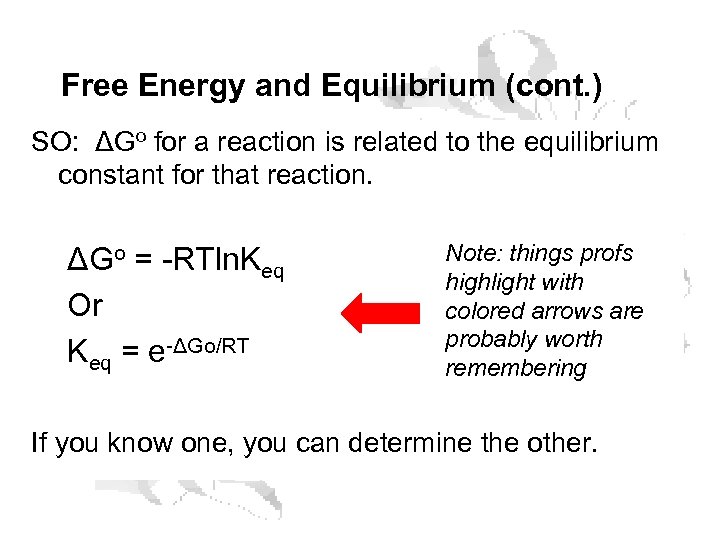
Free Energy and Equilibrium (cont. ) SO: ΔGo for a reaction is related to the equilibrium constant for that reaction. ΔGo = -RTln. Keq Or Keq = e-ΔGo/RT Note: things profs highlight with colored arrows are probably worth remembering If you know one, you can determine the other.
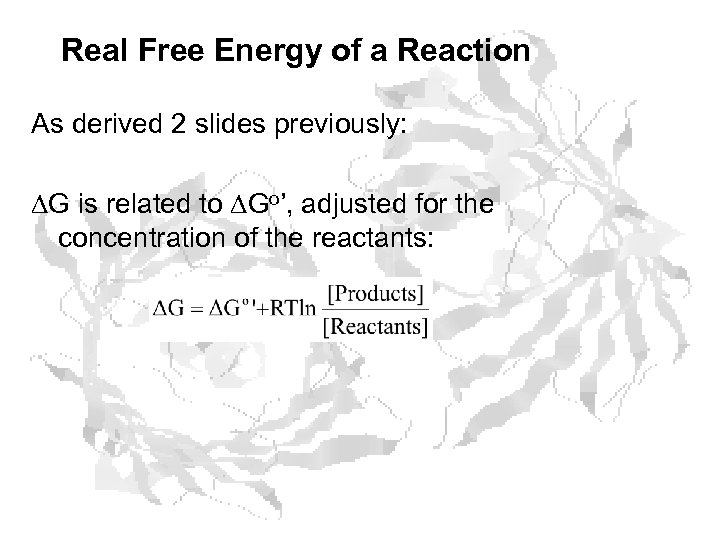
Real Free Energy of a Reaction As derived 2 slides previously: G is related to Go’, adjusted for the concentration of the reactants:
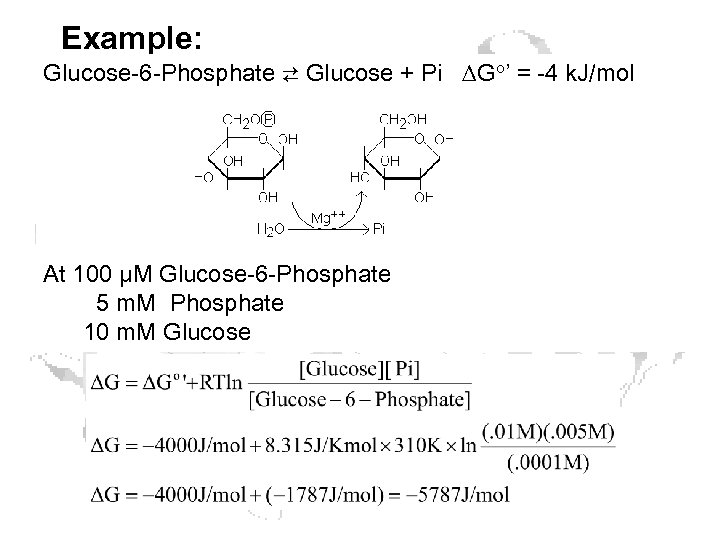
Example: Glucose-6 -Phosphate ⇄ Glucose + Pi ∆Go’ = -4 k. J/mol At 100 μM Glucose-6 -Phosphate 5 m. M Phosphate 10 m. M Glucose
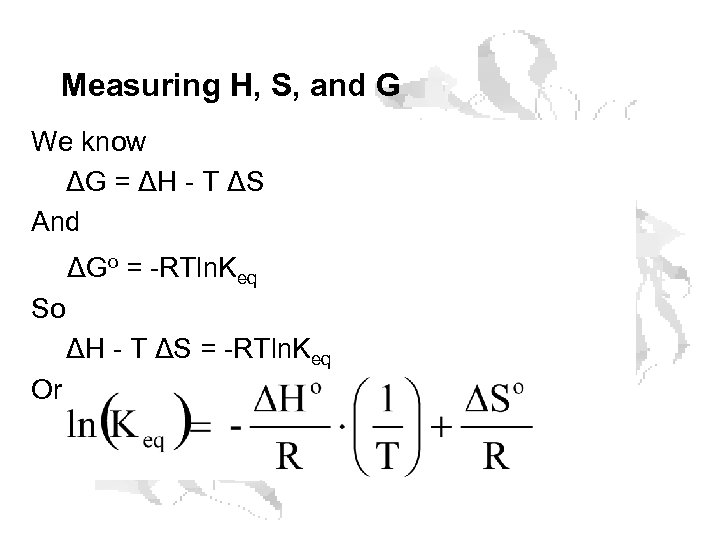
Measuring H, S, and G We know ΔG = ΔH - T ΔS And ΔGo = -RTln. Keq So ΔH - T ΔS = -RTln. Keq Or
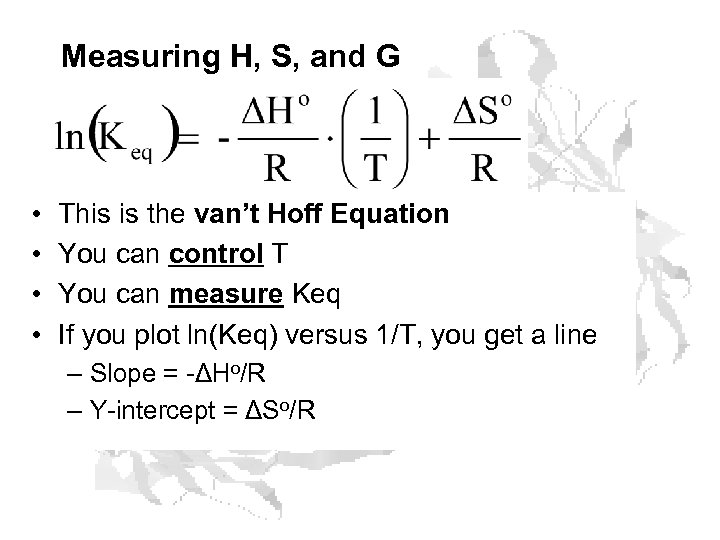
Measuring H, S, and G • • This is the van’t Hoff Equation You can control T You can measure Keq If you plot ln(Keq) versus 1/T, you get a line – Slope = -ΔHo/R – Y-intercept = ΔSo/R
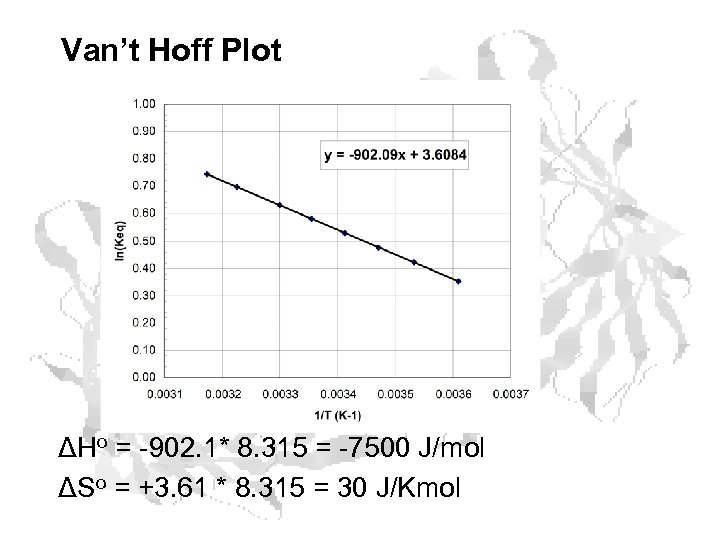
Van’t Hoff Plot ΔHo = -902. 1* 8. 315 = -7500 J/mol ΔSo = +3. 61 * 8. 315 = 30 J/Kmol
42a98830837b61a9109d96207df1cb5a.ppt