bc0d06856a504050961a48cbb8053e1b.ppt
- Количество слайдов: 9
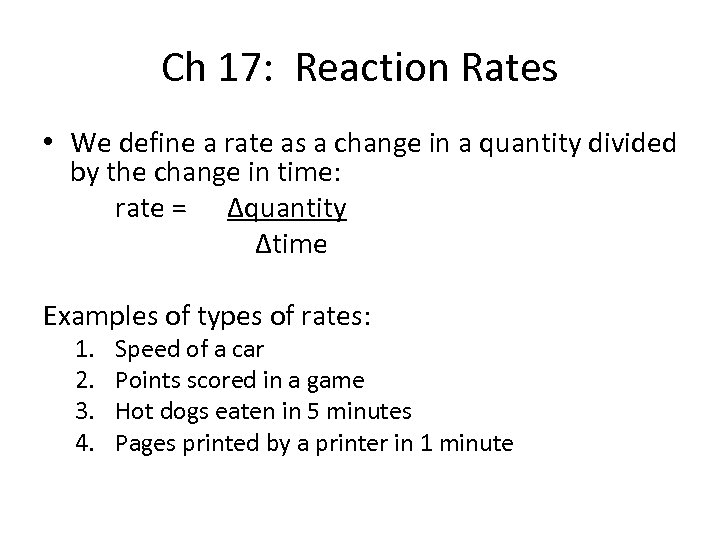 Ch 17: Reaction Rates • We define a rate as a change in a quantity divided by the change in time: rate = ∆quantity ∆time Examples of types of rates: 1. 2. 3. 4. Speed of a car Points scored in a game Hot dogs eaten in 5 minutes Pages printed by a printer in 1 minute
Ch 17: Reaction Rates • We define a rate as a change in a quantity divided by the change in time: rate = ∆quantity ∆time Examples of types of rates: 1. 2. 3. 4. Speed of a car Points scored in a game Hot dogs eaten in 5 minutes Pages printed by a printer in 1 minute
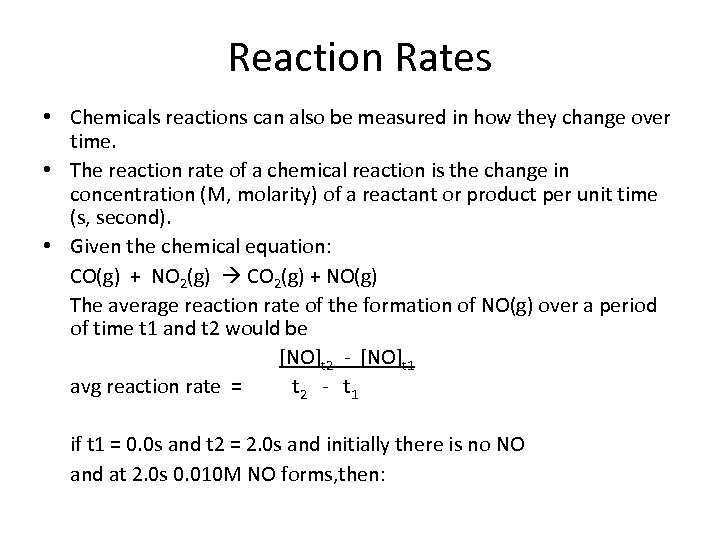 Reaction Rates • Chemicals reactions can also be measured in how they change over time. • The reaction rate of a chemical reaction is the change in concentration (M, molarity) of a reactant or product per unit time (s, second). • Given the chemical equation: CO(g) + NO 2(g) CO 2(g) + NO(g) The average reaction rate of the formation of NO(g) over a period of time t 1 and t 2 would be [NO]t 2 - [NO]t 1 avg reaction rate = t 2 - t 1 if t 1 = 0. 0 s and t 2 = 2. 0 s and initially there is no NO and at 2. 0 s 0. 010 M NO forms, then:
Reaction Rates • Chemicals reactions can also be measured in how they change over time. • The reaction rate of a chemical reaction is the change in concentration (M, molarity) of a reactant or product per unit time (s, second). • Given the chemical equation: CO(g) + NO 2(g) CO 2(g) + NO(g) The average reaction rate of the formation of NO(g) over a period of time t 1 and t 2 would be [NO]t 2 - [NO]t 1 avg reaction rate = t 2 - t 1 if t 1 = 0. 0 s and t 2 = 2. 0 s and initially there is no NO and at 2. 0 s 0. 010 M NO forms, then:
 Collision Theory • Reactions occur when molecules collide together • The collision theory says that: 1. atoms, ions, and molecules must collide in order to react. 2. Reacting substances must collide with the correct orientation 3. Reacting substances must collide with sufficient energy to form the activated complex • The activated complex (or transition state) is a temporary, unstable arrangement of atoms that may form products or may break apart to reform the reactants.
Collision Theory • Reactions occur when molecules collide together • The collision theory says that: 1. atoms, ions, and molecules must collide in order to react. 2. Reacting substances must collide with the correct orientation 3. Reacting substances must collide with sufficient energy to form the activated complex • The activated complex (or transition state) is a temporary, unstable arrangement of atoms that may form products or may break apart to reform the reactants.
 Activation Energy • In order for a reaction to occur, the reacting atoms, ions, and molecules must have sufficient energy when they collide in the right orientation. • The minimum amount of energy required for the activated complex to form and for the reaction to take places is called the activation energy (Ea). • Although important in determining if a reaction will occur, this tells us little about the actual speed/rate of reaction.
Activation Energy • In order for a reaction to occur, the reacting atoms, ions, and molecules must have sufficient energy when they collide in the right orientation. • The minimum amount of energy required for the activated complex to form and for the reaction to take places is called the activation energy (Ea). • Although important in determining if a reaction will occur, this tells us little about the actual speed/rate of reaction.
 Factors Affecting Reaction Rates • The compounds themselves • Concentration – Reactions speed up when the concentrations of reactants are increased. (Increasing the concentration increases the number of particles available to collide) • Surface Area – Reactions can occur faster when more area is exposed to take part in a reaction. (Increasing surface area increased the number of particles available to collide) • Temperature – Reactant particles require activation energy in order to react. Raising the temperature increases the average number of particles with sufficient activation energy, increasing the rate of reaction.
Factors Affecting Reaction Rates • The compounds themselves • Concentration – Reactions speed up when the concentrations of reactants are increased. (Increasing the concentration increases the number of particles available to collide) • Surface Area – Reactions can occur faster when more area is exposed to take part in a reaction. (Increasing surface area increased the number of particles available to collide) • Temperature – Reactant particles require activation energy in order to react. Raising the temperature increases the average number of particles with sufficient activation energy, increasing the rate of reaction.
 Catalysts and Inhibitors • A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed in the reaction. • Essentially, a catalyst helps lower the activation energy of the reaction. This means that more collisions will then have sufficient energy to react. • An inhibitor on the other hand is a substance that slows down reaction rates or prevents reactions from occurring at all.
Catalysts and Inhibitors • A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed in the reaction. • Essentially, a catalyst helps lower the activation energy of the reaction. This means that more collisions will then have sufficient energy to react. • An inhibitor on the other hand is a substance that slows down reaction rates or prevents reactions from occurring at all.
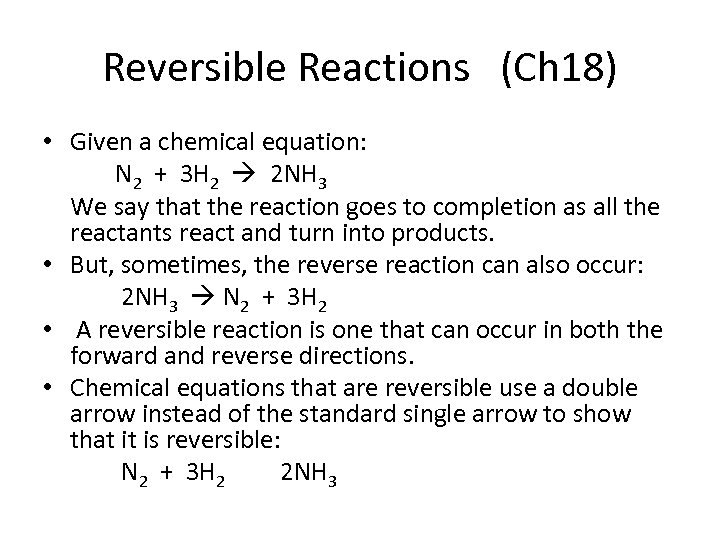 Reversible Reactions (Ch 18) • Given a chemical equation: N 2 + 3 H 2 2 NH 3 We say that the reaction goes to completion as all the reactants react and turn into products. • But, sometimes, the reverse reaction can also occur: 2 NH 3 N 2 + 3 H 2 • A reversible reaction is one that can occur in both the forward and reverse directions. • Chemical equations that are reversible use a double arrow instead of the standard single arrow to show that it is reversible: N 2 + 3 H 2 2 NH 3
Reversible Reactions (Ch 18) • Given a chemical equation: N 2 + 3 H 2 2 NH 3 We say that the reaction goes to completion as all the reactants react and turn into products. • But, sometimes, the reverse reaction can also occur: 2 NH 3 N 2 + 3 H 2 • A reversible reaction is one that can occur in both the forward and reverse directions. • Chemical equations that are reversible use a double arrow instead of the standard single arrow to show that it is reversible: N 2 + 3 H 2 2 NH 3
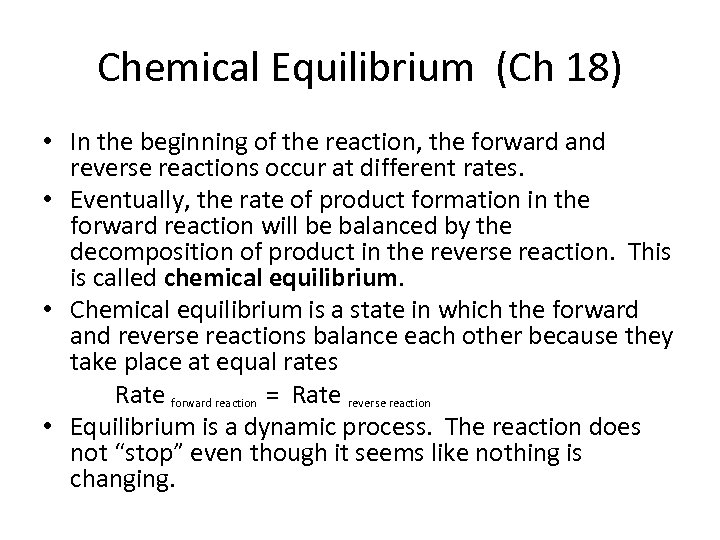 Chemical Equilibrium (Ch 18) • In the beginning of the reaction, the forward and reverse reactions occur at different rates. • Eventually, the rate of product formation in the forward reaction will be balanced by the decomposition of product in the reverse reaction. This is called chemical equilibrium. • Chemical equilibrium is a state in which the forward and reverse reactions balance each other because they take place at equal rates Rate forward reaction = Rate reverse reaction • Equilibrium is a dynamic process. The reaction does not “stop” even though it seems like nothing is changing.
Chemical Equilibrium (Ch 18) • In the beginning of the reaction, the forward and reverse reactions occur at different rates. • Eventually, the rate of product formation in the forward reaction will be balanced by the decomposition of product in the reverse reaction. This is called chemical equilibrium. • Chemical equilibrium is a state in which the forward and reverse reactions balance each other because they take place at equal rates Rate forward reaction = Rate reverse reaction • Equilibrium is a dynamic process. The reaction does not “stop” even though it seems like nothing is changing.
 Changes in Equilibrium (Ch 18) • Equilibrium of chemical reactions can “shift” in one direction or another. • Le Châtelier’s Principle states that if a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. – Increase reactants – forward reaction favored, making more product. The reaction “shifts to the right” – Increase products – reverse reaction favored, making more reactants. The reaction “shifts to the left” – Decrease reactants – reverse reaction favored, making more reactants. The reaction “shifts to the left” – Decrease producs – forward reaction favored, making more products. The reaction “shifts to the right” – Increase temperature – Endothermic reaction is favored. Reaction shifts towards the endothermic process – Add catalyst – no effect
Changes in Equilibrium (Ch 18) • Equilibrium of chemical reactions can “shift” in one direction or another. • Le Châtelier’s Principle states that if a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. – Increase reactants – forward reaction favored, making more product. The reaction “shifts to the right” – Increase products – reverse reaction favored, making more reactants. The reaction “shifts to the left” – Decrease reactants – reverse reaction favored, making more reactants. The reaction “shifts to the left” – Decrease producs – forward reaction favored, making more products. The reaction “shifts to the right” – Increase temperature – Endothermic reaction is favored. Reaction shifts towards the endothermic process – Add catalyst – no effect


