ffdf0e9c565d76c3f411300cfb91b6a1.ppt
- Количество слайдов: 33
 Cervical Cancer: An update on the guidelines for screening Shikha Bose M. D. Prof. of Pathology Cedars Sinai Medical Center & David Geffen School of Medicine, UCLA Global Cancer Congress Sept. 20, 2014
Cervical Cancer: An update on the guidelines for screening Shikha Bose M. D. Prof. of Pathology Cedars Sinai Medical Center & David Geffen School of Medicine, UCLA Global Cancer Congress Sept. 20, 2014
 Worldwide Cervical cancer q Cervical cancer is a considerable problem § 555, 100 new cases § 80% of these will be in low/middle income countries q Third leading cause of cancer death in women § 309, 800 deaths annually § 73, 000 deaths in India (1/4)
Worldwide Cervical cancer q Cervical cancer is a considerable problem § 555, 100 new cases § 80% of these will be in low/middle income countries q Third leading cause of cancer death in women § 309, 800 deaths annually § 73, 000 deaths in India (1/4)
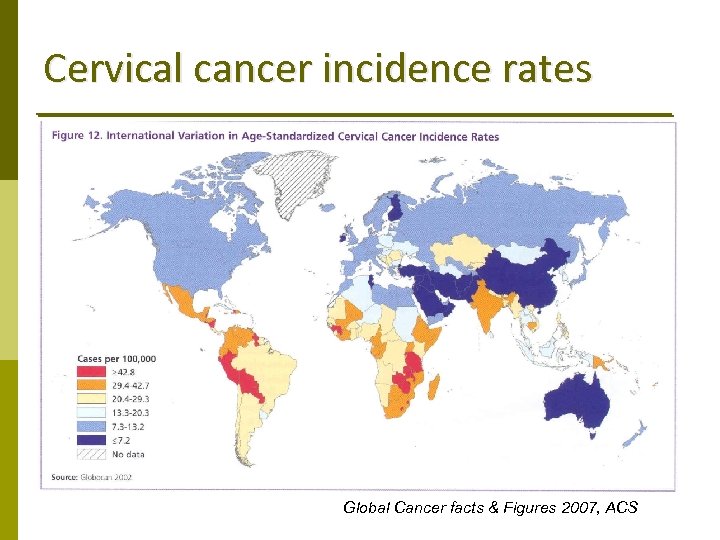 Cervical cancer incidence rates Global Cancer facts & Figures 2007, ACS
Cervical cancer incidence rates Global Cancer facts & Figures 2007, ACS
 HPV & Cervical CA
HPV & Cervical CA
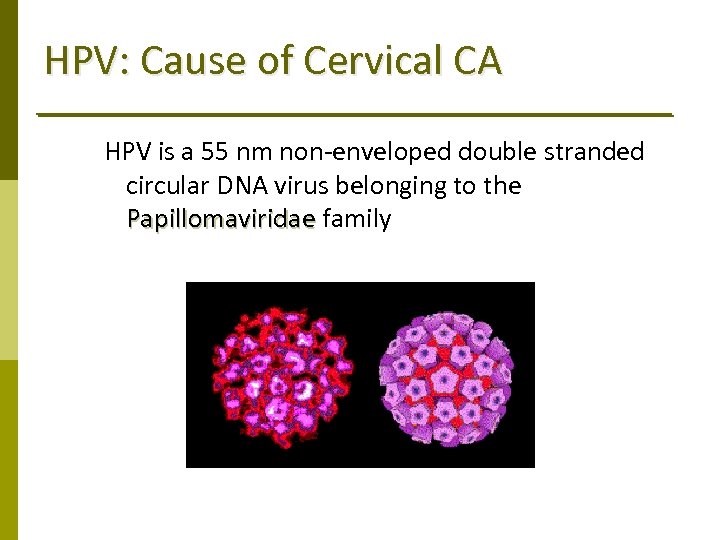 HPV: Cause of Cervical CA HPV is a 55 nm non‐enveloped double stranded circular DNA virus belonging to the Papillomaviridae family Papillomaviridae
HPV: Cause of Cervical CA HPV is a 55 nm non‐enveloped double stranded circular DNA virus belonging to the Papillomaviridae family Papillomaviridae
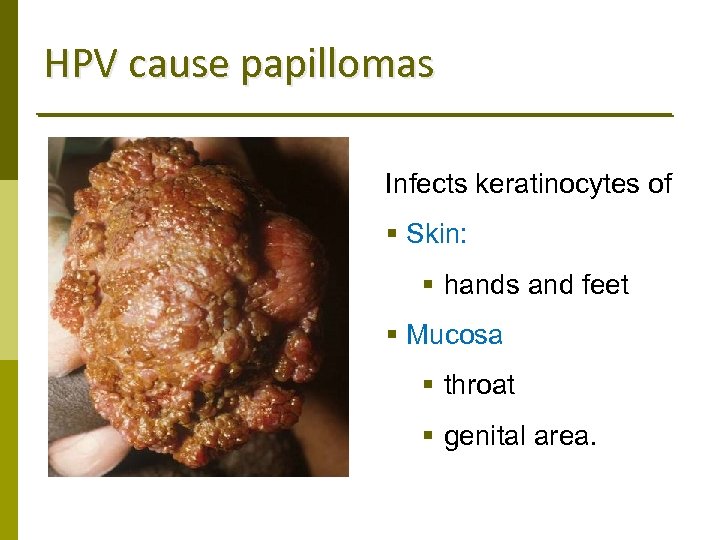 HPV cause papillomas Infects keratinocytes of § Skin: § hands and feet § Mucosa § throat § genital area.
HPV cause papillomas Infects keratinocytes of § Skin: § hands and feet § Mucosa § throat § genital area.
 HPV q q q Harald zur Hausen - 2008 Nobel Prize for Medicine Was first discovered in the 1950 s Stefania Ginsburg‐Jablonska, Polish dermatologist, Univ. of Warsaw first discovered the association between HPV & skin cancer in 1972 Harald zur Hausen, a German scientist and virologist was the first to show that HPV causes Cervical Ca and identified HPV 16 and HPV 18 as the responsible strains.
HPV q q q Harald zur Hausen - 2008 Nobel Prize for Medicine Was first discovered in the 1950 s Stefania Ginsburg‐Jablonska, Polish dermatologist, Univ. of Warsaw first discovered the association between HPV & skin cancer in 1972 Harald zur Hausen, a German scientist and virologist was the first to show that HPV causes Cervical Ca and identified HPV 16 and HPV 18 as the responsible strains.
 Anogenital HPV q Most common STD (6. 2 million new infections/yr in US) § Warts present in 1% of sexually active people § Subclinical infection >15% § 1 in 2 will acquire genital HPV in their lifetime q Worldwide age standardized prevalence of current HPV infection: 10. 5% q Varies between different regions: 1. 4% (Spain) to 25% (Nigeria)
Anogenital HPV q Most common STD (6. 2 million new infections/yr in US) § Warts present in 1% of sexually active people § Subclinical infection >15% § 1 in 2 will acquire genital HPV in their lifetime q Worldwide age standardized prevalence of current HPV infection: 10. 5% q Varies between different regions: 1. 4% (Spain) to 25% (Nigeria)
 HPV q >100 types; 40 infect anogenital mucosa § Low risk (LR)‐ HPV 6, 11 § Condyloma acuminata (~ 500, 000/yr) § Juvenile respiratory papillomatosis § Rare progression to cancer § High risk (HR) – at least 15 known § HPV 16, 18 – Commonly associated with cancer
HPV q >100 types; 40 infect anogenital mucosa § Low risk (LR)‐ HPV 6, 11 § Condyloma acuminata (~ 500, 000/yr) § Juvenile respiratory papillomatosis § Rare progression to cancer § High risk (HR) – at least 15 known § HPV 16, 18 – Commonly associated with cancer
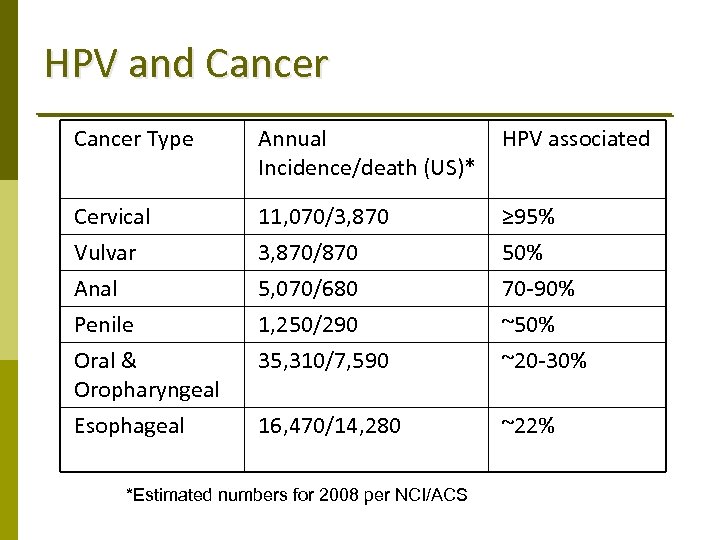 HPV and Cancer Type Annual Incidence/death (US)* HPV associated Cervical Vulvar Anal Penile Oral & Oropharyngeal Esophageal 11, 070/3, 870/870 5, 070/680 1, 250/290 35, 310/7, 590 ≥ 95% 50% 70‐ 90% ~50% ~20‐ 30% 16, 470/14, 280 ~22% *Estimated numbers for 2008 per NCI/ACS
HPV and Cancer Type Annual Incidence/death (US)* HPV associated Cervical Vulvar Anal Penile Oral & Oropharyngeal Esophageal 11, 070/3, 870/870 5, 070/680 1, 250/290 35, 310/7, 590 ≥ 95% 50% 70‐ 90% ~50% ~20‐ 30% 16, 470/14, 280 ~22% *Estimated numbers for 2008 per NCI/ACS
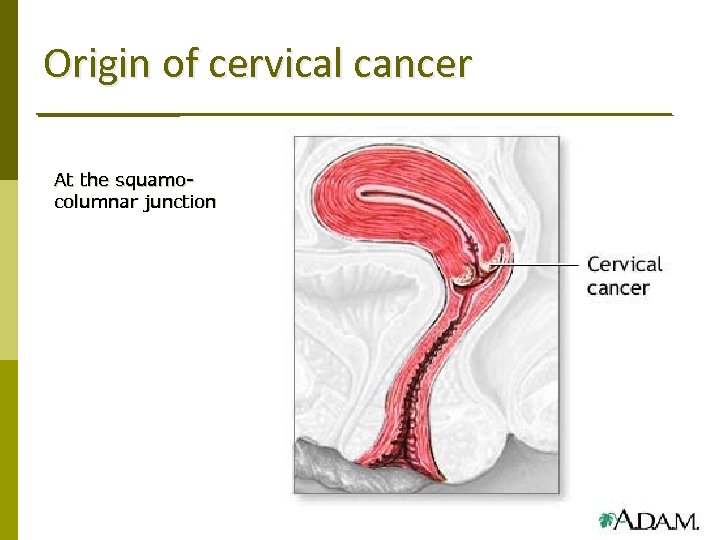 Origin of cervical cancer At the squamocolumnar junction
Origin of cervical cancer At the squamocolumnar junction
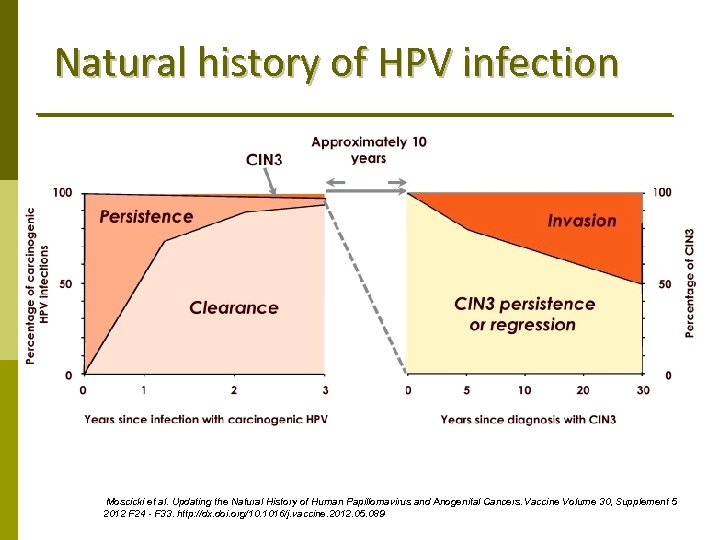 Natural history of HPV infection Moscicki et al. Updating the Natural History of Human Papillomavirus and Anogenital Cancers. Vaccine Volume 30, Supplement 5 2012 F 24 - F 33. http: //dx. doi. org/10. 1016/j. vaccine. 2012. 05. 089
Natural history of HPV infection Moscicki et al. Updating the Natural History of Human Papillomavirus and Anogenital Cancers. Vaccine Volume 30, Supplement 5 2012 F 24 - F 33. http: //dx. doi. org/10. 1016/j. vaccine. 2012. 05. 089
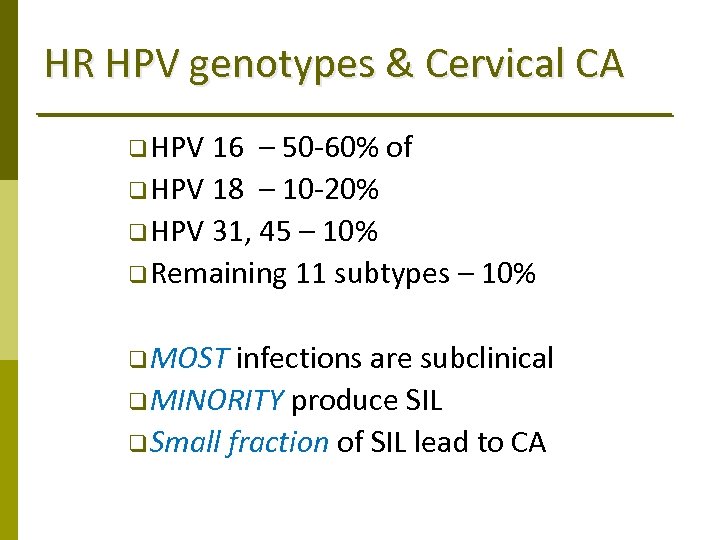 HR HPV genotypes & Cervical CA q HPV 16 – 50‐ 60% of q HPV 18 – 10‐ 20% q HPV 31, 45 – 10% q Remaining 11 subtypes – 10% q MOST infections are subclinical q MINORITY produce SIL q Small fraction of SIL lead to CA
HR HPV genotypes & Cervical CA q HPV 16 – 50‐ 60% of q HPV 18 – 10‐ 20% q HPV 31, 45 – 10% q Remaining 11 subtypes – 10% q MOST infections are subclinical q MINORITY produce SIL q Small fraction of SIL lead to CA
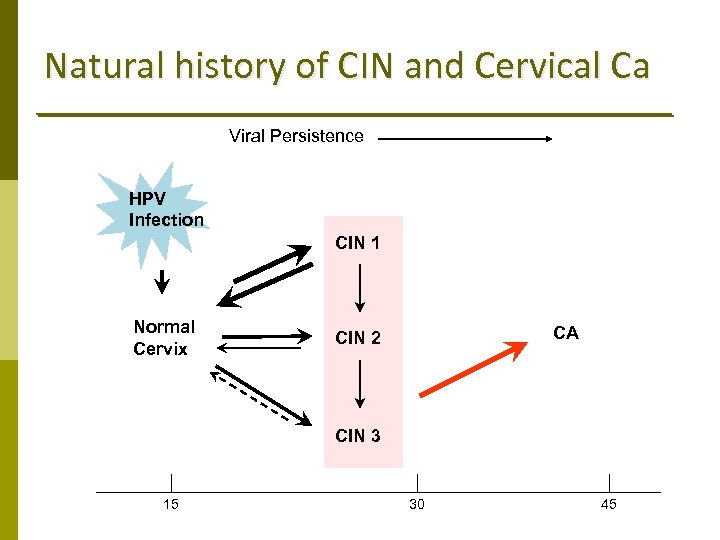 Natural history of CIN and Cervical Ca Viral Persistence HPV Infection CIN 1 Normal Cervix CA CIN 2 CIN 3 15 30 45
Natural history of CIN and Cervical Ca Viral Persistence HPV Infection CIN 1 Normal Cervix CA CIN 2 CIN 3 15 30 45
 CERVICAL CANCER SCREENING
CERVICAL CANCER SCREENING
 The Papanicolaou (Pap) Test q q q George Papanicolaou First announced the test in 1928 at a conference in Michigan. 1943 published “Diagnosis of Uterine Cancer by the Vaginal Smear” Best screening tool introduced for any cancer
The Papanicolaou (Pap) Test q q q George Papanicolaou First announced the test in 1928 at a conference in Michigan. 1943 published “Diagnosis of Uterine Cancer by the Vaginal Smear” Best screening tool introduced for any cancer
 The Pap Test q The Pap test, when combined with a regular screening program & appropriate follow‐up, can reduce cervical cancer deaths by up to 80%. q In the US of women with invasive cervical cancer: § >50% have never had a Pap smear § 10‐ 20% have not had a Pap smear in the preceding 5 yrs § 25% had an abnormal Pap smear, but did not get appropriate follow‐up (woman did not return for care, or clinician did not perform recommended tests or treatment)
The Pap Test q The Pap test, when combined with a regular screening program & appropriate follow‐up, can reduce cervical cancer deaths by up to 80%. q In the US of women with invasive cervical cancer: § >50% have never had a Pap smear § 10‐ 20% have not had a Pap smear in the preceding 5 yrs § 25% had an abnormal Pap smear, but did not get appropriate follow‐up (woman did not return for care, or clinician did not perform recommended tests or treatment)
 The Pap Test: Limitations q Good quality cytology‐based screening programs require highly trained personnel, specialized equipment and screening at regular intervals q Low sensitivity 50‐ 60% (Specificity – 95%) • High false negative associated with • patient compliance • sample collection • subjective assessments
The Pap Test: Limitations q Good quality cytology‐based screening programs require highly trained personnel, specialized equipment and screening at regular intervals q Low sensitivity 50‐ 60% (Specificity – 95%) • High false negative associated with • patient compliance • sample collection • subjective assessments
 Limitations of the Pap Test q Good quality cytology‐based screening programs require highly trained personnel and specialized equipment q Low sensitivity 50‐ 60% (Specificity – 95%) • High false negative • Increased liability • Requires screening at regular intervals q Subjective assessments
Limitations of the Pap Test q Good quality cytology‐based screening programs require highly trained personnel and specialized equipment q Low sensitivity 50‐ 60% (Specificity – 95%) • High false negative • Increased liability • Requires screening at regular intervals q Subjective assessments
 Newer modalities for Screening q Liquid based cytology q Computer ‐ assisted screening q Adjunctive molecular testing
Newer modalities for Screening q Liquid based cytology q Computer ‐ assisted screening q Adjunctive molecular testing
 Liquid based Pap Test q q q Currently Thin. Prep© (Hologic, Marlborough, MA) and Sure. Path© (Becton‐ Dickinson/Tri. Path, Burlington, NC) are the predominant LB Pap test types used in the US. Provide a more representative sampling Decrease pre‐analytic problems: transfer of sample, excess blood/inflammation, and air‐drying. Allows additional tests for HPV, STI (Chlamydia, Gonorrhea and Trichomonads) from the same vial. Allowed the incorporation of computer imaging technology. Meta‐analyses do not indicate an overall increase in HSIL detection. Arbyn M, Bergeron C, Klinkhamer P et al. Obstet Gynecol 111: 167, 2008
Liquid based Pap Test q q q Currently Thin. Prep© (Hologic, Marlborough, MA) and Sure. Path© (Becton‐ Dickinson/Tri. Path, Burlington, NC) are the predominant LB Pap test types used in the US. Provide a more representative sampling Decrease pre‐analytic problems: transfer of sample, excess blood/inflammation, and air‐drying. Allows additional tests for HPV, STI (Chlamydia, Gonorrhea and Trichomonads) from the same vial. Allowed the incorporation of computer imaging technology. Meta‐analyses do not indicate an overall increase in HSIL detection. Arbyn M, Bergeron C, Klinkhamer P et al. Obstet Gynecol 111: 167, 2008
 Computer imaging technology q Two FDAs approved devices to assist in cervical cytology screening: § The B. D. Focal. Point™ Slide Profiler Intelligent Pap Imaging™ system § incorporates initial computer evaluation followed by manual rescreening of the most likely abnormal cases; the least abnormal cases require no review. § The Hologic Thin. Prep™ Imaging System § involves automated pre‐screening and presentation of digitized images of the most abnormal 22 fields of view (FOV) to a reviewer, thus allowing a dual review: one full review from the Thin. Prep Imager and another from an experienced cytotechnologist
Computer imaging technology q Two FDAs approved devices to assist in cervical cytology screening: § The B. D. Focal. Point™ Slide Profiler Intelligent Pap Imaging™ system § incorporates initial computer evaluation followed by manual rescreening of the most likely abnormal cases; the least abnormal cases require no review. § The Hologic Thin. Prep™ Imaging System § involves automated pre‐screening and presentation of digitized images of the most abnormal 22 fields of view (FOV) to a reviewer, thus allowing a dual review: one full review from the Thin. Prep Imager and another from an experienced cytotechnologist
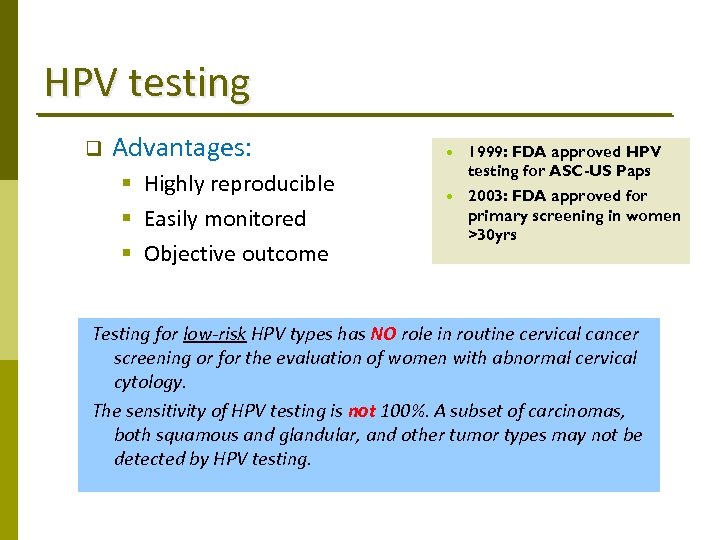 HPV testing q Advantages: § Highly reproducible § Easily monitored § Objective outcome 1999: FDA approved HPV testing for ASC-US Paps 2003: FDA approved for primary screening in women >30 yrs Testing for low-risk HPV types has NO role in routine cervical cancer screening or for the evaluation of women with abnormal cervical cytology. The sensitivity of HPV testing is not 100%. A subset of carcinomas, both squamous and glandular, and other tumor types may not be detected by HPV testing.
HPV testing q Advantages: § Highly reproducible § Easily monitored § Objective outcome 1999: FDA approved HPV testing for ASC-US Paps 2003: FDA approved for primary screening in women >30 yrs Testing for low-risk HPV types has NO role in routine cervical cancer screening or for the evaluation of women with abnormal cervical cytology. The sensitivity of HPV testing is not 100%. A subset of carcinomas, both squamous and glandular, and other tumor types may not be detected by HPV testing.
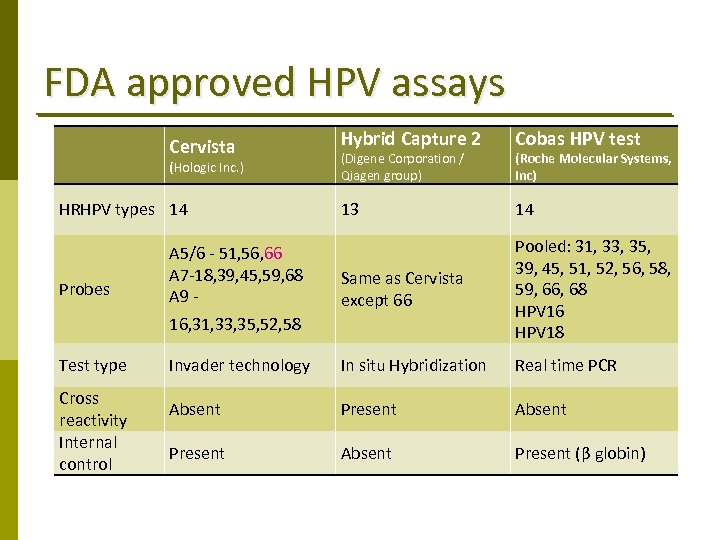 FDA approved HPV assays Hybrid Capture 2 Cobas HPV test 13 14 A 5/6 ‐ 51, 56, 66 A 7‐ 18, 39, 45, 59, 68 A 9 ‐ Same as Cervista except 66 Pooled: 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 HPV 16 HPV 18 Invader technology In situ Hybridization Real time PCR Absent Present (β globin) Cervista (Hologic Inc. ) HRHPV types 14 Probes 16, 31, 33, 35, 52, 58 Test type Cross reactivity Internal control (Digene Corporation / Qiagen group) (Roche Molecular Systems, Inc)
FDA approved HPV assays Hybrid Capture 2 Cobas HPV test 13 14 A 5/6 ‐ 51, 56, 66 A 7‐ 18, 39, 45, 59, 68 A 9 ‐ Same as Cervista except 66 Pooled: 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 HPV 16 HPV 18 Invader technology In situ Hybridization Real time PCR Absent Present (β globin) Cervista (Hologic Inc. ) HRHPV types 14 Probes 16, 31, 33, 35, 52, 58 Test type Cross reactivity Internal control (Digene Corporation / Qiagen group) (Roche Molecular Systems, Inc)
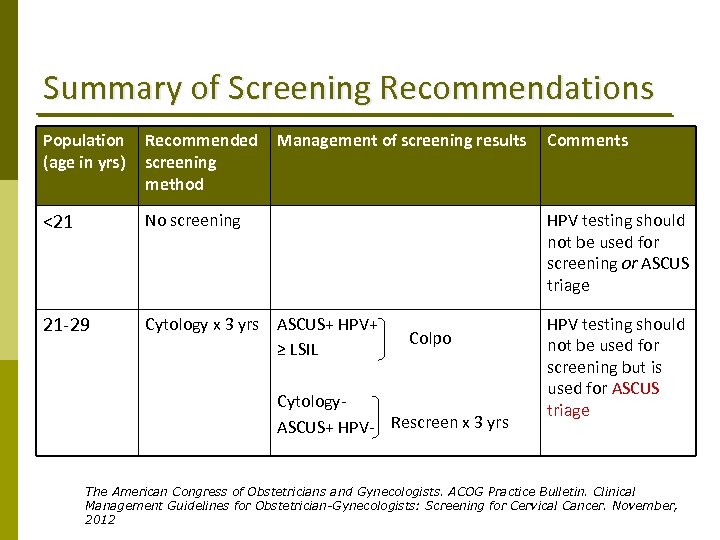 Summary of Screening Recommendations Population (age in yrs) Recommended screening method Management of screening results <21 No screening HPV testing should not be used for screening or ASCUS triage 21‐ 29 Cytology x 3 yrs ASCUS+ HPV+ ≥ LSIL HPV testing should not be used for screening but is used for ASCUS triage Colpo Cytology‐ ASCUS+ HPV‐ Rescreen x 3 yrs Comments The American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists: Screening for Cervical Cancer. November, 2012
Summary of Screening Recommendations Population (age in yrs) Recommended screening method Management of screening results <21 No screening HPV testing should not be used for screening or ASCUS triage 21‐ 29 Cytology x 3 yrs ASCUS+ HPV+ ≥ LSIL HPV testing should not be used for screening but is used for ASCUS triage Colpo Cytology‐ ASCUS+ HPV‐ Rescreen x 3 yrs Comments The American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists: Screening for Cervical Cancer. November, 2012
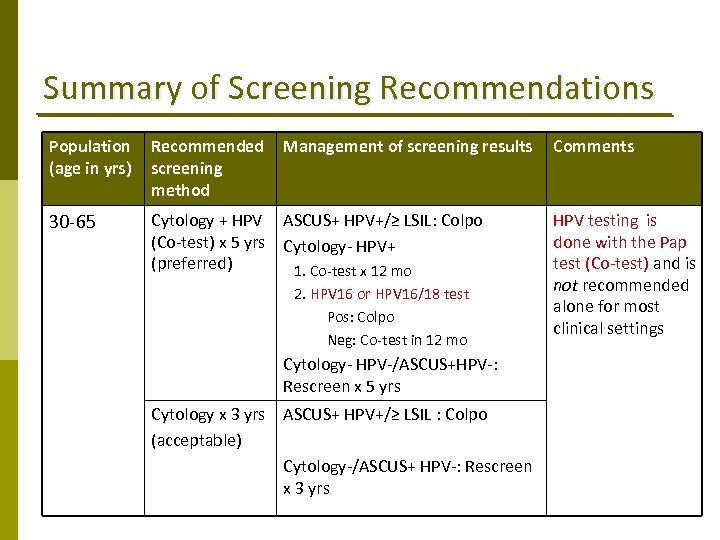 Summary of Screening Recommendations Population (age in yrs) Recommended screening method Management of screening results 30‐ 65 Cytology + HPV ASCUS+ HPV+/≥ LSIL: Colpo (Co‐test) x 5 yrs Cytology‐ HPV+ (preferred) 1. Co‐test x 12 mo 2. HPV 16 or HPV 16/18 test Pos: Colpo Neg: Co‐test in 12 mo Cytology‐ HPV‐/ASCUS+HPV‐: Rescreen x 5 yrs Cytology x 3 yrs ASCUS+ HPV+/≥ LSIL : Colpo (acceptable) Cytology‐/ASCUS+ HPV‐: Rescreen x 3 yrs Comments HPV testing is done with the Pap test (Co‐test) and is not recommended alone for most clinical settings
Summary of Screening Recommendations Population (age in yrs) Recommended screening method Management of screening results 30‐ 65 Cytology + HPV ASCUS+ HPV+/≥ LSIL: Colpo (Co‐test) x 5 yrs Cytology‐ HPV+ (preferred) 1. Co‐test x 12 mo 2. HPV 16 or HPV 16/18 test Pos: Colpo Neg: Co‐test in 12 mo Cytology‐ HPV‐/ASCUS+HPV‐: Rescreen x 5 yrs Cytology x 3 yrs ASCUS+ HPV+/≥ LSIL : Colpo (acceptable) Cytology‐/ASCUS+ HPV‐: Rescreen x 3 yrs Comments HPV testing is done with the Pap test (Co‐test) and is not recommended alone for most clinical settings
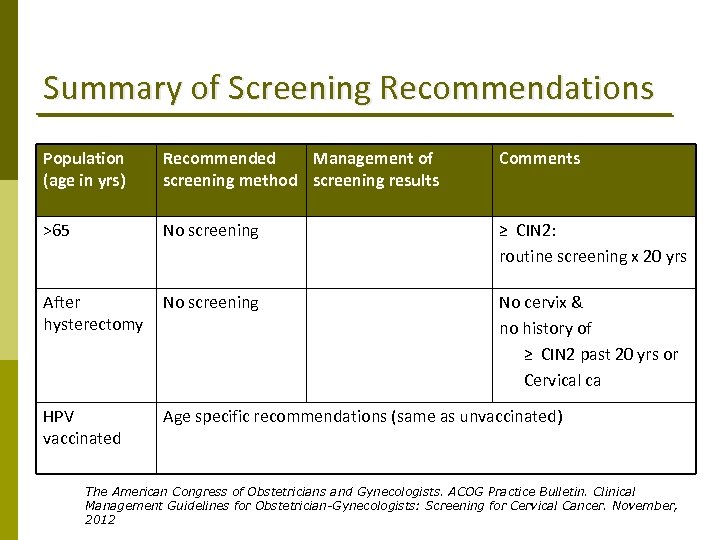 Summary of Screening Recommendations Population (age in yrs) Recommended Management of screening method screening results Comments >65 No screening ≥ CIN 2: routine screening x 20 yrs After hysterectomy No screening No cervix & no history of ≥ CIN 2 past 20 yrs or Cervical ca HPV vaccinated Age specific recommendations (same as unvaccinated) The American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists: Screening for Cervical Cancer. November, 2012
Summary of Screening Recommendations Population (age in yrs) Recommended Management of screening method screening results Comments >65 No screening ≥ CIN 2: routine screening x 20 yrs After hysterectomy No screening No cervix & no history of ≥ CIN 2 past 20 yrs or Cervical ca HPV vaccinated Age specific recommendations (same as unvaccinated) The American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists: Screening for Cervical Cancer. November, 2012
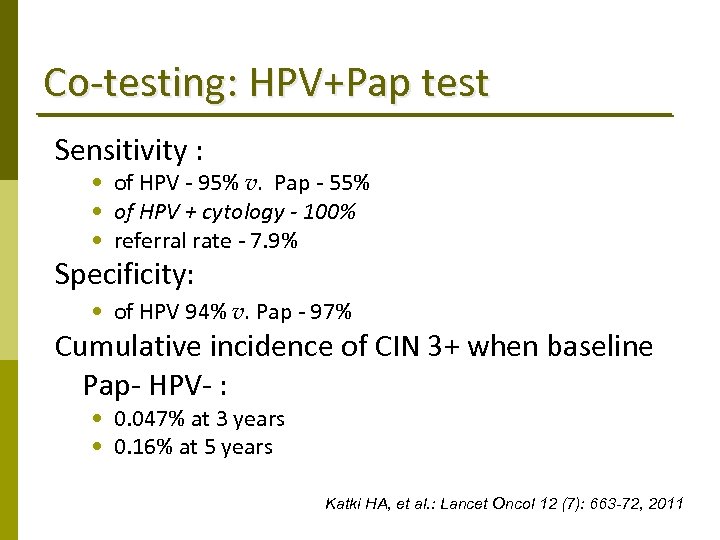 Co‐testing: HPV+Pap test Sensitivity : • of HPV ‐ 95% v. Pap ‐ 55% • of HPV + cytology - 100% • referral rate ‐ 7. 9% Specificity: • of HPV 94% v. Pap ‐ 97% Cumulative incidence of CIN 3+ when baseline Pap‐ HPV‐ : • 0. 047% at 3 years • 0. 16% at 5 years Katki HA, et al. : Lancet Oncol 12 (7): 663 -72, 2011
Co‐testing: HPV+Pap test Sensitivity : • of HPV ‐ 95% v. Pap ‐ 55% • of HPV + cytology - 100% • referral rate ‐ 7. 9% Specificity: • of HPV 94% v. Pap ‐ 97% Cumulative incidence of CIN 3+ when baseline Pap‐ HPV‐ : • 0. 047% at 3 years • 0. 16% at 5 years Katki HA, et al. : Lancet Oncol 12 (7): 663 -72, 2011
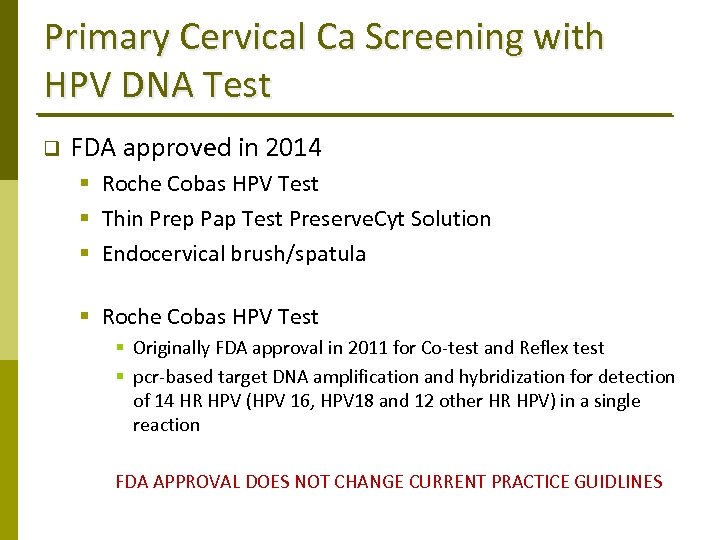 Primary Cervical Ca Screening with HPV DNA Test q FDA approved in 2014 § Roche Cobas HPV Test § Thin Prep Pap Test Preserve. Cyt Solution § Endocervical brush/spatula § Roche Cobas HPV Test § Originally FDA approval in 2011 for Co‐test and Reflex test § pcr‐based target DNA amplification and hybridization for detection of 14 HR HPV (HPV 16, HPV 18 and 12 other HR HPV) in a single reaction FDA APPROVAL DOES NOT CHANGE CURRENT PRACTICE GUIDLINES
Primary Cervical Ca Screening with HPV DNA Test q FDA approved in 2014 § Roche Cobas HPV Test § Thin Prep Pap Test Preserve. Cyt Solution § Endocervical brush/spatula § Roche Cobas HPV Test § Originally FDA approval in 2011 for Co‐test and Reflex test § pcr‐based target DNA amplification and hybridization for detection of 14 HR HPV (HPV 16, HPV 18 and 12 other HR HPV) in a single reaction FDA APPROVAL DOES NOT CHANGE CURRENT PRACTICE GUIDLINES
 HPV for primary screening q q Meta analysis of 4 European randomised trials comparing HPV‐ based with cytology‐based Cx Ca screening (Swedescreen, POBASCAM, ARTISTIC & NTCC 10) of >175 000 women HPV‐based screening resulted in: § 60– 70% reduction in invasive Cx Ca incidence § significant decreased incidence of invasive Cx Ca on longer follow‐up § observed cumulative incidence of invasive cervical cancer was lower 5· 5 years after a negative HPV test than 3· 5 years after a negative cytology test, indicating that 5‐year screen intervals with HPV‐based testing are safer than 3‐year intervals with conventional cytology § enhanced detection of CIN 2/3 in screened women, therefore enabling early treatment and reduction of invasive cervical cancer risk
HPV for primary screening q q Meta analysis of 4 European randomised trials comparing HPV‐ based with cytology‐based Cx Ca screening (Swedescreen, POBASCAM, ARTISTIC & NTCC 10) of >175 000 women HPV‐based screening resulted in: § 60– 70% reduction in invasive Cx Ca incidence § significant decreased incidence of invasive Cx Ca on longer follow‐up § observed cumulative incidence of invasive cervical cancer was lower 5· 5 years after a negative HPV test than 3· 5 years after a negative cytology test, indicating that 5‐year screen intervals with HPV‐based testing are safer than 3‐year intervals with conventional cytology § enhanced detection of CIN 2/3 in screened women, therefore enabling early treatment and reduction of invasive cervical cancer risk
 ATHENA trial q q Prospective study of 40, 901 women aged 25 years and older who underwent routine cervical exams Colposcopy and cervical tissue biopsy was performed on women with: § positive Pap test § positive HPV test § subset with negative Pap and HPV tests (~900 women). § Used to calculate verification biased adjustment (VBA) q q All biopsy results were compared with the Pap and cobas HPV Test results. 3 years of follow‐up
ATHENA trial q q Prospective study of 40, 901 women aged 25 years and older who underwent routine cervical exams Colposcopy and cervical tissue biopsy was performed on women with: § positive Pap test § positive HPV test § subset with negative Pap and HPV tests (~900 women). § Used to calculate verification biased adjustment (VBA) q q All biopsy results were compared with the Pap and cobas HPV Test results. 3 years of follow‐up
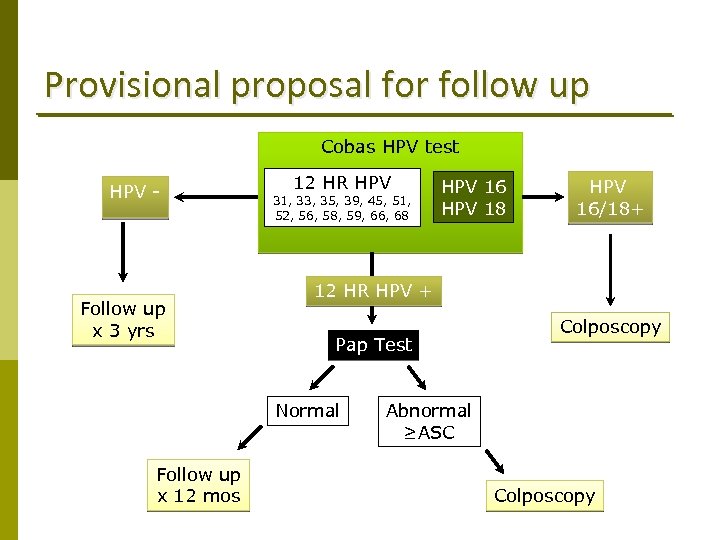 Provisional proposal for follow up Cobas HPV test HPV - Follow up x 3 yrs 12 HR HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 HPV 16/18+ 12 HR HPV + Pap Test Normal Follow up x 12 mos HPV 16 HPV 18 Colposcopy Abnormal ≥ASC Colposcopy
Provisional proposal for follow up Cobas HPV test HPV - Follow up x 3 yrs 12 HR HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 HPV 16/18+ 12 HR HPV + Pap Test Normal Follow up x 12 mos HPV 16 HPV 18 Colposcopy Abnormal ≥ASC Colposcopy



