d9df160ddc5a50786e9982a2440979ad.ppt
- Количество слайдов: 23
 Centro de Construcción de Cardioestimuladores
Centro de Construcción de Cardioestimuladores
 Overview n On February 3, 1960, Prof. Orestes Fiandra performed the first effective pacemaker implant in the world. n CCC was founded in 1970 dedicated to the development and manufacture of pacemakers n We have 30 years of experience in the medical devices field and we are expanding activities to other engineering areas.
Overview n On February 3, 1960, Prof. Orestes Fiandra performed the first effective pacemaker implant in the world. n CCC was founded in 1970 dedicated to the development and manufacture of pacemakers n We have 30 years of experience in the medical devices field and we are expanding activities to other engineering areas.
 A brief history n 70’s: Design and manufacture of pacemakers for the domestic market n 80’s: Assembly of kits supplied by Exonix n 90 -94: Development of APEX series n 95: Starting international business n 97: Starting engineering and OEM business n 02: Offices in Europe (i 2 m)
A brief history n 70’s: Design and manufacture of pacemakers for the domestic market n 80’s: Assembly of kits supplied by Exonix n 90 -94: Development of APEX series n 95: Starting international business n 97: Starting engineering and OEM business n 02: Offices in Europe (i 2 m)
 Today n Some years of experience in the global market n Two divisions: Pacemakers n Engineering n
Today n Some years of experience in the global market n Two divisions: Pacemakers n Engineering n
 Engineering Division n We aim at the development of specific projects through direct agreements with customers, or by participating in agreements with other solution supplier companies. n We have developed a substantial set of framework solutions and thoroughly tested modules; so, we can offer short lead times with uncompromising quality. n We can offer rapid development of prototypes and new products together with high quality standards and low cost n Our work is not limited to the medical devices field
Engineering Division n We aim at the development of specific projects through direct agreements with customers, or by participating in agreements with other solution supplier companies. n We have developed a substantial set of framework solutions and thoroughly tested modules; so, we can offer short lead times with uncompromising quality. n We can offer rapid development of prototypes and new products together with high quality standards and low cost n Our work is not limited to the medical devices field
 Electronics n Design, prototyping and circuit manufacture according to applicable international medical quality standards. n Design and manufacture of monitoring devices. n Design and manufacture of micro-power implantable circuits. n Design of Application Specific Integrated Circuits (ASICs).
Electronics n Design, prototyping and circuit manufacture according to applicable international medical quality standards. n Design and manufacture of monitoring devices. n Design and manufacture of micro-power implantable circuits. n Design of Application Specific Integrated Circuits (ASICs).
 Firmware n Development of embedded systems software, including real-time systems, signal processing, and communications. n Development of complex systems involving real-time parallel processing. n Development of microcontroller-based ultra-low power consumption solutions.
Firmware n Development of embedded systems software, including real-time systems, signal processing, and communications. n Development of complex systems involving real-time parallel processing. n Development of microcontroller-based ultra-low power consumption solutions.
 High-level Software n Development of high-level software, including Graphical User Interfaces and software for the command of embedded systems. n Development of special-purpose languages aimed at simplifying maintenance and operation tasks. n Development of communications software, used with devices designed by CCC or by third parties n Remote operation through the Internet. n Integration of Data Base services.
High-level Software n Development of high-level software, including Graphical User Interfaces and software for the command of embedded systems. n Development of special-purpose languages aimed at simplifying maintenance and operation tasks. n Development of communications software, used with devices designed by CCC or by third parties n Remote operation through the Internet. n Integration of Data Base services.
 Leads and Encapsulation n Design and Manufacture of special purpose, chronically-implantable leads. n Medical-grade encapsulations including titanium and epoxy enclosures. n Mechanical solutions for medical devices n Medical-grade packaging, including blister packs and ETO sterilization.
Leads and Encapsulation n Design and Manufacture of special purpose, chronically-implantable leads. n Medical-grade encapsulations including titanium and epoxy enclosures. n Mechanical solutions for medical devices n Medical-grade packaging, including blister packs and ETO sterilization.
 Project Leadership & Consulting n Project management and leadership of missioncritical projects, integrating our capacity and engineering capabilities with the customer’s resources. n Wide experience in international multi-center projects. n Feasibility studies in areas such as micro-power electronics, real time software development. n Consulting services in Software Engineering and SQA.
Project Leadership & Consulting n Project management and leadership of missioncritical projects, integrating our capacity and engineering capabilities with the customer’s resources. n Wide experience in international multi-center projects. n Feasibility studies in areas such as micro-power electronics, real time software development. n Consulting services in Software Engineering and SQA.
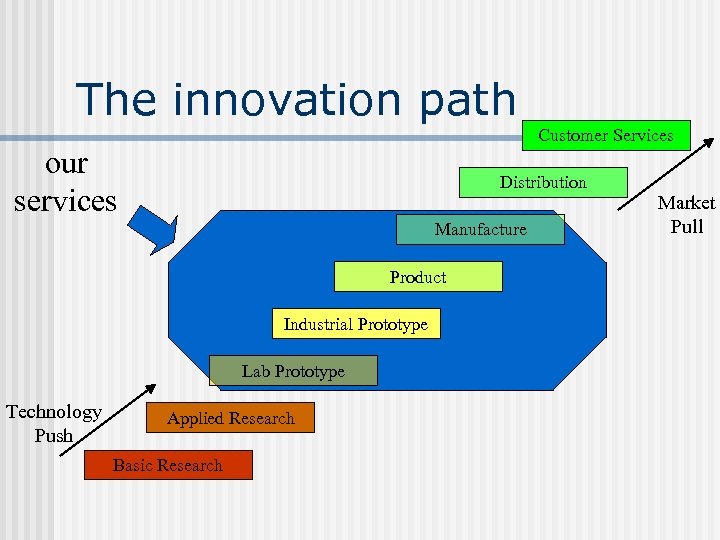 The innovation path Customer Services our services Distribution Manufacture Product Industrial Prototype Lab Prototype Technology Push Applied Research Basic Research Market Pull
The innovation path Customer Services our services Distribution Manufacture Product Industrial Prototype Lab Prototype Technology Push Applied Research Basic Research Market Pull
 Some clients and partners Illini Group Neurostream
Some clients and partners Illini Group Neurostream
 Example n n n From the lab to CE mark and FDA approval Start-up company from US - Israel CCC: n n n 1998: lab prototype 1999: 1 st implantable prototype (animals) 2000: refinements, prototypes 2001: clinical trials (Italy) - Starting CE Mark 2002: CE Mark 2003: developing a new model
Example n n n From the lab to CE mark and FDA approval Start-up company from US - Israel CCC: n n n 1998: lab prototype 1999: 1 st implantable prototype (animals) 2000: refinements, prototypes 2001: clinical trials (Italy) - Starting CE Mark 2002: CE Mark 2003: developing a new model
 Pacemaker Division n Pacemakers & Leads: n n n Uruguay: direct sales Latin America: Distributors Circuits & Parts: n Russia n India
Pacemaker Division n Pacemakers & Leads: n n n Uruguay: direct sales Latin America: Distributors Circuits & Parts: n Russia n India
 Pacemaker Division n We are focused in design and manufacture activities n We are expanding Sales capabilities n We are looking for partners able to promote and commercialize our products
Pacemaker Division n We are focused in design and manufacture activities n We are expanding Sales capabilities n We are looking for partners able to promote and commercialize our products
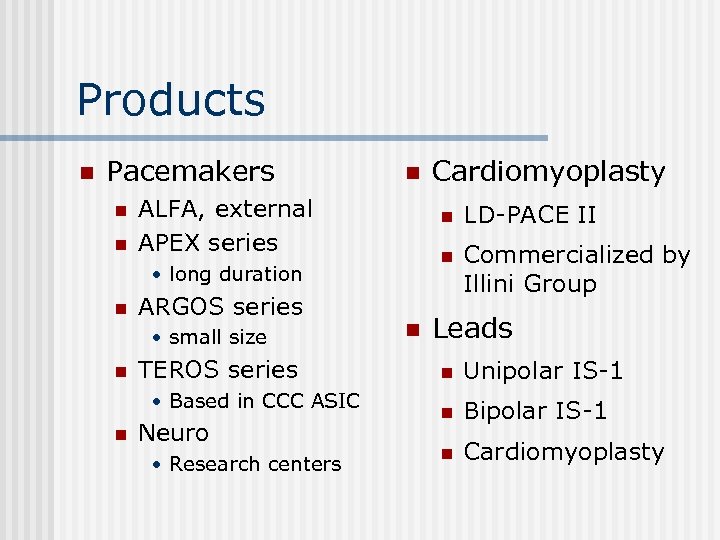 Products n Pacemakers n n n ALFA, external APEX series n ARGOS series • small size n TEROS series • Based in CCC ASIC n Neuro • Research centers n LD-PACE II n • long duration n Cardiomyoplasty Commercialized by Illini Group Leads n Unipolar IS-1 n Bipolar IS-1 n Cardiomyoplasty
Products n Pacemakers n n n ALFA, external APEX series n ARGOS series • small size n TEROS series • Based in CCC ASIC n Neuro • Research centers n LD-PACE II n • long duration n Cardiomyoplasty Commercialized by Illini Group Leads n Unipolar IS-1 n Bipolar IS-1 n Cardiomyoplasty
 Argos Series n n n SSI – SSIR Hybrid circuits Small size
Argos Series n n n SSI – SSIR Hybrid circuits Small size
 External n Pacemakers n Custom Holters n Signal acquisition n Specific stimulators
External n Pacemakers n Custom Holters n Signal acquisition n Specific stimulators
 Programmer n One programmer for all series Easy to use n Log n ECG display n Remote monitoring n Custom data base developments n
Programmer n One programmer for all series Easy to use n Log n ECG display n Remote monitoring n Custom data base developments n
 New developments n Teros DDD, DDDR n n Teros SSI, SSIR n n 2 nd quarter 2004 First implant, October 2003 Implantable Defibrillator: starting 4 th quarter 2003
New developments n Teros DDD, DDDR n n Teros SSI, SSIR n n 2 nd quarter 2004 First implant, October 2003 Implantable Defibrillator: starting 4 th quarter 2003
 Quality Assurance n n n ISO 9000: 2000 ISO 13485 Inspections and reviews n n n SQA n n Design reviews Quality Systems Audited by the Client Audited by DHC Associates British Standards Institute n Successfully audited in March 2002, March 2003
Quality Assurance n n n ISO 9000: 2000 ISO 13485 Inspections and reviews n n n SQA n n Design reviews Quality Systems Audited by the Client Audited by DHC Associates British Standards Institute n Successfully audited in March 2002, March 2003
 Strategic Partners and Clients n Impulse Dynamics – USA, Israel n CNM, Europe (i 2 m) LMT – Russia n Aeromedical, Argentina n
Strategic Partners and Clients n Impulse Dynamics – USA, Israel n CNM, Europe (i 2 m) LMT – Russia n Aeromedical, Argentina n
 Thank you. . .
Thank you. . .


