71fa9e6f08f47d6d13deb4a4941472ca.ppt
- Количество слайдов: 48

Centocor Presentation REMICADE® (infliximab) 1

Agenda of Speakers REMICADE® Jerome A. Boscia, MD Safety Review Vice President, Clinical Research & Development Centocor Risk Management Thomas F. Schaible, Ph. D and Efficacy Vice President, Medical Affairs Centocor 2

Consultants Roger Cohen, MD Paul Stang, Ph. D Fox Chase Cancer Center Galt Associates Fox Chase, Pennsylvania Sterling, Virginia Susan Fisher, Ph. D University of Rochester, New York E. William St. Clair, MD Duke University Medical Center Durham, North Carolina Stephen Hanauer, MD Frederick Wolfe, MD University of Chicago Medical Center Arthritis Research Center Foundation Chicago, Illinois Wichita, Kansas Milton Packer, MD Columbia University College of Physicians and Surgeons New York, New York 3
Burden of Disease • Rheumatoid Arthritis (RA) – 90% of patients with aggressive disease become significantly disabled within 20 years – Reduced life expectancy compared with the general population • Crohn’s Disease (CD) – Debilitating disease affecting young adults – Detrimental impact on employment and productivity in 50% of patients – 90% of patients require surgical intervention 4
REMICADE Overview • REMICADE is indicated for patients with RA and CD who have had an inadequate response to conventional therapies • Fulfills previously unmet medical need • Profound benefit in a majority of patients • Safety profile continues to be characterized – Arthritis Advisory Committee (AAC) Safety Update August 2001 – New data from clinical trials, registries, spontaneous adverse event reports 5
REMICADE® Experience • 15 completed clinical trials with ~1700 REMICADE treated patients and 3445 pt-yrs of follow-up • 14 ongoing clinical trials with ~3100 REMICADE treated patients • Approximately 365, 000 patients treated commercially with REMICADE with more than 554, 000 pt-yrs since first exposure (~64% in the U. S. ) – 198, 000 RA patients – 157, 000 CD patients 6
Background Increased Risk of Lymphoma • Comparisons typically made with the SEER database which represents the general population • Lymphomas are more common in the overall RA population than in the general population (SIR = 2 -3) – Elevated relative risk associated with: • High inflammatory activity (26 -fold) • Functional Class III/IV (5 -fold) • Small and large joint involvement (9 -fold) • Use of conventional immunosuppressants (e. g. azathioprine) increases risk • Possible increased risk of lymphoma in CD 7
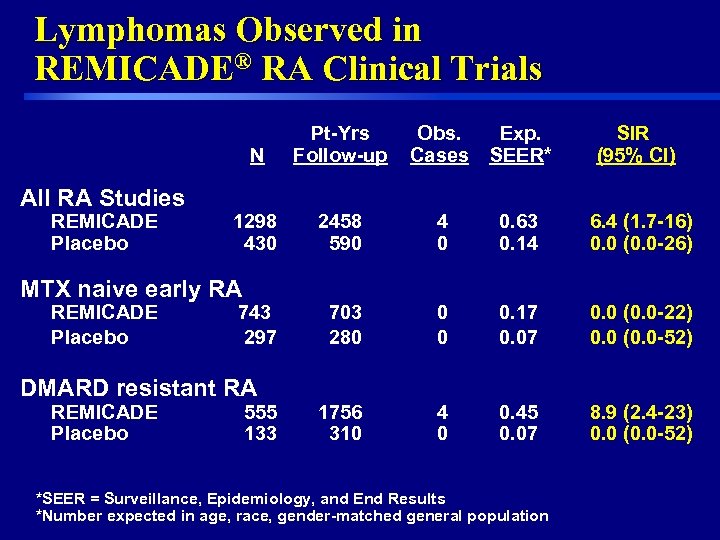
Lymphomas Observed in REMICADE® RA Clinical Trials N All RA Studies REMICADE Placebo Pt-Yrs Follow-up Obs. Cases Exp. SEER* SIR (95% CI) 1298 430 2458 590 4 0 0. 63 0. 14 6. 4 (1. 7 -16) 0. 0 (0. 0 -26) 703 280 0 0 0. 17 0. 0 (0. 0 -22) 0. 0 (0. 0 -52) 1756 310 4 0 0. 45 0. 07 8. 9 (2. 4 -23) 0. 0 (0. 0 -52) MTX naive early RA REMICADE Placebo 743 297 DMARD resistant RA REMICADE Placebo 8 555 133 *SEER = Surveillance, Epidemiology, and End Results *Number expected in age, race, gender-matched general population
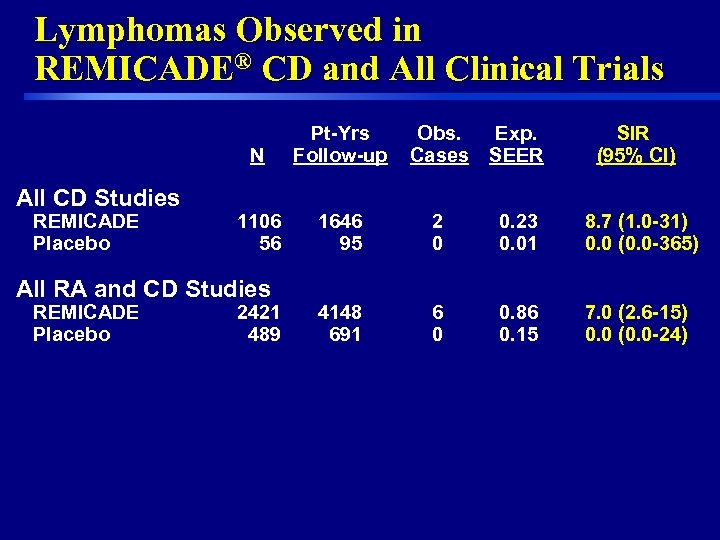
Lymphomas Observed in REMICADE® CD and All Clinical Trials N All CD Studies REMICADE Placebo Pt-Yrs Follow-up Obs. Cases Exp. SEER SIR (95% CI) 1106 56 1646 95 2 0 0. 23 0. 01 8. 7 (1. 0 -31) 0. 0 (0. 0 -365) 4148 691 6 0 0. 86 0. 15 7. 0 (2. 6 -15) 0. 0 (0. 0 -24) All RA and CD Studies REMICADE Placebo 9 2421 489
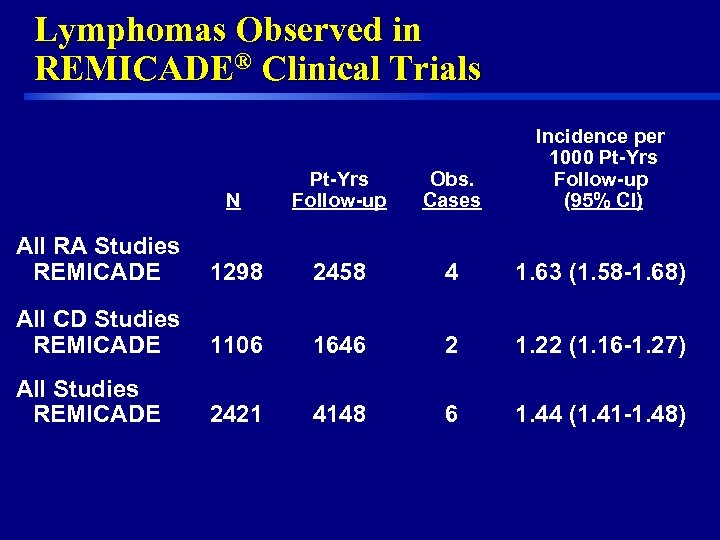
Lymphomas Observed in REMICADE® Clinical Trials N Pt-Yrs Follow-up Obs. Cases Incidence per 1000 Pt-Yrs Follow-up (95% CI) All RA Studies REMICADE 1298 2458 4 1. 63 (1. 58 -1. 68) All CD Studies REMICADE 1106 1646 2 1. 22 (1. 16 -1. 27) All Studies REMICADE 2421 4148 6 1. 44 (1. 41 -1. 48) 10
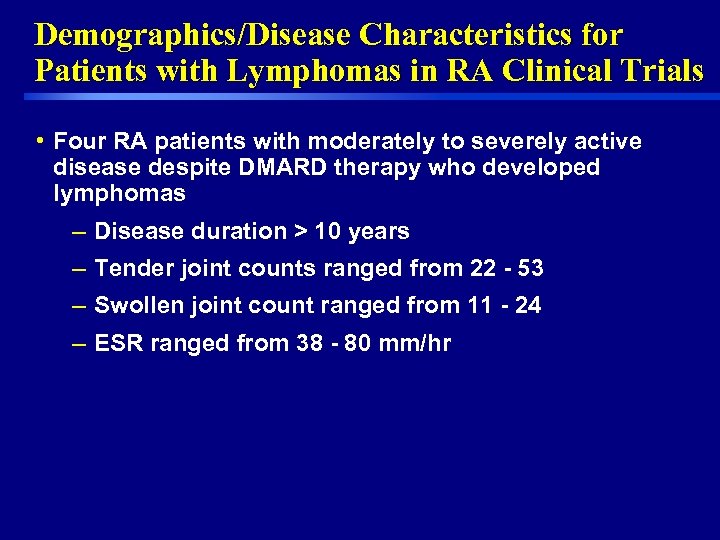
Demographics/Disease Characteristics for Patients with Lymphomas in RA Clinical Trials • Four RA patients with moderately to severely active disease despite DMARD therapy who developed lymphomas – Disease duration > 10 years – Tender joint counts ranged from 22 - 53 – Swollen joint count ranged from 11 - 24 – ESR ranged from 38 - 80 mm/hr 11
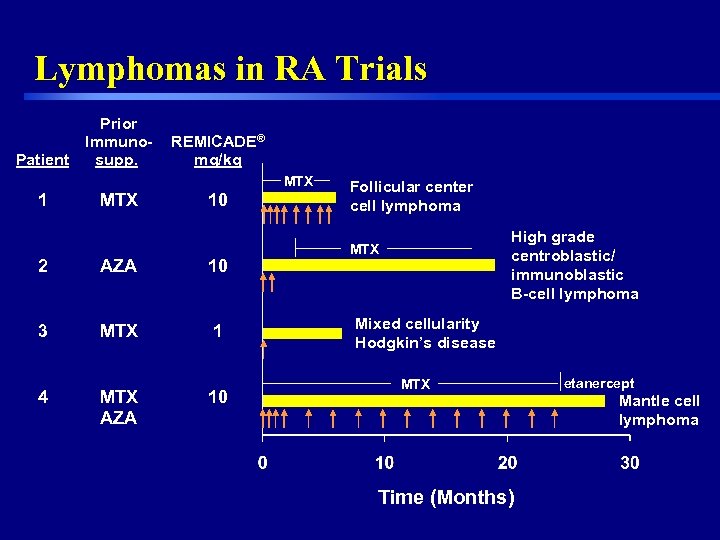
Lymphomas in RA Trials Patient Prior Immunosupp. REMICADE® mg/kg MTX 10 2 AZA 10 3 MTX 1 4 MTX AZA 10 Follicular center cell lymphoma High grade centroblastic/ immunoblastic B-cell lymphoma MTX Mixed cellularity Hodgkin’s disease MTX Time (Months) 12 etanercept Mantle cell lymphoma
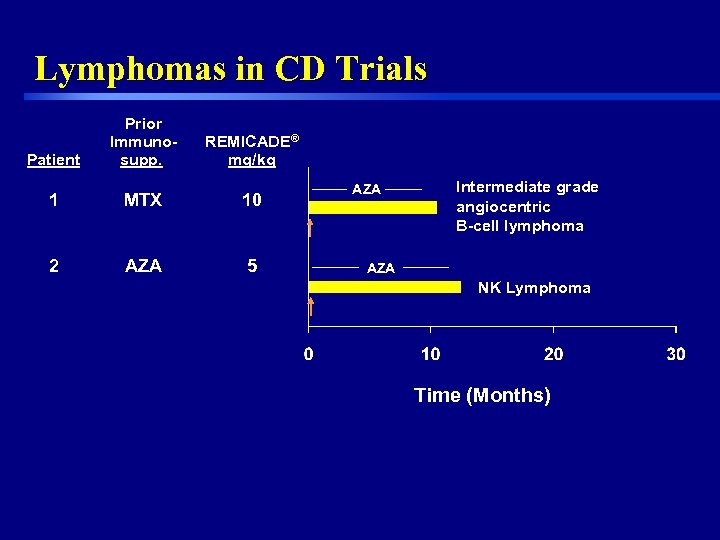
Lymphomas in CD Trials Patient Prior Immunosupp. REMICADE® mg/kg 1 MTX 10 2 AZA 5 AZA Intermediate grade angiocentric B-cell lymphoma AZA NK Lymphoma Time (Months) 13

National Data Bank for Rheumatic Diseases (NDRD) • Long term study of outcomes in 18, 557 patients with RA (1998 -2002) – RA patients recruited from the practices of 908 U. S. rheumatologists – Biannual assessment – Validation process includes MD, hospital and death records to maximize accuracy and reliability – ~8% attrition annually 14
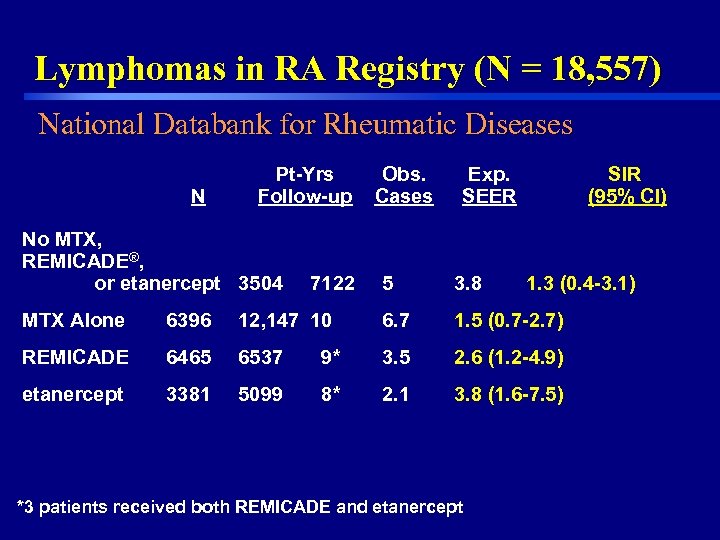
Lymphomas in RA Registry (N = 18, 557) National Databank for Rheumatic Diseases N Pt-Yrs Follow-up No MTX, REMICADE®, or etanercept 3504 7122 Obs. Cases Exp. SEER 5 3. 8 SIR (95% CI) 1. 3 (0. 4 -3. 1) MTX Alone 6396 12, 147 10 6. 7 1. 5 (0. 7 -2. 7) REMICADE 6465 6537 9* 3. 5 2. 6 (1. 2 -4. 9) etanercept 3381 5099 8* 2. 1 3. 8 (1. 6 -7. 5) *3 patients received both REMICADE and etanercept 15
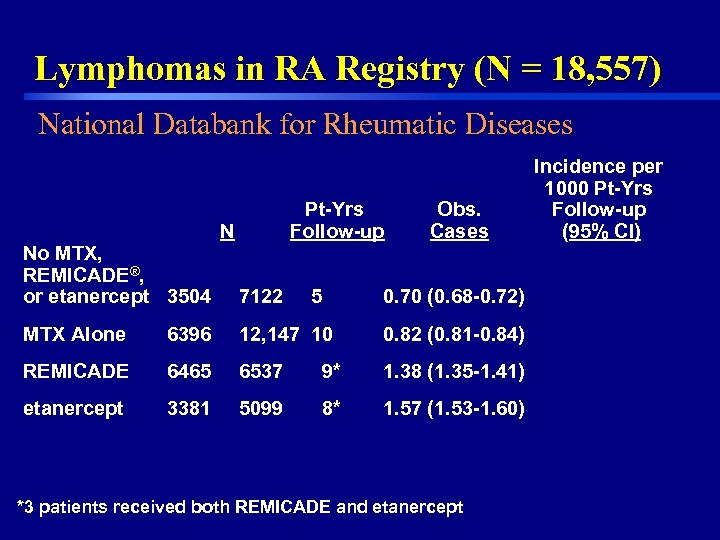
Lymphomas in RA Registry (N = 18, 557) National Databank for Rheumatic Diseases No MTX, REMICADE®, or etanercept 3504 Pt-Yrs Follow-up N 7122 5 Obs. Cases 0. 70 (0. 68 -0. 72) MTX Alone 6396 12, 147 10 0. 82 (0. 81 -0. 84) REMICADE 6465 6537 9* 1. 38 (1. 35 -1. 41) etanercept 3381 5099 8* 1. 57 (1. 53 -1. 60) *3 patients received both REMICADE and etanercept 16 Incidence per 1000 Pt-Yrs Follow-up (95% CI)

The Crohn’s Therapy, Resource, Evaluation and Assessment Tool (TREAT) Registry • CD patients ( 18 years) eligible (need to participate for a minimum of 5 years) • At baseline and every subsequent 6 months: – Patients complete a health status questionnaire – Data collected: • • 17 Demographics and disease characteristics Medications Adverse events Health resource utilization

Lymphomas in Crohn’s Disease Registry TREAT Registry Treatment Patients REMICADE® Lymphoma 1108 1 Not exposed 1291 1 to REMICADE 18

Lymphoma Cases in Spontaneous Adverse Event Reporting • 71 cases of lymphoma have been reported since launch – 45 reports in RA – 20 reports in CD – 6 reports in other diseases 19
Lymphoma Risk with REMICADE® • Lymphomas are more common in the overall RA population than in the general population (SIR = 2 -3) • In RA clinical trials, a SIR of 6. 4 for lymphoma was observed in REMICADE treated patients compared with the general population – All cases occurred in high risk patients • In NDRD a SIR of 2. 6 for lymphoma was observed in REMICADE treated patients compared with the general population • Current evidence is insufficient to reach conclusions on whether REMICADE increases the risk of lymphomas 20

Non-Lymphoma Malignancies in RA and Crohn’s Disease Background • Large RA cohorts have not reported increased risk of non-lymphoma cancers • Long-standing CD predisposes to cancer of both the small and large intestine • Risk of colon cancer in Crohn’s colitis may be comparable to risk in ulcerative colitis 21
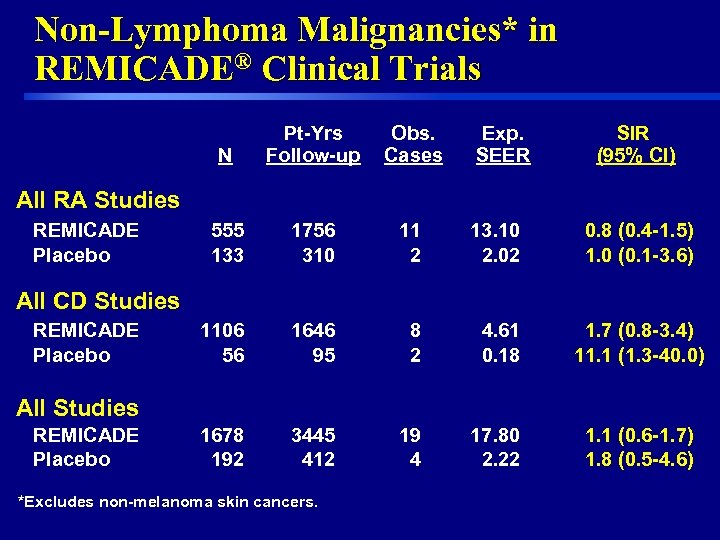
Non-Lymphoma Malignancies* in REMICADE® Clinical Trials N Pt-Yrs Follow-up Obs. Cases Exp. SEER SIR (95% CI) 555 133 1756 310 11 2 13. 10 2. 02 0. 8 (0. 4 -1. 5) 1. 0 (0. 1 -3. 6) 1106 56 1646 95 8 2 4. 61 0. 18 1. 7 (0. 8 -3. 4) 11. 1 (1. 3 -40. 0) 1678 192 3445 412 19 4 17. 80 2. 22 1. 1 (0. 6 -1. 7) 1. 8 (0. 5 -4. 6) All RA Studies REMICADE Placebo All CD Studies REMICADE Placebo All Studies REMICADE Placebo *Excludes non-melanoma skin cancers. 22

Non-Lymphoma Malignancies in Spontaneous Adverse Event Reporting • 354 cases of non-lymphoma malignancies have been reported since launch – 230 reports in RA – 68 reports in CD – 15 reports in other diseases – 41 indication not reported 23

Tuberculosis (TB) Update • Reviewed in detail at August 2001 AAC meeting – Box warning added to prescribing information – Dear Healthcare Professional letter sent • Implemented education program on TB risk and screening for latent TB – Education provided to 7500 rheumatologists and gastroenterologists – Follow-up indicates most physicians perform pre-REMICADE TB screening • Decreased number of spontaneous reports of TB despite increased patient exposure 24
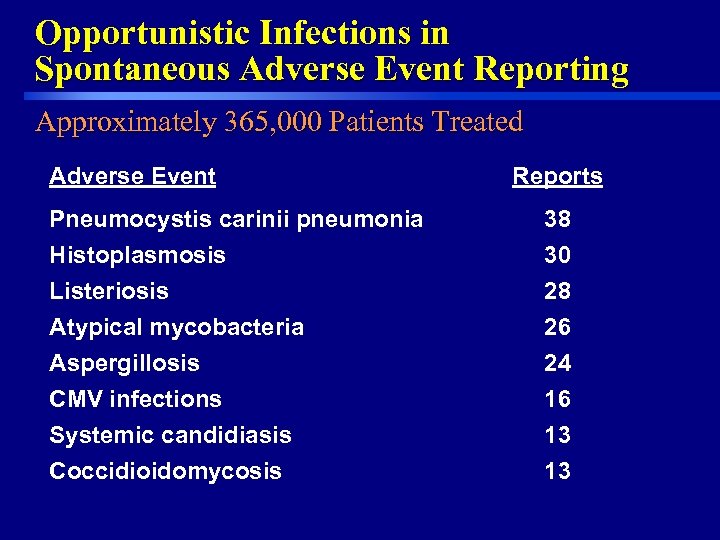
Opportunistic Infections in Spontaneous Adverse Event Reporting Approximately 365, 000 Patients Treated Adverse Event Reports Pneumocystis carinii pneumonia Histoplasmosis Listeriosis Atypical mycobacteria Aspergillosis CMV infections Systemic candidiasis 13 Coccidioidomycosis 25 38 30 28 26 24 16 13

Opportunistic Infections – Risk Management • Histoplasmosis and coccidioidomycosis cases occurred primarily in endemic areas • For patients who have resided in histoplasmosis or coccidioidomycosis endemic areas – Careful Benefit: Risk assessment prior to REMICADE® treatment • Careful monitoring of patients during and after REMICADE therapy – Route of administration allows for regular follow-up 26
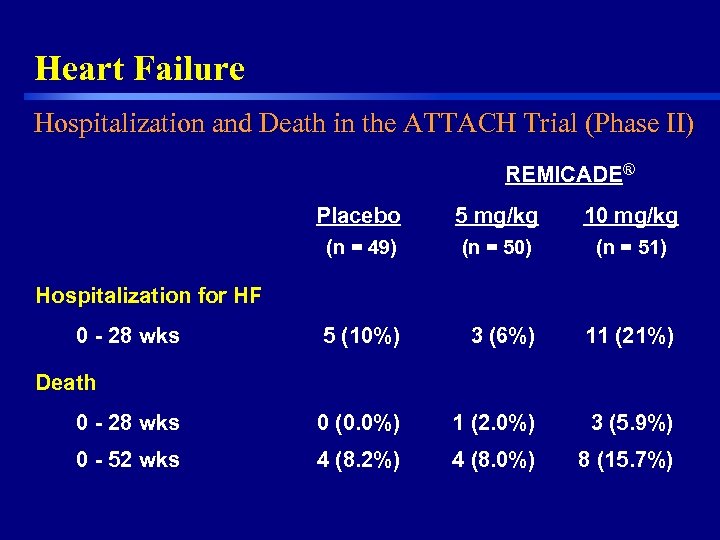
Heart Failure Hospitalization and Death in the ATTACH Trial (Phase II) REMICADE® Placebo 5 mg/kg 10 mg/kg (n = 49) (n = 50) (n = 51) 5 (10%) 3 (6%) 11 (21%) 0 - 28 wks 0 (0. 0%) 1 (2. 0%) 3 (5. 9%) 0 - 52 wks 4 (8. 2%) 4 (8. 0%) 8 (15. 7%) Hospitalization for HF 0 - 28 wks Death 27

REMICADE® Prescribing Information Heart Failure • Contraindications – Patients with Class III/IV heart failure • Warnings – Use with caution in patients with Class I/II heart failure – Dose should not exceed 5 mg/kg – Closely monitor patients and discontinue REMICADE if new or worsening symptoms of heart failure appear 28
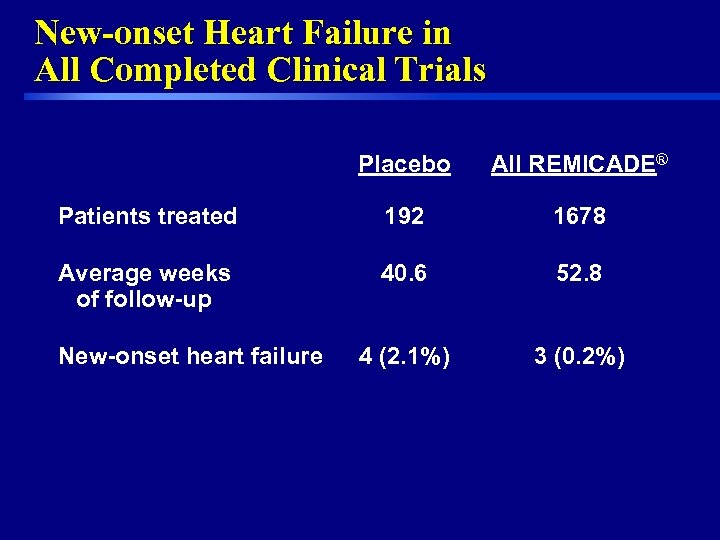
New-onset Heart Failure in All Completed Clinical Trials Placebo All REMICADE® Patients treated 192 1678 Average weeks of follow-up 40. 6 52. 8 4 (2. 1%) 3 (0. 2%) New-onset heart failure 29

New-onset Heart Failure in Spontaneous Adverse Event Reporting • 158 spontaneous adverse event reports of heart failure – 28 patients with no known history of heart failure, acute precipitating event, or risk factor – Confounded by incomplete information and lack of a control group 30

Agenda of Speakers REMICADE® Jerome A. Boscia, MD Safety Review Vice President, Clinical Research & Development Centocor Risk Management Thomas F. Schaible, Ph. D and Efficacy Vice President, Medical Affairs Centocor 31

Continuing Safety Commitment • Centocor is committed to obtaining long-term prospective safety information – Progress since August 2001 AAC • Ongoing safety assessment programs • New programs – Expansion of safety databases – Specific follow-up on lymphoma cases • Programs collect data in patients receiving and not receiving REMICADE – Important to differentiate safety signals 32
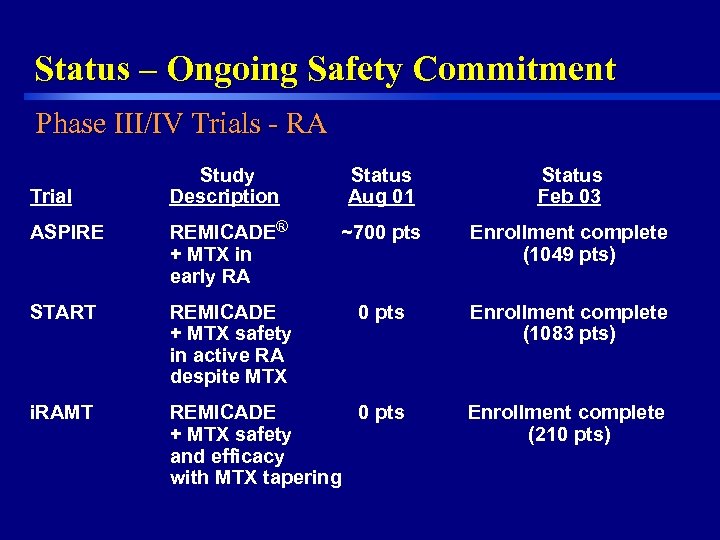
Status – Ongoing Safety Commitment Phase III/IV Trials - RA Study Description Status Aug 01 Status Feb 03 ASPIRE REMICADE® + MTX in early RA ~700 pts Enrollment complete (1049 pts) START REMICADE + MTX safety in active RA despite MTX 0 pts Enrollment complete (1083 pts) i. RAMT REMICADE 0 pts + MTX safety and efficacy with MTX tapering Enrollment complete (210 pts) Trial 33
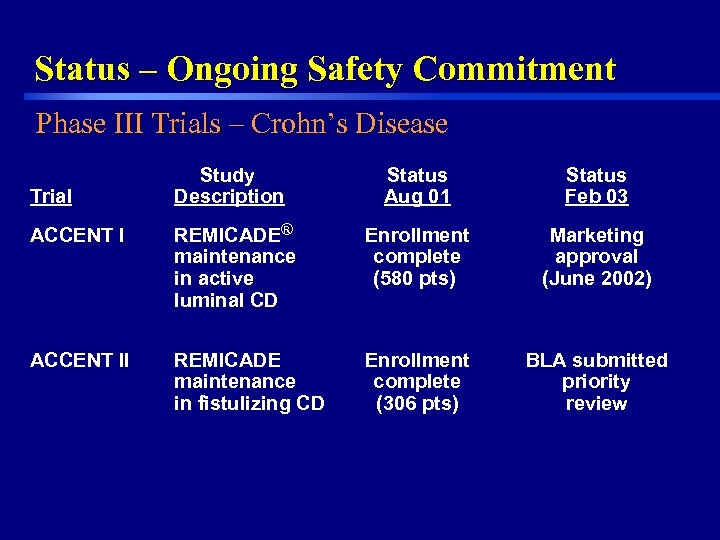
Status – Ongoing Safety Commitment Phase III Trials – Crohn’s Disease Trial Study Description Status Aug 01 Status Feb 03 ACCENT I Enrollment complete (580 pts) Marketing approval (June 2002) ACCENT II 34 REMICADE® maintenance in active luminal CD REMICADE maintenance in fistulizing CD Enrollment complete (306 pts) BLA submitted priority review
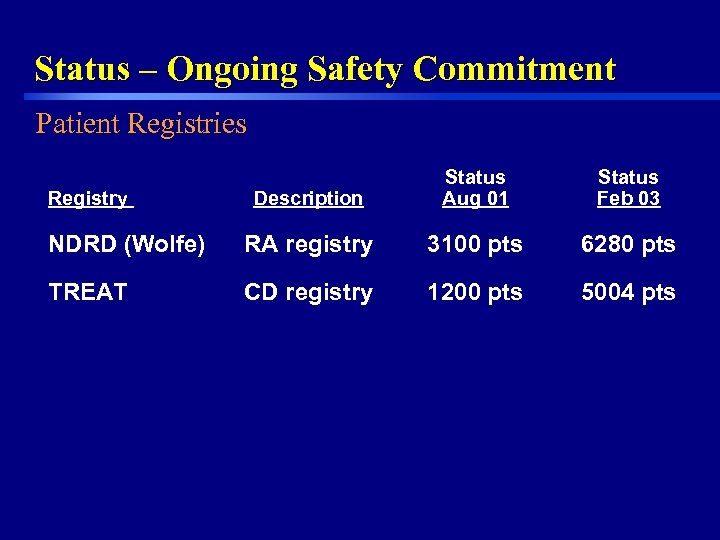
Status – Ongoing Safety Commitment Patient Registries Description Status Aug 01 Status Feb 03 NDRD (Wolfe) RA registry 3100 pts 6280 pts TREAT CD registry 1200 pts 5004 pts Registry 35
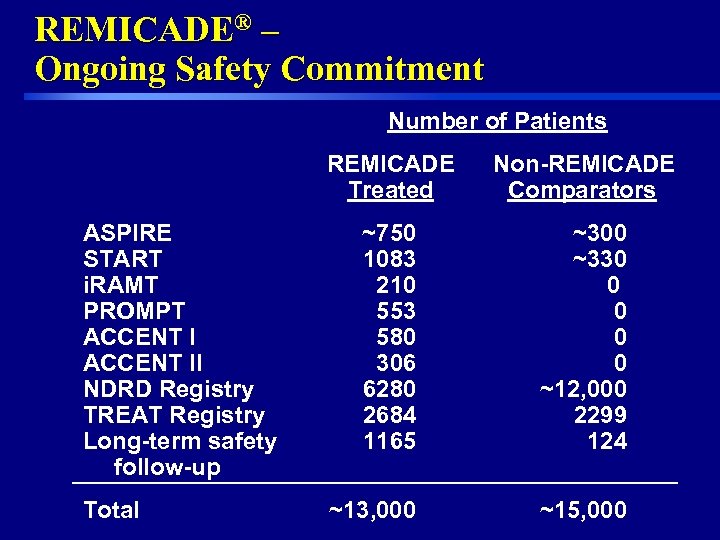
REMICADE® – Ongoing Safety Commitment Number of Patients REMICADE Treated ASPIRE START i. RAMT PROMPT ACCENT II NDRD Registry TREAT Registry Long-term safety follow-up 36 Total Non-REMICADE Comparators ~750 1083 210 553 580 306 6280 2684 1165 ~300 ~330 0 0 ~12, 000 2299 124 ~13, 000 ~15, 000

Safety Commitment – New Programs Patient Registries • APART registry – 2500 -patient RA registry in U. S. • European RA registries – Registries in Spain, Germany, Sweden and UK • European CD registry – 4000 -patient registry • All registries enroll REMICADE and non-REMICADE treated patients 37

Safety Commitment – New Programs Additional Lymphoma Follow-up • Registries provide sources to obtain additional details on reported lymphomas – Compare lymphoma profiles with REMICADE +/- immunosuppressants (MTX, AZA) – More fully characterize lymphomas • Initiate surveillance in multiple healthcare delivery systems – Further quantify lymphoma risk and contributing factors 38

Risk Management – Physicians Using REMICADE® • REMICADE is used primarily by and continues to be promoted to sub-specialists – Best able to make Benefit: Risk decisions • This sub-specialist population is readily targeted by risk management initiatives – e. g. REMICADE TB education program 39

Safety Commitment Conclusions • Conduct risk management programs as specific safety issues arise • Expand prospective safety databases – Phase III/IV clinical studies, international patient registries, long-term safety follow-up – Follow-up in REMICADE® and non-REMICADE treated patients (approaching 30, 000) 40

Efficacy FDA Ad. Comm 030403
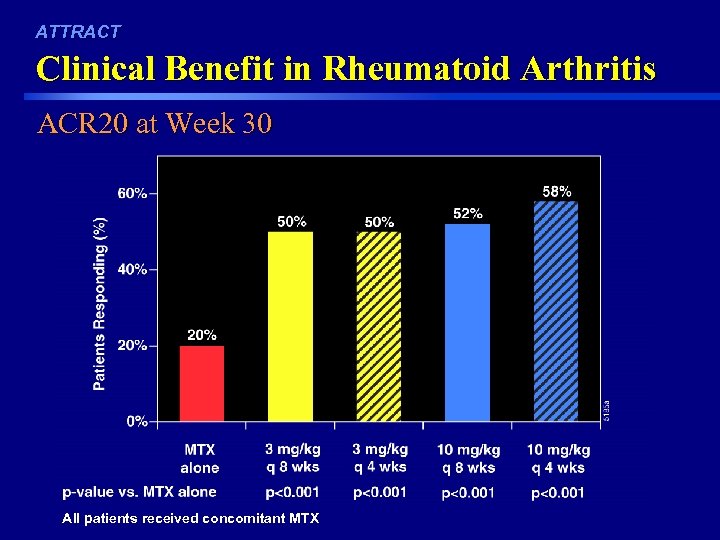
ATTRACT Clinical Benefit in Rheumatoid Arthritis ACR 20 at Week 30 42 All patients received concomitant MTX
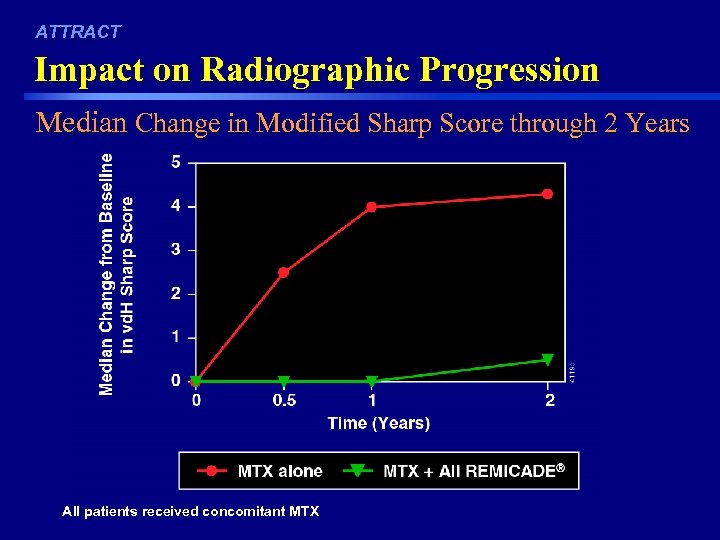
ATTRACT Impact on Radiographic Progression Median Change in Modified Sharp Score through 2 Years 43 All patients received concomitant MTX
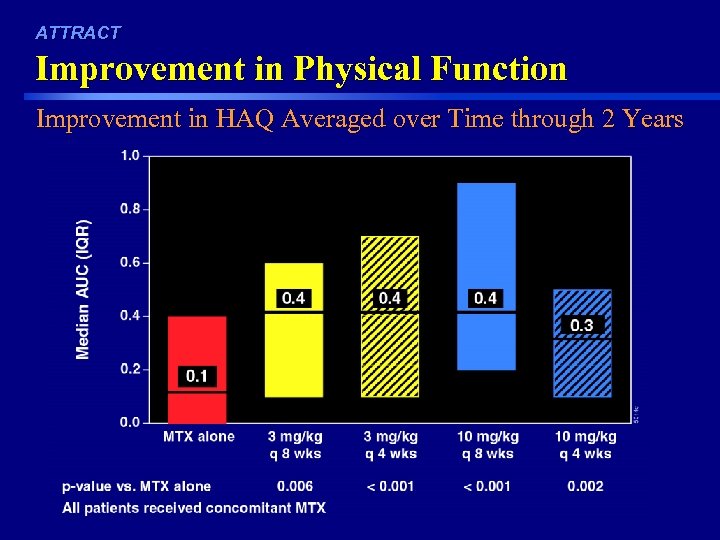
ATTRACT Improvement in Physical Function Improvement in HAQ Averaged over Time through 2 Years 44
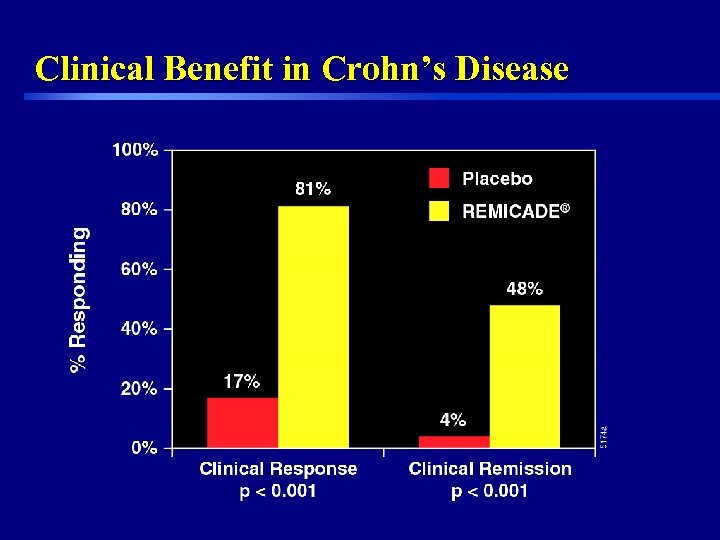
Clinical Benefit in Crohn’s Disease 45
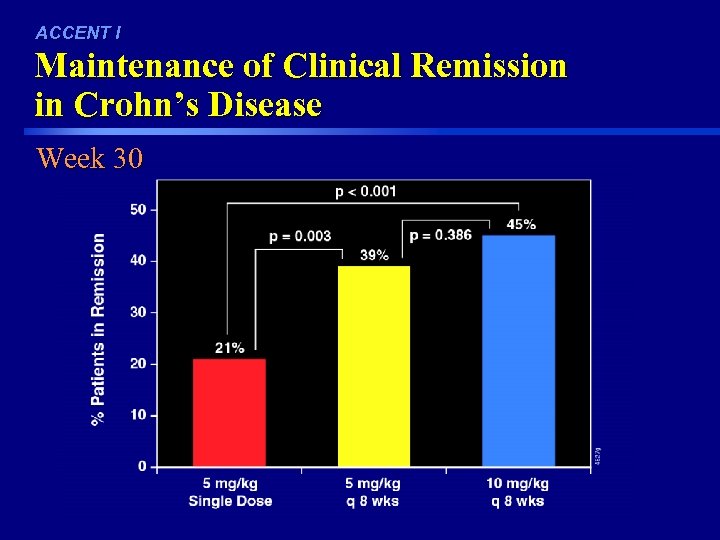
ACCENT I Maintenance of Clinical Remission in Crohn’s Disease Week 30 46 FDA Ad. Comm 030403
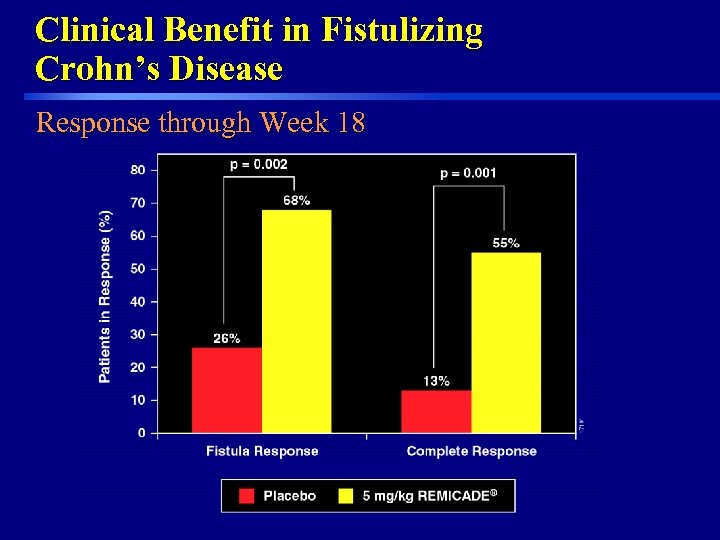
Clinical Benefit in Fistulizing Crohn’s Disease Response through Week 18 47

Benefit: Risk of REMICADE® Therapy • REMICADE is highly effective in RA and CD patients who have failed conventional therapies • Treatment related serious adverse events are infrequent • Centocor remains committed to continuing safety assessment and risk management programs as needed • Benefit: Risk for REMICADE in both RA and CD continues to be excellent 48
71fa9e6f08f47d6d13deb4a4941472ca.ppt