87eade782ea975a5def2e8cc9a12c87e.ppt
- Количество слайдов: 21
 Center for Devices and Radiological Health Mass. Medic May 2006 Larry Kessler, Sc. D. Director, Office of Science and Engineering Laboratories, CDRH
Center for Devices and Radiological Health Mass. Medic May 2006 Larry Kessler, Sc. D. Director, Office of Science and Engineering Laboratories, CDRH
 The State of the World: CDRH • How are we doing premarket? • How are we doing on compliance efforts? • How are we doing on surveillance? • How are we doing on communication? • What are new scientific challenges and how do they connect to FDA Critical Path Initiative?
The State of the World: CDRH • How are we doing premarket? • How are we doing on compliance efforts? • How are we doing on surveillance? • How are we doing on communication? • What are new scientific challenges and how do they connect to FDA Critical Path Initiative?
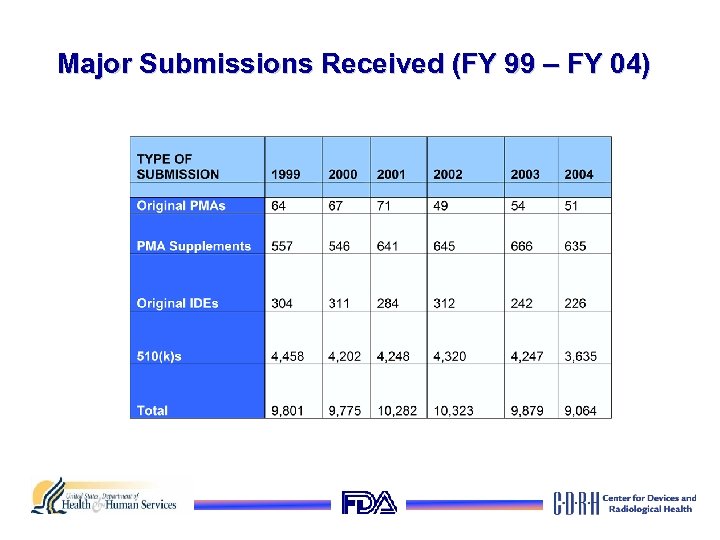 Major Submissions Received (FY 99 – FY 04)
Major Submissions Received (FY 99 – FY 04)
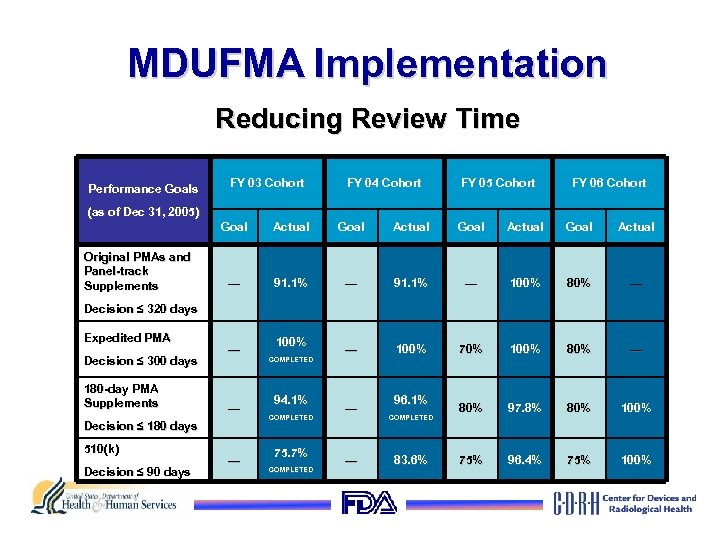 MDUFMA Implementation Reducing Review Time Performance Goals FY 03 Cohort FY 04 Cohort FY 05 Cohort FY 06 Cohort (as of Dec 31, 2005) Goal Original PMAs and Panel-track Supplements Actual Goal Actual — 91. 1% — 100% 80% — — 100% 70% 100% 80% — 80% 97. 8% 80% 100% 75% 96. 4% 75% 100% Decision ≤ 320 days Expedited PMA Decision ≤ 300 days 180 -day PMA Supplements — — Decision ≤ 180 days 510(k) Decision ≤ 90 days — 100% COMPLETED 94. 1% COMPLETED 75. 7% COMPLETED — — 96. 1% COMPLETED 83. 6%
MDUFMA Implementation Reducing Review Time Performance Goals FY 03 Cohort FY 04 Cohort FY 05 Cohort FY 06 Cohort (as of Dec 31, 2005) Goal Original PMAs and Panel-track Supplements Actual Goal Actual — 91. 1% — 100% 80% — — 100% 70% 100% 80% — 80% 97. 8% 80% 100% 75% 96. 4% 75% 100% Decision ≤ 320 days Expedited PMA Decision ≤ 300 days 180 -day PMA Supplements — — Decision ≤ 180 days 510(k) Decision ≤ 90 days — 100% COMPLETED 94. 1% COMPLETED 75. 7% COMPLETED — — 96. 1% COMPLETED 83. 6%
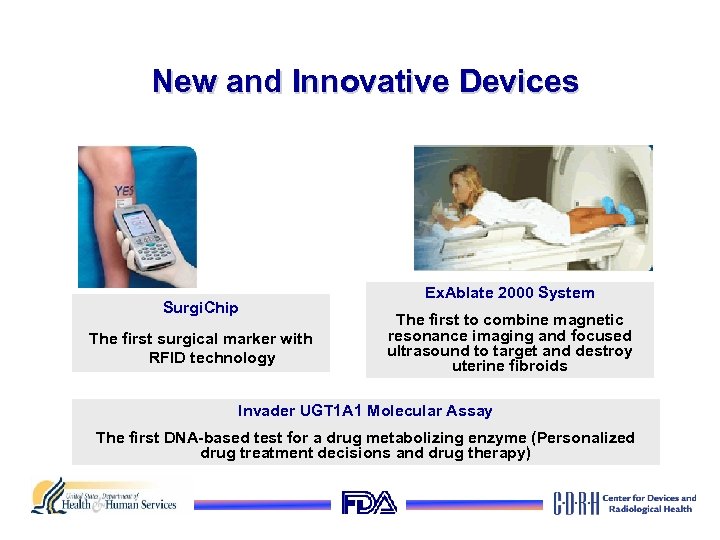 New and Innovative Devices Surgi. Chip The first surgical marker with RFID technology Ex. Ablate 2000 System The first to combine magnetic resonance imaging and focused ultrasound to target and destroy uterine fibroids Invader UGT 1 A 1 Molecular Assay The first DNA-based test for a drug metabolizing enzyme (Personalized drug treatment decisions and drug therapy)
New and Innovative Devices Surgi. Chip The first surgical marker with RFID technology Ex. Ablate 2000 System The first to combine magnetic resonance imaging and focused ultrasound to target and destroy uterine fibroids Invader UGT 1 A 1 Molecular Assay The first DNA-based test for a drug metabolizing enzyme (Personalized drug treatment decisions and drug therapy)
 Premarket Review Quality Assessment Pilot Program Ensuring Quality in Premarket Review • The goal is to assess quality of review of premarket submissions using retrospective (post-decision) peer assessments • Pilot focuses on 3 areas of review: – Biocompatibility – Sterilization/Packaging – Statistics
Premarket Review Quality Assessment Pilot Program Ensuring Quality in Premarket Review • The goal is to assess quality of review of premarket submissions using retrospective (post-decision) peer assessments • Pilot focuses on 3 areas of review: – Biocompatibility – Sterilization/Packaging – Statistics
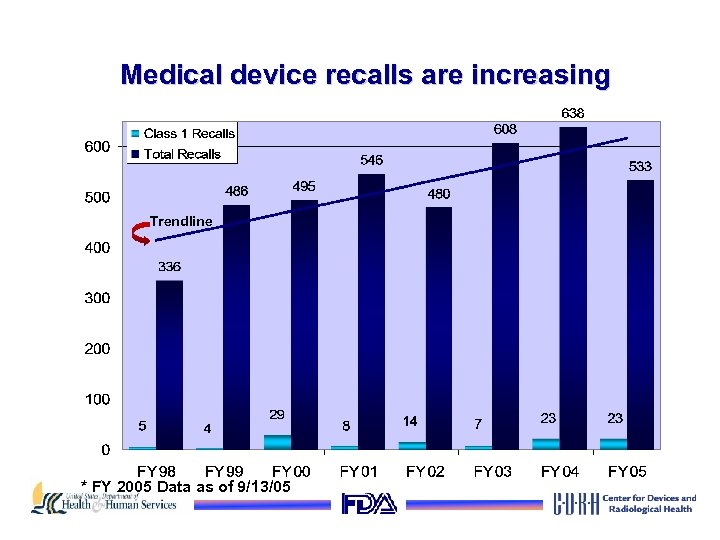 Medical device recalls are increasing Trendline * FY 2005 Data as of 9/13/05
Medical device recalls are increasing Trendline * FY 2005 Data as of 9/13/05
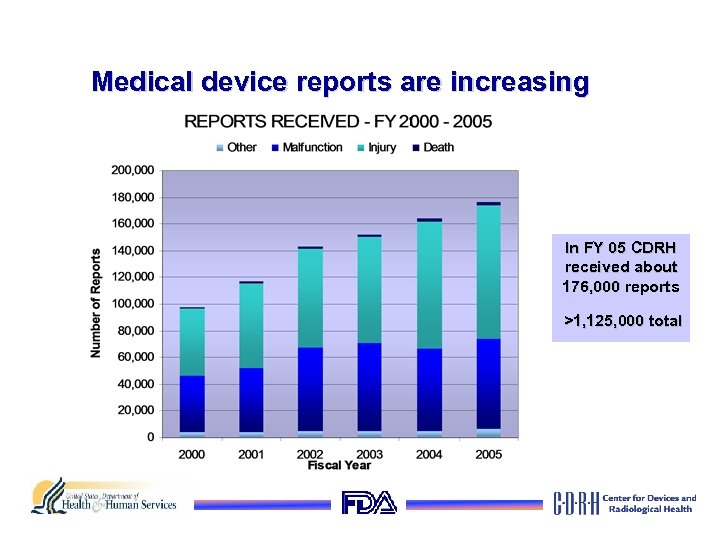 Medical device reports are increasing In FY 05 CDRH received about 176, 000 reports >1, 125, 000 total
Medical device reports are increasing In FY 05 CDRH received about 176, 000 reports >1, 125, 000 total
 We are working on critical postmarket issues • Strengthening Condition of Approval studies • Improving targeted surveillance systems: Med. Sun • Focusing on risk-based inspections • Implementing third party inspections • Better communicating risk/benefit information • Improving our automated information systems
We are working on critical postmarket issues • Strengthening Condition of Approval studies • Improving targeted surveillance systems: Med. Sun • Focusing on risk-based inspections • Implementing third party inspections • Better communicating risk/benefit information • Improving our automated information systems
 Working together to improve postmarket safety… • FDA collaborated in a postmarket workshop with Adva. Med • FDA, the Heart Rhythm Society and industry reps are exploring opportunities to improve Product Performance reports, PMA Annual reports and standardize our Dear Doctor letter format • FDA’s Defibrillation Working group focus on postmarket • Additional areas of active collaboration include recalls and unique device identifiers
Working together to improve postmarket safety… • FDA collaborated in a postmarket workshop with Adva. Med • FDA, the Heart Rhythm Society and industry reps are exploring opportunities to improve Product Performance reports, PMA Annual reports and standardize our Dear Doctor letter format • FDA’s Defibrillation Working group focus on postmarket • Additional areas of active collaboration include recalls and unique device identifiers

 Our Communication Strategy We're improving our automated information systems. . . • Easy-to-use databases of regulated products • Routinely-asked questions and answers • Developing methods of collecting and disseminating (“pushing out”) CDRH news • Improving CDRH web pages: – Medical Device Recalls - a web-friendly, plain language overview of medical device recalls http: //www. accessdata. fda. gov/scripts/cdrh/cfdocs /cf. Topic/medicaldevicesafety/recalls. cfm
Our Communication Strategy We're improving our automated information systems. . . • Easy-to-use databases of regulated products • Routinely-asked questions and answers • Developing methods of collecting and disseminating (“pushing out”) CDRH news • Improving CDRH web pages: – Medical Device Recalls - a web-friendly, plain language overview of medical device recalls http: //www. accessdata. fda. gov/scripts/cdrh/cfdocs /cf. Topic/medicaldevicesafety/recalls. cfm
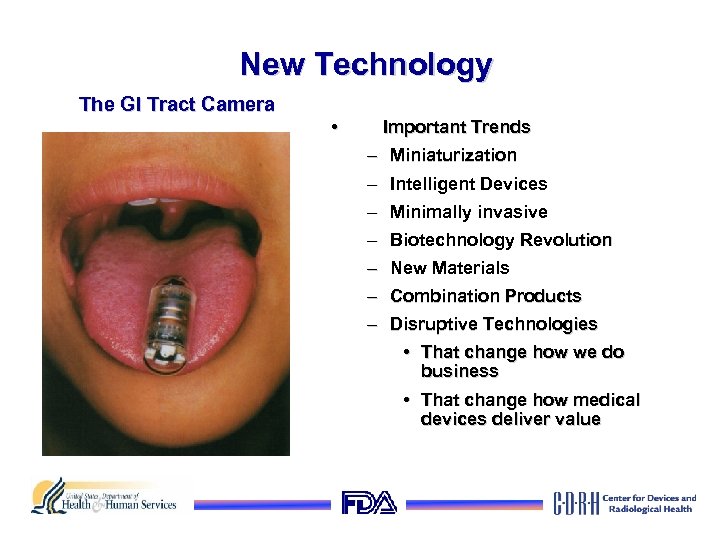 New Technology The GI Tract Camera • Important Trends – Miniaturization – Intelligent Devices – Minimally invasive – Biotechnology Revolution – New Materials – Combination Products – Disruptive Technologies • That change how we do business • That change how medical devices deliver value
New Technology The GI Tract Camera • Important Trends – Miniaturization – Intelligent Devices – Minimally invasive – Biotechnology Revolution – New Materials – Combination Products – Disruptive Technologies • That change how we do business • That change how medical devices deliver value
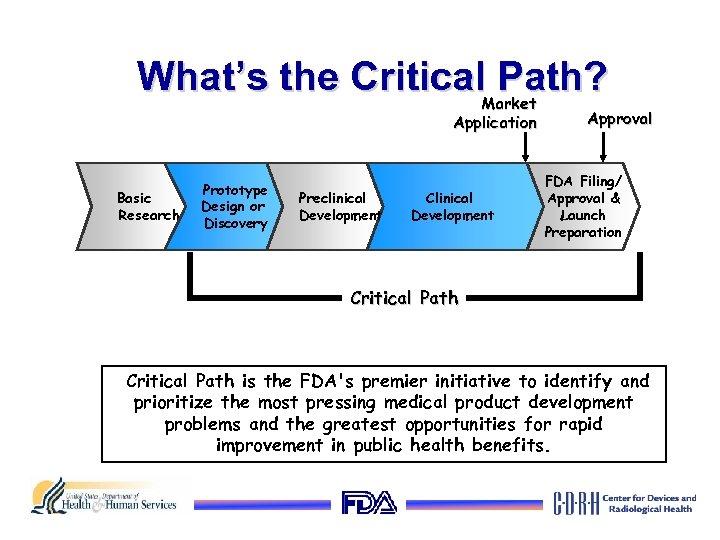 What’s the Critical Path? Market Application Basic Research Prototype Design or Discovery Preclinical Development Clinical Development Approval FDA Filing/ Approval & Launch Preparation Critical Path is the FDA's premier initiative to identify and prioritize the most pressing medical product development problems and the greatest opportunities for rapid improvement in public health benefits.
What’s the Critical Path? Market Application Basic Research Prototype Design or Discovery Preclinical Development Clinical Development Approval FDA Filing/ Approval & Launch Preparation Critical Path is the FDA's premier initiative to identify and prioritize the most pressing medical product development problems and the greatest opportunities for rapid improvement in public health benefits.
 CDRH Research Program (Efficient, Effective, and Predictable Product Development) High Intensity Focused Ultrasound (HIFU) ISSUE The lack of standardized methods to assess the acoustic and thermal characteristics of the focused beams ACCOMPLISHMENTS In a device for the ablation of uterine fibroids, CDRHdeveloped computational modeling to predict the performance of the device under conditions that would have been difficult to investigate experimentally, thus shortening the review time
CDRH Research Program (Efficient, Effective, and Predictable Product Development) High Intensity Focused Ultrasound (HIFU) ISSUE The lack of standardized methods to assess the acoustic and thermal characteristics of the focused beams ACCOMPLISHMENTS In a device for the ablation of uterine fibroids, CDRHdeveloped computational modeling to predict the performance of the device under conditions that would have been difficult to investigate experimentally, thus shortening the review time
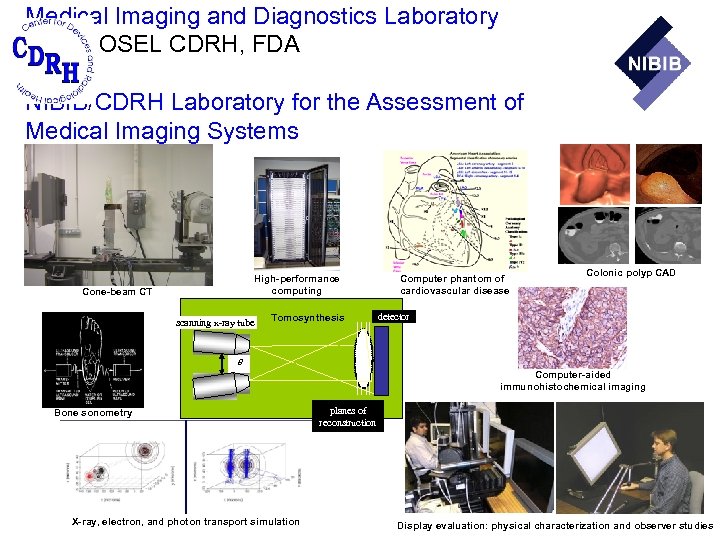 Medical Imaging and Diagnostics Laboratory DIAM, OSEL CDRH, FDA and NIBIB/CDRH Laboratory for the Assessment of Medical Imaging Systems High-performance computing Cone-beam CT scanning x-ray tube Tomosynthesis Computer phantom of cardiovascular disease Colonic polyp CAD detector q Computer-aided immunohistochemical imaging Bone sonometry X-ray, electron, and photon transport simulation planes of reconstruction Display evaluation: physical characterization and observer studies
Medical Imaging and Diagnostics Laboratory DIAM, OSEL CDRH, FDA and NIBIB/CDRH Laboratory for the Assessment of Medical Imaging Systems High-performance computing Cone-beam CT scanning x-ray tube Tomosynthesis Computer phantom of cardiovascular disease Colonic polyp CAD detector q Computer-aided immunohistochemical imaging Bone sonometry X-ray, electron, and photon transport simulation planes of reconstruction Display evaluation: physical characterization and observer studies
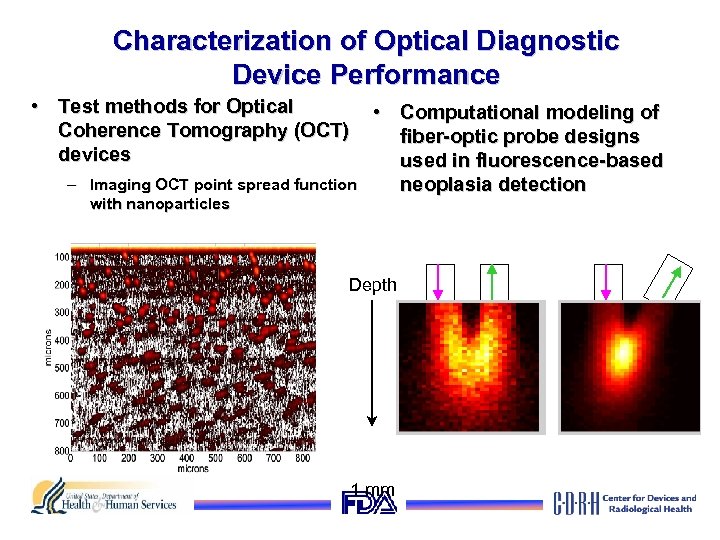 Characterization of Optical Diagnostic Device Performance • Test methods for Optical Coherence Tomography (OCT) devices – Imaging OCT point spread function with nanoparticles • Computational modeling of fiber-optic probe designs used in fluorescence-based neoplasia detection Depth 1 mm
Characterization of Optical Diagnostic Device Performance • Test methods for Optical Coherence Tomography (OCT) devices – Imaging OCT point spread function with nanoparticles • Computational modeling of fiber-optic probe designs used in fluorescence-based neoplasia detection Depth 1 mm
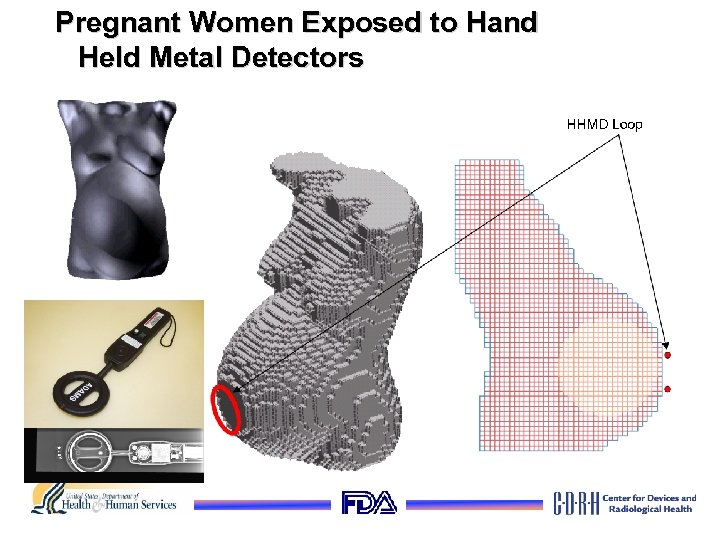 Pregnant Women Exposed to Hand Held Metal Detectors
Pregnant Women Exposed to Hand Held Metal Detectors
 Information Technology Using and Developing Improved IT Systems put into production in 2005 and 2006: • Three CTS (Center Tracking System) “subsystems” – Device Nomenclature Management System (DNMS) – Condition of Approval Tracking System (COATS) – e. Consult • OIVD Turbo 510(k) Systems under development: • e. MDR (electronic submittal receipt of adverse event reports) • PPOMP (Pre-market Program Operations Modernization Project) • OC Tracking – This will also be a “subsystem” within CTS
Information Technology Using and Developing Improved IT Systems put into production in 2005 and 2006: • Three CTS (Center Tracking System) “subsystems” – Device Nomenclature Management System (DNMS) – Condition of Approval Tracking System (COATS) – e. Consult • OIVD Turbo 510(k) Systems under development: • e. MDR (electronic submittal receipt of adverse event reports) • PPOMP (Pre-market Program Operations Modernization Project) • OC Tracking – This will also be a “subsystem” within CTS



