73eedaa514745544fd953285d228a0f0.ppt
- Количество слайдов: 17
 CE marking and European IVD Directive
CE marking and European IVD Directive
 Current regulatory system – Joint Action Plan – Signal Detection Proposals for change – Classification – Clinical evidence – In house manufacturing – Companion diagnostics 2
Current regulatory system – Joint Action Plan – Signal Detection Proposals for change – Classification – Clinical evidence – In house manufacturing – Companion diagnostics 2
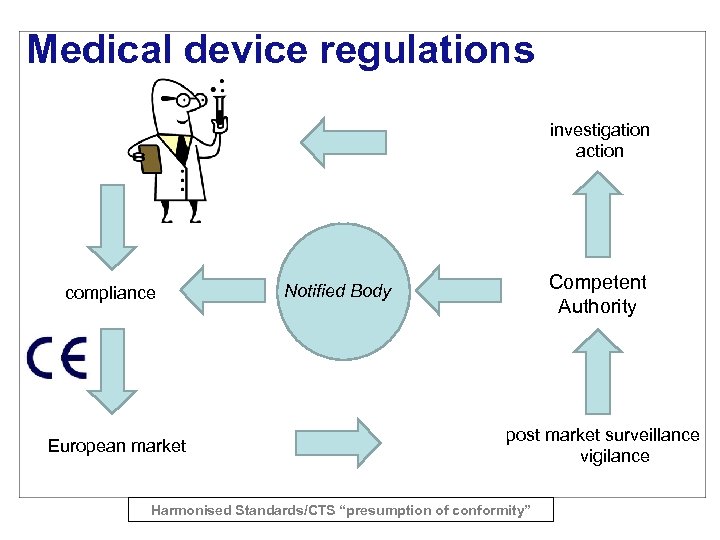 Medical device regulations investigation action compliance European market Competent Authority Notified Body post market surveillance vigilance Harmonised Standards/CTS “presumption of conformity”
Medical device regulations investigation action compliance European market Competent Authority Notified Body post market surveillance vigilance Harmonised Standards/CTS “presumption of conformity”
 4
4
 Joint Action Plan • Qualifications of NB re-assessed • Joint Audits of NB • Unannounced visits of manufacturers • Vigilance teleconference of Competent Authorities
Joint Action Plan • Qualifications of NB re-assessed • Joint Audits of NB • Unannounced visits of manufacturers • Vigilance teleconference of Competent Authorities
 Signal Detection • Significant improvements in adverse incident reporting by UK healthcare • New signal detection software • Increased focus on signals
Signal Detection • Significant improvements in adverse incident reporting by UK healthcare • New signal detection software • Increased focus on signals
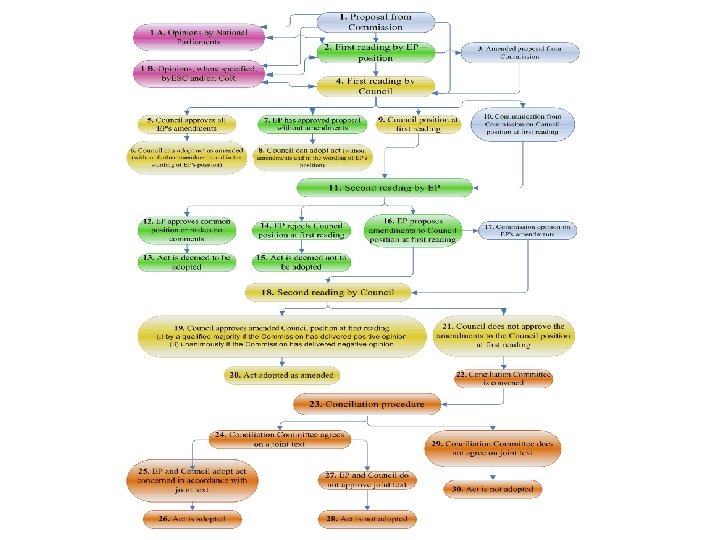
 Proposed new regulations • Proposals published in September 2012 • Medical Devices Regulation • IVD Regulation – – – Move to risk-based classification rules Clinical evidence & clinical investigation requirements Changes to ‘in-house’ exemption Companion diagnostics Many other aspects aligned across both regulations (notified bodies, unique device identifiers)
Proposed new regulations • Proposals published in September 2012 • Medical Devices Regulation • IVD Regulation – – – Move to risk-based classification rules Clinical evidence & clinical investigation requirements Changes to ‘in-house’ exemption Companion diagnostics Many other aspects aligned across both regulations (notified bodies, unique device identifiers)
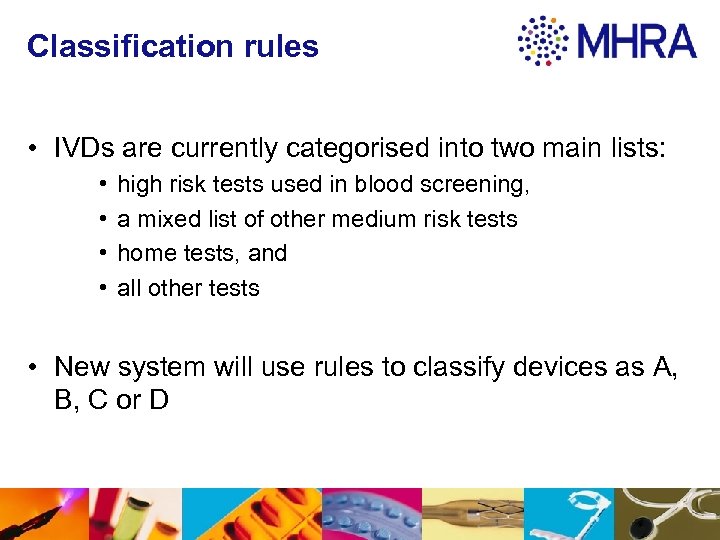 Classification rules • IVDs are currently categorised into two main lists: • • high risk tests used in blood screening, a mixed list of other medium risk tests home tests, and all other tests • New system will use rules to classify devices as A, B, C or D
Classification rules • IVDs are currently categorised into two main lists: • • high risk tests used in blood screening, a mixed list of other medium risk tests home tests, and all other tests • New system will use rules to classify devices as A, B, C or D
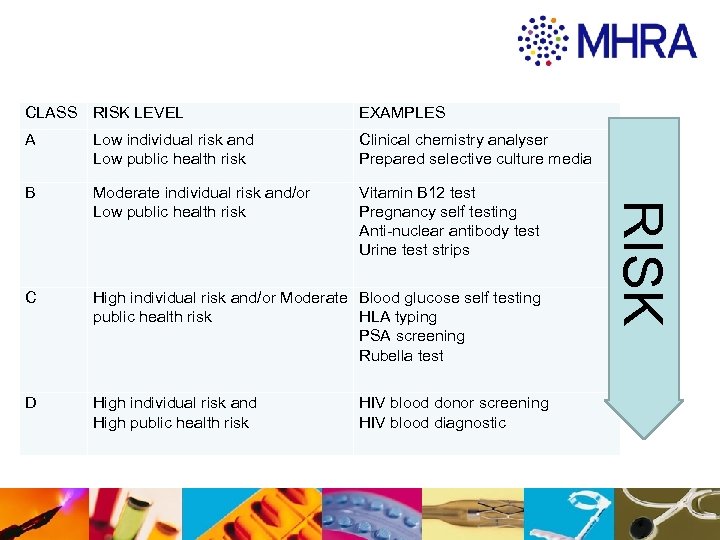 EXAMPLES A Low individual risk and Low public health risk Clinical chemistry analyser Prepared selective culture media B Moderate individual risk and/or Low public health risk Vitamin B 12 test Pregnancy self testing Anti-nuclear antibody test Urine test strips C High individual risk and/or Moderate Blood glucose self testing public health risk HLA typing PSA screening Rubella test D High individual risk and High public health risk HIV blood donor screening HIV blood diagnostic RISK CLASS RISK LEVEL
EXAMPLES A Low individual risk and Low public health risk Clinical chemistry analyser Prepared selective culture media B Moderate individual risk and/or Low public health risk Vitamin B 12 test Pregnancy self testing Anti-nuclear antibody test Urine test strips C High individual risk and/or Moderate Blood glucose self testing public health risk HLA typing PSA screening Rubella test D High individual risk and High public health risk HIV blood donor screening HIV blood diagnostic RISK CLASS RISK LEVEL
 Clinical Evidence • Clinical evidence now required (though exceptions possible) – documented in a clinical evidence report • Concepts of analytical performance scientific validity, and clinical performance introduced • Post-market surveillance required to keep clinical evidence up to date
Clinical Evidence • Clinical evidence now required (though exceptions possible) – documented in a clinical evidence report • Concepts of analytical performance scientific validity, and clinical performance introduced • Post-market surveillance required to keep clinical evidence up to date
 Clinical Evidence Terminology Analytical performance: the ability of an IVD to detect or measure a particular analyte Scientific validity: the association of an analyte to a clinical condition or a physiological state Clinical performance: the ability of an IVD to give results that are related to the clinical condition/ physiological state in the target population and intended user
Clinical Evidence Terminology Analytical performance: the ability of an IVD to detect or measure a particular analyte Scientific validity: the association of an analyte to a clinical condition or a physiological state Clinical performance: the ability of an IVD to give results that are related to the clinical condition/ physiological state in the target population and intended user
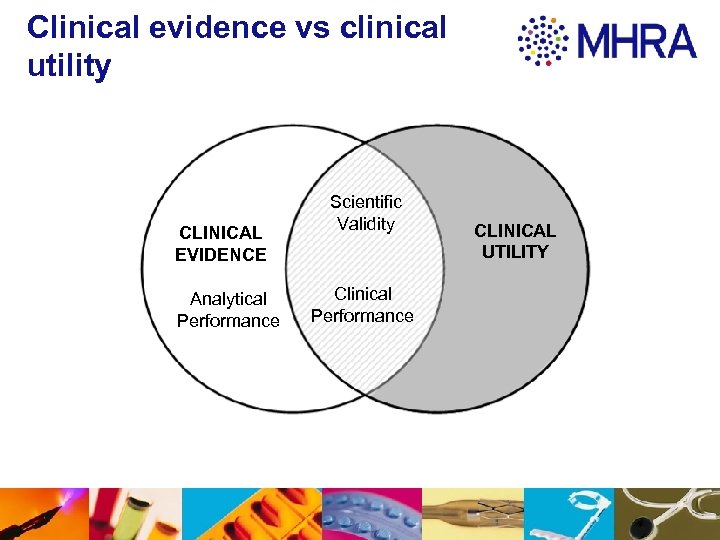 Clinical evidence vs clinical utility CLINICAL EVIDENCE Analytical Performance Scientific Validity Clinical Performance CLINICAL UTILITY
Clinical evidence vs clinical utility CLINICAL EVIDENCE Analytical Performance Scientific Validity Clinical Performance CLINICAL UTILITY
 Clinical performance studies • New concept – when gathering data to support CE marking • General requirements on all studies • Specific requirements – including competent authority approval – on: – ‘interventional’ studies – affecting patient management decisions; and – studies that involve invasive procedures or other risks for patients.
Clinical performance studies • New concept – when gathering data to support CE marking • General requirements on all studies • Specific requirements – including competent authority approval – on: – ‘interventional’ studies – affecting patient management decisions; and – studies that involve invasive procedures or other risks for patients.
 The ‘in-house’ exemption • Currently – blanket exemption for all IVDs manufactured and used within same health institution • Commission’s proposal: – single quality management system – accreditation to ISO 15189 – vigilance reporting – exclusion of highest risk (‘class D’) IVDs
The ‘in-house’ exemption • Currently – blanket exemption for all IVDs manufactured and used within same health institution • Commission’s proposal: – single quality management system – accreditation to ISO 15189 – vigilance reporting – exclusion of highest risk (‘class D’) IVDs
 Companion Diagnostics • Class C • “specifically intended to select patients with a previously diagnosed condition or predisposition as eligible for a targeted therapy” • The Notified Body will consult with the county’s medicines authority
Companion Diagnostics • Class C • “specifically intended to select patients with a previously diagnosed condition or predisposition as eligible for a targeted therapy” • The Notified Body will consult with the county’s medicines authority
 Thank you for listening Steve Lee 020 3080 7309 Stephen. Lee@mhra. gsi. gov. uk
Thank you for listening Steve Lee 020 3080 7309 Stephen. Lee@mhra. gsi. gov. uk


