7dee71701a6dd726e9cfe157695522f7.ppt
- Количество слайдов: 12

CE mark issues -PEI view Micha Nübling, PEI
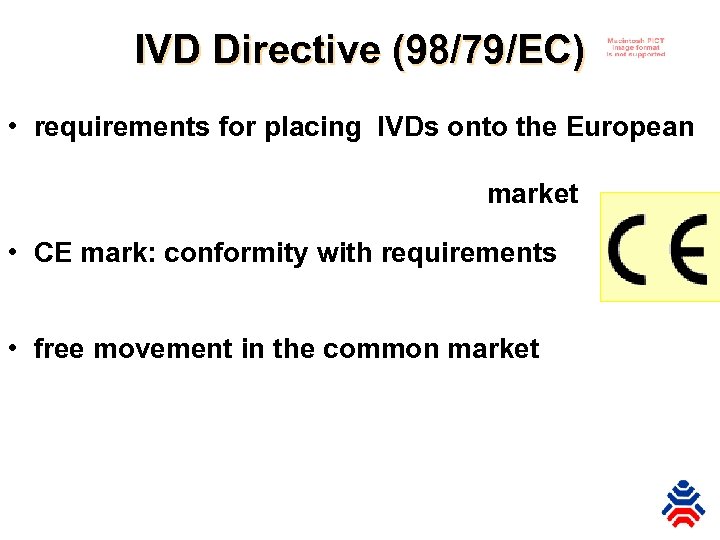
IVD Directive (98/79/EC) • requirements for placing IVDs onto the European market • CE mark: conformity with requirements • free movement in the common market
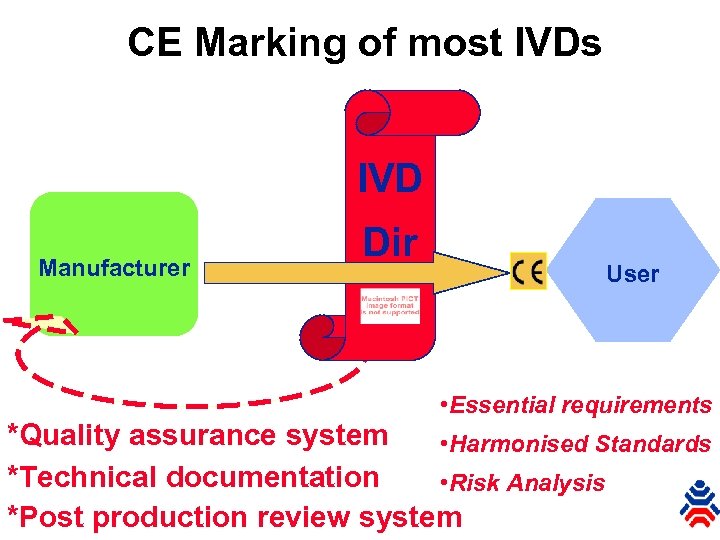
CE Marking of most IVDs IVD Manufacturer Dir User • Essential requirements *Quality assurance system • Harmonised Standards *Technical documentation • Risk Analysis *Post production review system
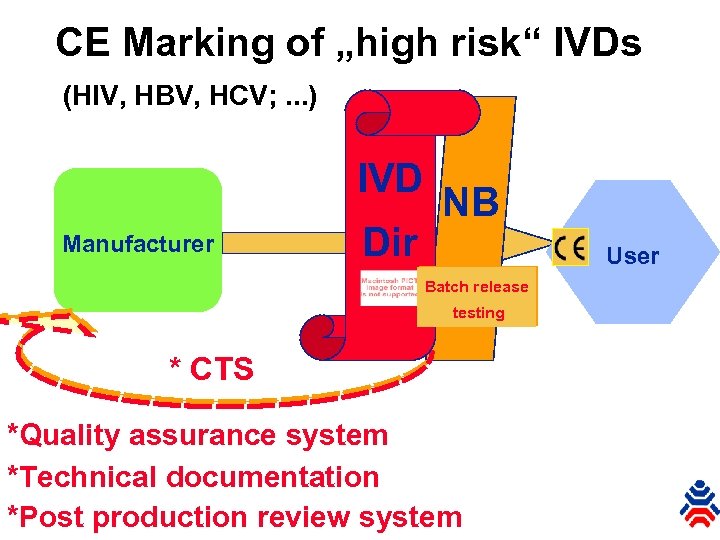
CE Marking of „high risk“ IVDs (HIV, HBV, HCV; . . . ) IVD Manufacturer Dir NB User Batch release testing * CTS *Quality assurance system *Technical documentation *Post production review system
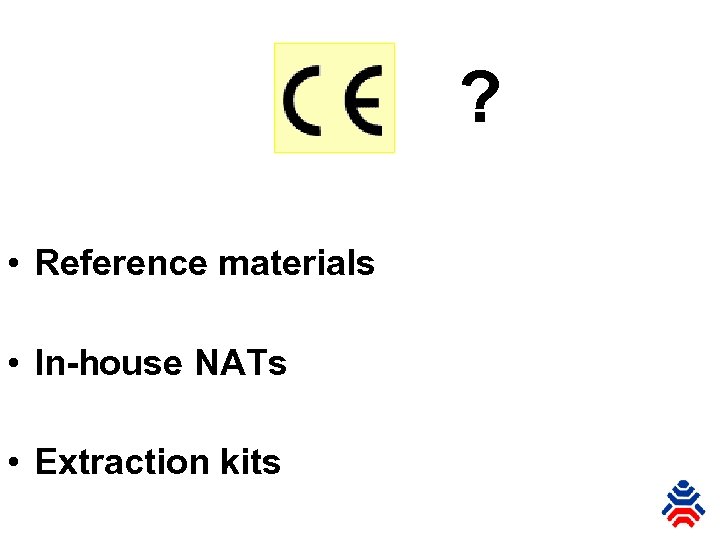
? • Reference materials • In-house NATs • Extraction kits
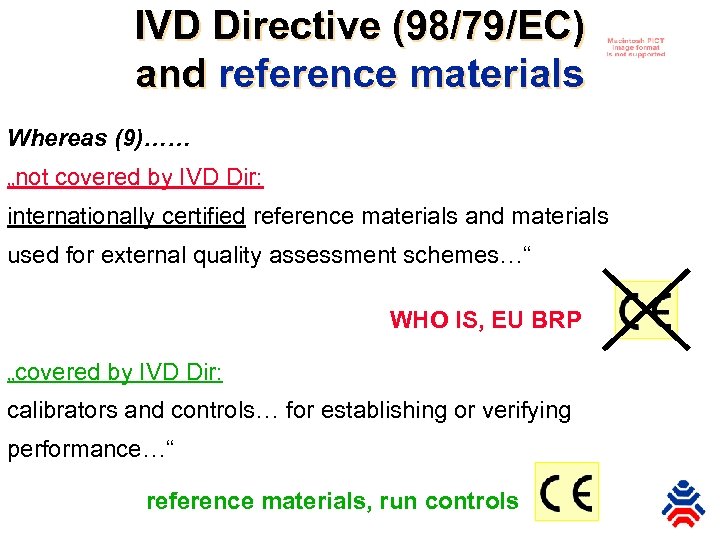
IVD Directive (98/79/EC) and reference materials Whereas (9)…… „not covered by IVD Dir: internationally certified reference materials and materials used for external quality assessment schemes…“ WHO IS, EU BRP „covered by IVD Dir: calibrators and controls… for establishing or verifying performance…“ reference materials, run controls
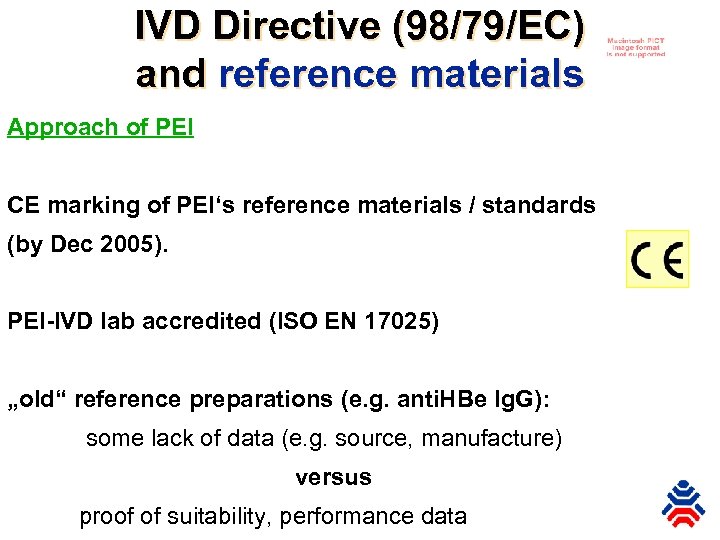
IVD Directive (98/79/EC) and reference materials Approach of PEI CE marking of PEI‘s reference materials / standards (by Dec 2005). PEI-IVD lab accredited (ISO EN 17025) „old“ reference preparations (e. g. anti. HBe Ig. G): some lack of data (e. g. source, manufacture) versus proof of suitability, performance data

IVD Directive (98/79/EC) and in-house NATs Whereas (10)…… not covered by IVD Dir: reagents produced within health institution laboratories for use in that environment…and not subject to commercial transactions“ Art 1 (5) Member State has the right to…. appropriate protection requirements.
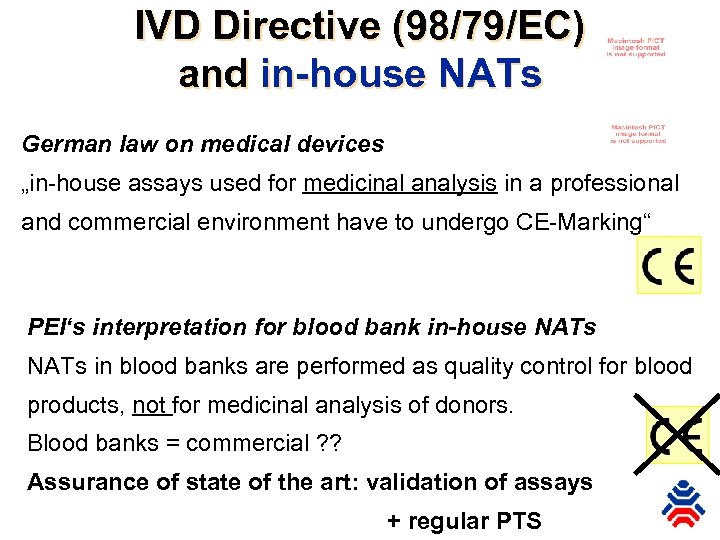
IVD Directive (98/79/EC) and in-house NATs German law on medical devices „in-house assays used for medicinal analysis in a professional and commercial environment have to undergo CE-Marking“ PEI‘s interpretation for blood bank in-house NATs in blood banks are performed as quality control for blood products, not for medicinal analysis of donors. Blood banks = commercial ? ? Assurance of state of the art: validation of assays + regular PTS

IVD Directive (98/79/EC) and extraction kits Art 1 (2 b) Scope, definitions „IVD = reagent (product), kit, instrument, …. …used alone or in combination …intended by the manufacturer to be used for in vitro examination for the purpose of providing diagnostic information…“ CE mark for extraction kits for diagnostic NATs ? ?

IVD Directive (98/79/EC) and extraction kits PEI‘s interpretation: Extraction kits for diagnostic NATs = „reagents …used in combination…for in vitro examination for the purpose of providing diagnostic information“ = critical part of NAT systems EDMA‘s interpretation: Extraction kits are no IVDs since they cannot provide diagnostic information on ist own.
? • Reference materials • In-house NATs • Extraction kits ? ?
7dee71701a6dd726e9cfe157695522f7.ppt