eeed53a672fc87a3622dec87b7cabe82.ppt
- Количество слайдов: 44
 CDM Update – 2014 – Session 5 Drugs, Biologicals, Vaccines January 2014 Jean C. Russell, MS, RHIT jrussell@epochhealth. com Richard Cooley, BA, CCS rcooley@epochhealth. com Matthew H. Lawney MSPT, MBA, CHC, mlawney@epochhealth. com 518 -430 -1144
CDM Update – 2014 – Session 5 Drugs, Biologicals, Vaccines January 2014 Jean C. Russell, MS, RHIT jrussell@epochhealth. com Richard Cooley, BA, CCS rcooley@epochhealth. com Matthew H. Lawney MSPT, MBA, CHC, mlawney@epochhealth. com 518 -430 -1144
 2 Agenda • • • Payment Basics Code & Payment Changes 2014 -2015 • Drugs • Vaccines Self Administered Drugs Drug Wastage Billing units
2 Agenda • • • Payment Basics Code & Payment Changes 2014 -2015 • Drugs • Vaccines Self Administered Drugs Drug Wastage Billing units
 3 Outpatient Payment Medicare Outpatient Drugs, biologicals and vaccines are paid under APCs Roughly 900 drugs, biologicals and vaccines are identified by HCPCS code Roughly 320 are paid while the rest are packaged or non-covered Paid drugs are Status G (pass-through), K (nonpass through), or L and F (reasonable cost)
3 Outpatient Payment Medicare Outpatient Drugs, biologicals and vaccines are paid under APCs Roughly 900 drugs, biologicals and vaccines are identified by HCPCS code Roughly 320 are paid while the rest are packaged or non-covered Paid drugs are Status G (pass-through), K (nonpass through), or L and F (reasonable cost)
 4 Medicare IP Some Drugs paid in addition to DRGs Report clotting factors Use rev code 636 Report Vaccines Use rev code 636 Use bill type 12 x (inpatient part B) rather than type 11 x (inpatient bill) Chapter 18, Preventative Services, Medicare Claims Processing Manual, website: http: //www. cms. gov/Regulations-and. Guidance/Manuals/Downloads/clm 104 c 18. pdf
4 Medicare IP Some Drugs paid in addition to DRGs Report clotting factors Use rev code 636 Report Vaccines Use rev code 636 Use bill type 12 x (inpatient part B) rather than type 11 x (inpatient bill) Chapter 18, Preventative Services, Medicare Claims Processing Manual, website: http: //www. cms. gov/Regulations-and. Guidance/Manuals/Downloads/clm 104 c 18. pdf
 5 Payment Medicaid APG- CLASS PHARMACOTHERAPY Report drug HCPCS on claim Paid by weight x rate (based on rate code) http: //www. health. ny. gov/health_care/medicaid/rates/apg/rates/hospital/ index. htm Most OP drugs billed under 1432 – Clinic 1402 - ER Carve-out drugs reported separately
5 Payment Medicaid APG- CLASS PHARMACOTHERAPY Report drug HCPCS on claim Paid by weight x rate (based on rate code) http: //www. health. ny. gov/health_care/medicaid/rates/apg/rates/hospital/ index. htm Most OP drugs billed under 1432 – Clinic 1402 - ER Carve-out drugs reported separately
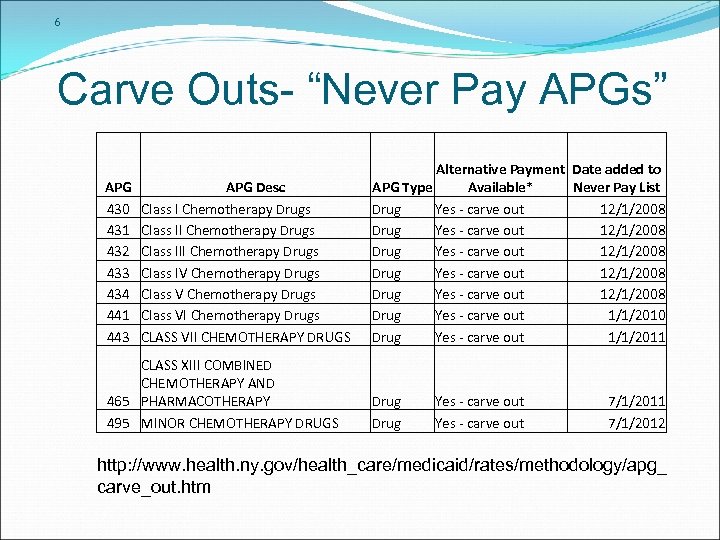 6 Carve Outs- “Never Pay APGs” APG 430 431 432 433 434 441 443 APG Desc Class I Chemotherapy Drugs Class III Chemotherapy Drugs Class IV Chemotherapy Drugs Class VI Chemotherapy Drugs CLASS VII CHEMOTHERAPY DRUGS CLASS XIII COMBINED CHEMOTHERAPY AND 465 PHARMACOTHERAPY 495 MINOR CHEMOTHERAPY DRUGS Alternative Payment Date added to APG Type Available* Never Pay List Drug Yes - carve out 12/1/2008 Drug Yes - carve out 12/1/2008 Drug Yes - carve out 1/1/2010 Drug Yes - carve out 1/1/2011 Drug Yes - carve out 7/1/2011 7/1/2012 http: //www. health. ny. gov/health_care/medicaid/rates/methodology/apg_ carve_out. htm
6 Carve Outs- “Never Pay APGs” APG 430 431 432 433 434 441 443 APG Desc Class I Chemotherapy Drugs Class III Chemotherapy Drugs Class IV Chemotherapy Drugs Class VI Chemotherapy Drugs CLASS VII CHEMOTHERAPY DRUGS CLASS XIII COMBINED CHEMOTHERAPY AND 465 PHARMACOTHERAPY 495 MINOR CHEMOTHERAPY DRUGS Alternative Payment Date added to APG Type Available* Never Pay List Drug Yes - carve out 12/1/2008 Drug Yes - carve out 12/1/2008 Drug Yes - carve out 1/1/2010 Drug Yes - carve out 1/1/2011 Drug Yes - carve out 7/1/2011 7/1/2012 http: //www. health. ny. gov/health_care/medicaid/rates/methodology/apg_ carve_out. htm
 7 Carve Outs Report as referred ambulatory- no rate code Report with National Drug Code (NDC) NDC maintained by pharmacist in formulary Report with Acquisition Cost
7 Carve Outs Report as referred ambulatory- no rate code Report with National Drug Code (NDC) NDC maintained by pharmacist in formulary Report with Acquisition Cost
 8 Rev Codes 25 x 250 General Pharmacy 251 Generic Drugs Pharmacy 252 Nongeneric Drugs Pharmacy 253 Take Home Drugs Pharmacy 254 Drugs Incident To Other Diagnostic Services 255 Drugs Incident To Radiology 256 Experimental Drugs 257 Nonprescription Drugs 258 IV Solutions Pharmacy 259 Other Pharmacy 636 - Drugs Requiring Detailed Coding 637 - Self-Administrable Drugs
8 Rev Codes 25 x 250 General Pharmacy 251 Generic Drugs Pharmacy 252 Nongeneric Drugs Pharmacy 253 Take Home Drugs Pharmacy 254 Drugs Incident To Other Diagnostic Services 255 Drugs Incident To Radiology 256 Experimental Drugs 257 Nonprescription Drugs 258 IV Solutions Pharmacy 259 Other Pharmacy 636 - Drugs Requiring Detailed Coding 637 - Self-Administrable Drugs
 9 Code & Payment Changes 2014 -2015
9 Code & Payment Changes 2014 -2015
 10 Drug Cost Threshold Moved from $80 in 2013 to $90 for 2014 Drugs greater than $90 per day cost (national average) will be paid Used ASP+6 percent per unit payment amount across all dosage levels of a specific drug or biological by the estimated units per day
10 Drug Cost Threshold Moved from $80 in 2013 to $90 for 2014 Drugs greater than $90 per day cost (national average) will be paid Used ASP+6 percent per unit payment amount across all dosage levels of a specific drug or biological by the estimated units per day
 11 2014 Payment for Drugs, Biologicals, and Radiopharmaceuticals Pass- through drugs will be paid at Average Sale Price plus 6% If ASP data is not available then Wholesale Acquisition Cost plus 6% If no WAC then 95% of most recent Average Wholesale Price
11 2014 Payment for Drugs, Biologicals, and Radiopharmaceuticals Pass- through drugs will be paid at Average Sale Price plus 6% If ASP data is not available then Wholesale Acquisition Cost plus 6% If no WAC then 95% of most recent Average Wholesale Price
 12 Pass-Through Drugs Temporary “at least” 2 to “not more than” 3 year “pass-through” of cost for new drugs Fourteen pass-through drugs and biologicals will expire, five became packaged, the rest became status K (non pass-through) Fourteen new pass-through drugs in 2014
12 Pass-Through Drugs Temporary “at least” 2 to “not more than” 3 year “pass-through” of cost for new drugs Fourteen pass-through drugs and biologicals will expire, five became packaged, the rest became status K (non pass-through) Fourteen new pass-through drugs in 2014
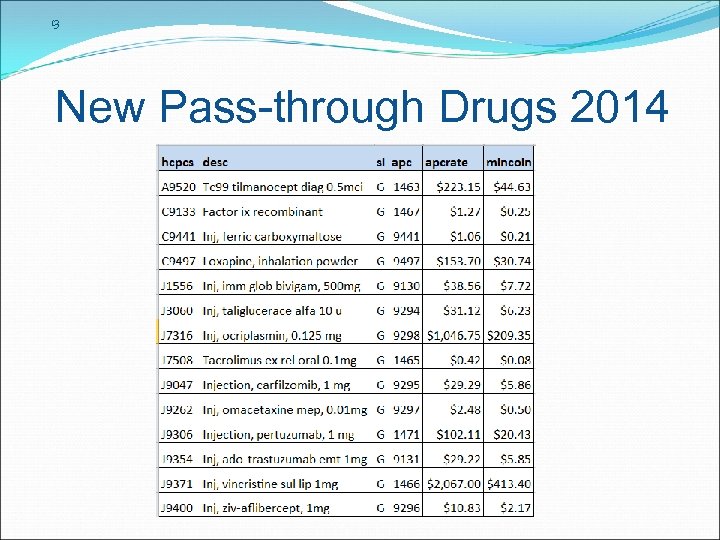 13 New Pass-through Drugs 2014
13 New Pass-through Drugs 2014
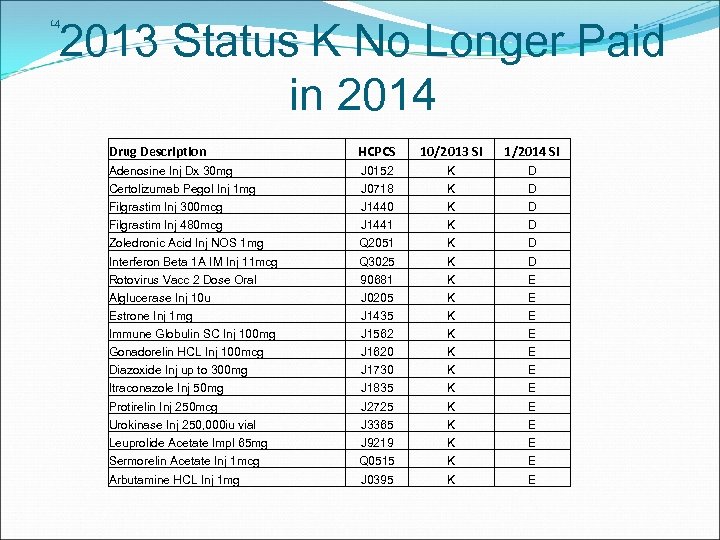 14 2013 Status K No Longer Paid in 2014 Drug Description HCPCS 10/2013 SI 1/2014 SI Adenosine Inj Dx 30 mg J 0152 K D Certolizumab Pegol Inj 1 mg J 0718 K D Filgrastim Inj 300 mcg J 1440 K D Filgrastim Inj 480 mcg J 1441 K D Zoledronic Acid Inj NOS 1 mg Q 2051 K D Interferon Beta 1 A IM Inj 11 mcg Q 3025 K D Rotovirus Vacc 2 Dose Oral 90681 K E Alglucerase Inj 10 u J 0205 K E Estrone Inj 1 mg J 1435 K E Immune Globulin SC Inj 100 mg J 1562 K E Gonadorelin HCL Inj 100 mcg J 1620 K E Diazoxide Inj up to 300 mg J 1730 K E Itraconazole Inj 50 mg J 1835 K E Protirelin Inj 250 mcg J 2725 K E Urokinase Inj 250, 000 iu vial J 3365 K E Leuprolide Acetate Impl 65 mg J 9219 K E Sermorelin Acetate Inj 1 mcg Q 0515 K E Arbutamine HCL Inj 1 mg J 0395 K E
14 2013 Status K No Longer Paid in 2014 Drug Description HCPCS 10/2013 SI 1/2014 SI Adenosine Inj Dx 30 mg J 0152 K D Certolizumab Pegol Inj 1 mg J 0718 K D Filgrastim Inj 300 mcg J 1440 K D Filgrastim Inj 480 mcg J 1441 K D Zoledronic Acid Inj NOS 1 mg Q 2051 K D Interferon Beta 1 A IM Inj 11 mcg Q 3025 K D Rotovirus Vacc 2 Dose Oral 90681 K E Alglucerase Inj 10 u J 0205 K E Estrone Inj 1 mg J 1435 K E Immune Globulin SC Inj 100 mg J 1562 K E Gonadorelin HCL Inj 100 mcg J 1620 K E Diazoxide Inj up to 300 mg J 1730 K E Itraconazole Inj 50 mg J 1835 K E Protirelin Inj 250 mcg J 2725 K E Urokinase Inj 250, 000 iu vial J 3365 K E Leuprolide Acetate Impl 65 mg J 9219 K E Sermorelin Acetate Inj 1 mcg Q 0515 K E Arbutamine HCL Inj 1 mg J 0395 K E
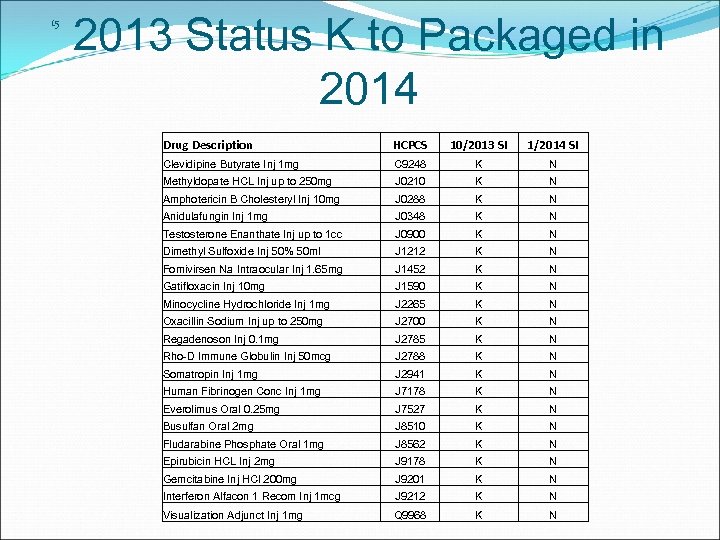 15 2013 Status K to Packaged in 2014 Drug Description HCPCS 10/2013 SI 1/2014 SI Clevidipine Butyrate Inj 1 mg C 9248 K N Methyldopate HCL Inj up to 250 mg J 0210 K N Amphotericin B Cholesteryl Inj 10 mg J 0288 K N Anidulafungin Inj 1 mg J 0348 K N Testosterone Enanthate Inj up to 1 cc J 0900 K N Dimethyl Sulfoxide Inj 50% 50 ml J 1212 K N Fomivirsen Na Intraocular Inj 1. 65 mg J 1452 K N Gatifloxacin Inj 10 mg J 1590 K N Minocycline Hydrochloride Inj 1 mg J 2265 K N Oxacillin Sodium Inj up to 250 mg J 2700 K N Regadenoson Inj 0. 1 mg J 2785 K N Rho-D Immune Globulin Inj 50 mcg J 2788 K N Somatropin Inj 1 mg J 2941 K N Human Fibrinogen Conc Inj 1 mg J 7178 K N Everolimus Oral 0. 25 mg J 7527 K N Busulfan Oral 2 mg J 8510 K N Fludarabine Phosphate Oral 1 mg J 8562 K N Epirubicin HCL Inj 2 mg J 9178 K N Gemcitabine Inj HCl 200 mg J 9201 K N Interferon Alfacon 1 Recom Inj 1 mcg J 9212 K N Visualization Adjunct Inj 1 mg Q 9968 K N
15 2013 Status K to Packaged in 2014 Drug Description HCPCS 10/2013 SI 1/2014 SI Clevidipine Butyrate Inj 1 mg C 9248 K N Methyldopate HCL Inj up to 250 mg J 0210 K N Amphotericin B Cholesteryl Inj 10 mg J 0288 K N Anidulafungin Inj 1 mg J 0348 K N Testosterone Enanthate Inj up to 1 cc J 0900 K N Dimethyl Sulfoxide Inj 50% 50 ml J 1212 K N Fomivirsen Na Intraocular Inj 1. 65 mg J 1452 K N Gatifloxacin Inj 10 mg J 1590 K N Minocycline Hydrochloride Inj 1 mg J 2265 K N Oxacillin Sodium Inj up to 250 mg J 2700 K N Regadenoson Inj 0. 1 mg J 2785 K N Rho-D Immune Globulin Inj 50 mcg J 2788 K N Somatropin Inj 1 mg J 2941 K N Human Fibrinogen Conc Inj 1 mg J 7178 K N Everolimus Oral 0. 25 mg J 7527 K N Busulfan Oral 2 mg J 8510 K N Fludarabine Phosphate Oral 1 mg J 8562 K N Epirubicin HCL Inj 2 mg J 9178 K N Gemcitabine Inj HCl 200 mg J 9201 K N Interferon Alfacon 1 Recom Inj 1 mcg J 9212 K N Visualization Adjunct Inj 1 mg Q 9968 K N
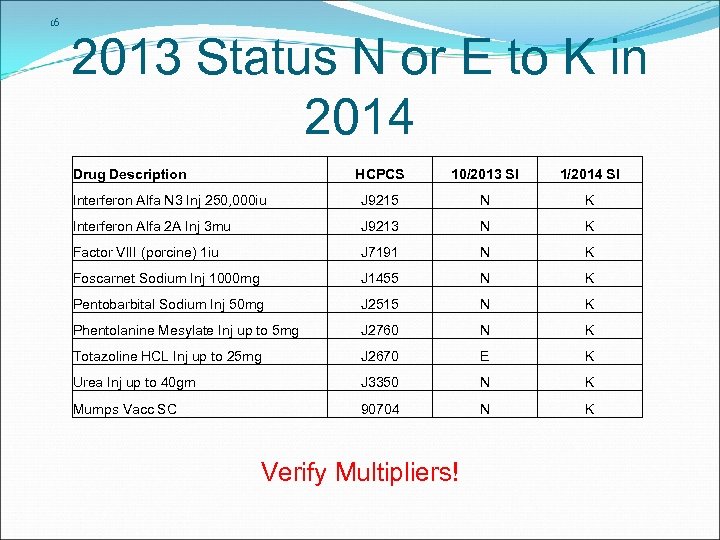 16 2013 Status N or E to K in 2014 Drug Description HCPCS 10/2013 SI 1/2014 SI Interferon Alfa N 3 Inj 250, 000 iu J 9215 N K Interferon Alfa 2 A Inj 3 mu J 9213 N K Factor VIII (porcine) 1 iu J 7191 N K Foscarnet Sodium Inj 1000 mg J 1455 N K Pentobarbital Sodium Inj 50 mg J 2515 N K Phentolanine Mesylate Inj up to 5 mg J 2760 N K Totazoline HCL Inj up to 25 mg J 2670 E K Urea Inj up to 40 gm J 3350 N K Mumps Vacc SC 90704 N K Verify Multipliers!
16 2013 Status N or E to K in 2014 Drug Description HCPCS 10/2013 SI 1/2014 SI Interferon Alfa N 3 Inj 250, 000 iu J 9215 N K Interferon Alfa 2 A Inj 3 mu J 9213 N K Factor VIII (porcine) 1 iu J 7191 N K Foscarnet Sodium Inj 1000 mg J 1455 N K Pentobarbital Sodium Inj 50 mg J 2515 N K Phentolanine Mesylate Inj up to 5 mg J 2760 N K Totazoline HCL Inj up to 25 mg J 2670 E K Urea Inj up to 40 gm J 3350 N K Mumps Vacc SC 90704 N K Verify Multipliers!
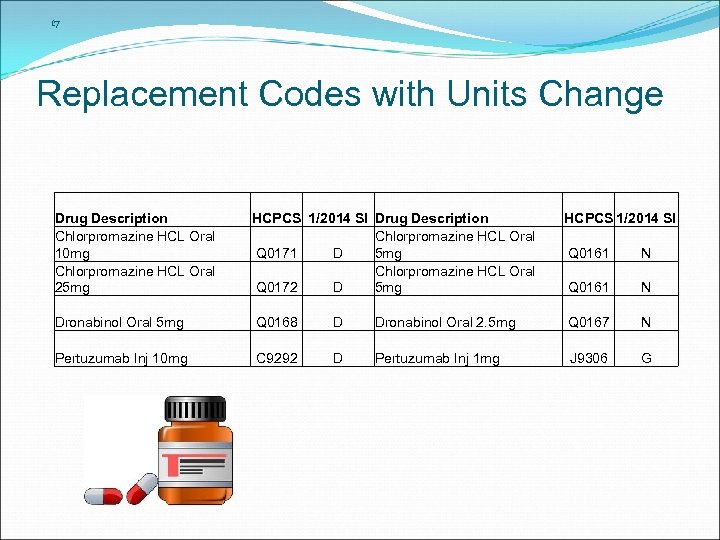 17 Replacement Codes with Units Change Drug Description Chlorpromazine HCL Oral 10 mg Chlorpromazine HCL Oral 25 mg HCPCS 1/2014 SI Drug Description Chlorpromazine HCL Oral Q 0171 D 5 mg Chlorpromazine HCL Oral Q 0172 D 5 mg HCPCS 1/2014 SI Dronabinol Oral 5 mg Q 0168 D Pertuzumab Inj 10 mg C 9292 D Q 0161 N Dronabinol Oral 2. 5 mg Q 0167 N Pertuzumab Inj 1 mg J 9306 G
17 Replacement Codes with Units Change Drug Description Chlorpromazine HCL Oral 10 mg Chlorpromazine HCL Oral 25 mg HCPCS 1/2014 SI Drug Description Chlorpromazine HCL Oral Q 0171 D 5 mg Chlorpromazine HCL Oral Q 0172 D 5 mg HCPCS 1/2014 SI Dronabinol Oral 5 mg Q 0168 D Pertuzumab Inj 10 mg C 9292 D Q 0161 N Dronabinol Oral 2. 5 mg Q 0167 N Pertuzumab Inj 1 mg J 9306 G
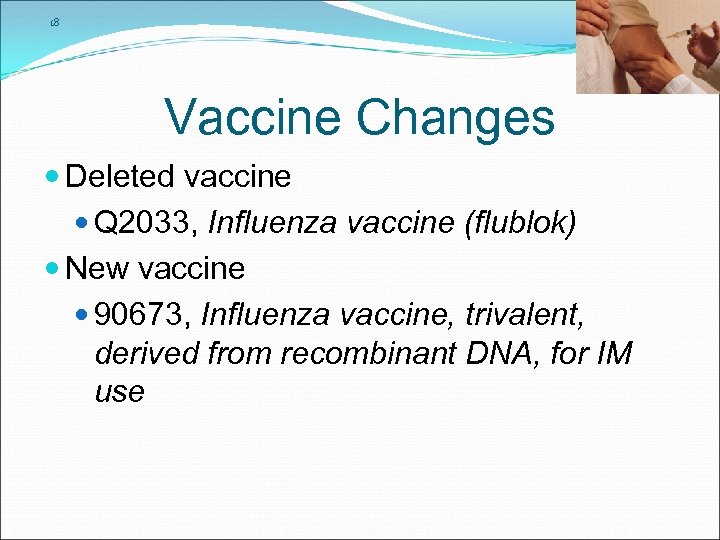 18 Vaccine Changes Deleted vaccine Q 2033, Influenza vaccine (flublok) New vaccine 90673, Influenza vaccine, trivalent, derived from recombinant DNA, for IM use
18 Vaccine Changes Deleted vaccine Q 2033, Influenza vaccine (flublok) New vaccine 90673, Influenza vaccine, trivalent, derived from recombinant DNA, for IM use
 19 Drug Administration No major changes Continue to reimburse for the add-on procedures, with the exception of add-on vaccination codes and pump reset 90472, immunization, each additional 90474, immunize oral/nasal, each additional 96371, therapeutic infusion SC reset pump All three are now unconditionally packaged (SI N)
19 Drug Administration No major changes Continue to reimburse for the add-on procedures, with the exception of add-on vaccination codes and pump reset 90472, immunization, each additional 90474, immunize oral/nasal, each additional 96371, therapeutic infusion SC reset pump All three are now unconditionally packaged (SI N)
 20 Proposed Rule 2014 Create 29 Comprehensive APCs with one payment made for the primary service plus all adjunctive services performed to support that service These were developed from the 29 highest cost device dependent APCs There will be a new status indicator (J 1) to identify the 136 HCPCS codes which map to the 29 comprehensive APCs
20 Proposed Rule 2014 Create 29 Comprehensive APCs with one payment made for the primary service plus all adjunctive services performed to support that service These were developed from the 29 highest cost device dependent APCs There will be a new status indicator (J 1) to identify the 136 HCPCS codes which map to the 29 comprehensive APCs
 21 Proposed Rule A single payment will be made that includes the following when performed as part of the service: All DME items Rehab codes, including PT/OT/ST All drugs, except pass-through drugs (status G), including self-administered drugs Recovery and extended recovery and observation services Two or more comprehensive APC procedures will result in payment for the higher paid procedure Add-on procedures
21 Proposed Rule A single payment will be made that includes the following when performed as part of the service: All DME items Rehab codes, including PT/OT/ST All drugs, except pass-through drugs (status G), including self-administered drugs Recovery and extended recovery and observation services Two or more comprehensive APC procedures will result in payment for the higher paid procedure Add-on procedures
 22 Final Rule 2014 Comprehensive APCs have been FINALIZED, But delayed until 1/1/2015 Extra time to allow hospitals to perform a thorough analysis of the impact of this change so that they can implement changes CMS will apply a “degree of complexity” to each J 1 procedure
22 Final Rule 2014 Comprehensive APCs have been FINALIZED, But delayed until 1/1/2015 Extra time to allow hospitals to perform a thorough analysis of the impact of this change so that they can implement changes CMS will apply a “degree of complexity” to each J 1 procedure
 23 Drugs/Biologicals/Radiopharm Dx Tests In 2013, the following drugs are APC status N (unconditionally packaged) unless status G (passthru): Drugs with a per day cost less than threshold Diagnostic radiopharmaceuticals Contrast agents Anesthesia drugs Drugs used as a supply Implanted biologicals
23 Drugs/Biologicals/Radiopharm Dx Tests In 2013, the following drugs are APC status N (unconditionally packaged) unless status G (passthru): Drugs with a per day cost less than threshold Diagnostic radiopharmaceuticals Contrast agents Anesthesia drugs Drugs used as a supply Implanted biologicals
 24 Drugs/Biologicals/Radiopharm Dx Tests For 2014, CMS is adding more categories of diagnostic drugs unconditionally packaged Drugs, biologicals, and radiopharmaceuticals that function as supplies when used in diagnostic tests or procedures Drugs and biologicals when used as supplies in surgical procedures
24 Drugs/Biologicals/Radiopharm Dx Tests For 2014, CMS is adding more categories of diagnostic drugs unconditionally packaged Drugs, biologicals, and radiopharmaceuticals that function as supplies when used in diagnostic tests or procedures Drugs and biologicals when used as supplies in surgical procedures
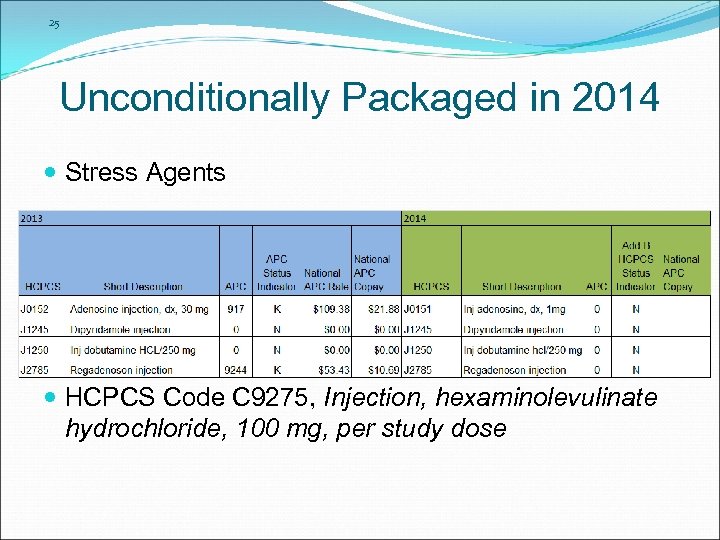 25 Unconditionally Packaged in 2014 Stress Agents HCPCS Code C 9275, Injection, hexaminolevulinate hydrochloride, 100 mg, per study dose
25 Unconditionally Packaged in 2014 Stress Agents HCPCS Code C 9275, Injection, hexaminolevulinate hydrochloride, 100 mg, per study dose
 26 Self Administered Drugs Self-administered drugs (SAD) are considered a statutory exclusion from Medicare benefits Reported in the non-covered portion of the outpatient bill Use Rev Code 637 for OP billing For most commercial payers report with a 250 revenue code
26 Self Administered Drugs Self-administered drugs (SAD) are considered a statutory exclusion from Medicare benefits Reported in the non-covered portion of the outpatient bill Use Rev Code 637 for OP billing For most commercial payers report with a 250 revenue code
 27 SAD Medicare Part B does not cover drugs that are “usually” (i. e. , more than 50% of the time) selfadministered by the patient It is a “benefit category” denial and not a denial based on medical necessity An Advance Beneficiary Notice (“ABN”) is not required Therefore providers may charge the beneficiary for an excluded drug If Hospital pharmacy participates (most don’t) in Part D drug plan, then some SAD may be covered
27 SAD Medicare Part B does not cover drugs that are “usually” (i. e. , more than 50% of the time) selfadministered by the patient It is a “benefit category” denial and not a denial based on medical necessity An Advance Beneficiary Notice (“ABN”) is not required Therefore providers may charge the beneficiary for an excluded drug If Hospital pharmacy participates (most don’t) in Part D drug plan, then some SAD may be covered
 28 NGS SAD List Contractors (FI/MACs) must publish a list of the injectable drugs that are subject to the selfadministered exclusion on their Web site Link to NGS SAD list-- http: //www. cms. gov/medicare-coveragedatabase/indexes/articlelist. aspx? Cntrctr=63&name=National%20 Government%20 Services, %20 Inc. %20%20( 00450, %20 FI)&Doc. Status=SAD&Contr. Num=00450&Cntrctr. Type=FI&LCntrctr=63&bc =BAACAACAAAAA&#Results. Anchor J 1675 INJECTION, HISTRELIN ACETATE, 10 MICROGRAMS Histrelin acetate 10 mg J 1815 INJECTION, INSULIN, PER 5 UNITS Insulin J 1817 INSULIN FOR ADMINISTRATION THROUGH DME (I. E. , INSULIN PUMP) PER 50 UNITS Insulin for administration
28 NGS SAD List Contractors (FI/MACs) must publish a list of the injectable drugs that are subject to the selfadministered exclusion on their Web site Link to NGS SAD list-- http: //www. cms. gov/medicare-coveragedatabase/indexes/articlelist. aspx? Cntrctr=63&name=National%20 Government%20 Services, %20 Inc. %20%20( 00450, %20 FI)&Doc. Status=SAD&Contr. Num=00450&Cntrctr. Type=FI&LCntrctr=63&bc =BAACAACAAAAA&#Results. Anchor J 1675 INJECTION, HISTRELIN ACETATE, 10 MICROGRAMS Histrelin acetate 10 mg J 1815 INJECTION, INSULIN, PER 5 UNITS Insulin J 1817 INSULIN FOR ADMINISTRATION THROUGH DME (I. E. , INSULIN PUMP) PER 50 UNITS Insulin for administration
 29 Don’t Report Admin Do not report the injection administration with the SAD list drugs
29 Don’t Report Admin Do not report the injection administration with the SAD list drugs
 30 Billing for Wastage The CMS encourages physicians, hospitals and other providers and suppliers to care for and administer to patients in such a way that they can use drugs or biologicals most efficiently When a provider must discard the remainder of a single use vial or other single use package after administering a drug or biological to a Medicare patient, the program provides payment for the amount of drug or biological discarded as well as the dose administered, up to the amount of the drug or biological as indicated on the vial or package label Medicare Claims Processing Manual Chapter 17 - Drugs and Biologicals
30 Billing for Wastage The CMS encourages physicians, hospitals and other providers and suppliers to care for and administer to patients in such a way that they can use drugs or biologicals most efficiently When a provider must discard the remainder of a single use vial or other single use package after administering a drug or biological to a Medicare patient, the program provides payment for the amount of drug or biological discarded as well as the dose administered, up to the amount of the drug or biological as indicated on the vial or package label Medicare Claims Processing Manual Chapter 17 - Drugs and Biologicals
 31 Wastage Document what wasted Can be per patient documentation Can be included in a drug wastage policy Don’t bill waste for multi-use vials OIG has recommended FIs set up an edit that looks for drug billing units equal to full vials for “multi-use” vial drugs
31 Wastage Document what wasted Can be per patient documentation Can be included in a drug wastage policy Don’t bill waste for multi-use vials OIG has recommended FIs set up an edit that looks for drug billing units equal to full vials for “multi-use” vial drugs
 32 Multi Use Vials Herceptin comes in a multiuse vial of 440 milligrams Herceptin, when reconstituted with BWFI and stored properly, can be used for up to 28 days For multiuse vials, Medicare pays only for the amount administered to a beneficiary and does not pay for any discarded drug A payment for an entire multiuse vial is likely to be incorrect This audit is part of a nationwide review of the drug Herceptin Report by THE OFFICE OF INSPECTOR GENERAL- December 2012 A-05 -11 -00112
32 Multi Use Vials Herceptin comes in a multiuse vial of 440 milligrams Herceptin, when reconstituted with BWFI and stored properly, can be used for up to 28 days For multiuse vials, Medicare pays only for the amount administered to a beneficiary and does not pay for any discarded drug A payment for an entire multiuse vial is likely to be incorrect This audit is part of a nationwide review of the drug Herceptin Report by THE OFFICE OF INSPECTOR GENERAL- December 2012 A-05 -11 -00112
 33 JW Modifier The JW modifier is only applied to the amount of drug or biological that is discarded Not required by NGS Some Hospitals use the JW modifier as part of there wastage documentation program
33 JW Modifier The JW modifier is only applied to the amount of drug or biological that is discarded Not required by NGS Some Hospitals use the JW modifier as part of there wastage documentation program
 34 NDC Review National Drug Code maintained in the formulary by the pharmacist 11 digit code represents brand (labeler), drug and dose, vial size NDC is used for billing for some payers e. g. , Medicaid Periodic review is important https: //www. emedny. org/info/formfile. aspx
34 NDC Review National Drug Code maintained in the formulary by the pharmacist 11 digit code represents brand (labeler), drug and dose, vial size NDC is used for billing for some payers e. g. , Medicaid Periodic review is important https: //www. emedny. org/info/formfile. aspx
 35 Billing Units Maintain Medicare billing units definition in CDM, not vial size from formulary E. g. , J 3246 Tirofiban HCL CDM description- Tirofiban HCL Inj 0. 25 mg Formulary description- Tirofiban HCL Inj 12. 5 mg 1 - 12. 5 mg vial = 50 billing units-- J 3246 x 50 Round up partial units to whole billing units
35 Billing Units Maintain Medicare billing units definition in CDM, not vial size from formulary E. g. , J 3246 Tirofiban HCL CDM description- Tirofiban HCL Inj 0. 25 mg Formulary description- Tirofiban HCL Inj 12. 5 mg 1 - 12. 5 mg vial = 50 billing units-- J 3246 x 50 Round up partial units to whole billing units
 36 Formulary to CDM Review at least annually Join Formulary to CDM Reconcile Formulary items not linked to CDM Drug CDM items with no formulary link Review multipliers
36 Formulary to CDM Review at least annually Join Formulary to CDM Reconcile Formulary items not linked to CDM Drug CDM items with no formulary link Review multipliers
 37 Pyxis Review the pharmacy Pyxis (or other automated dispensing system) Links to the CDM need to be verified Pull sample claims and verify charge flow to billing
37 Pyxis Review the pharmacy Pyxis (or other automated dispensing system) Links to the CDM need to be verified Pull sample claims and verify charge flow to billing
 38 Nuclear Medicine Procedure -to-Radiolabled Product Edit CMS finalized the proposal to discontinue this edit There will no longer be an edit to ensure that nuclear medicine drugs are reported with nuclear medicine procedures Therefore care must be taken to ensure these drugs are correctly reported with the appropriate charges
38 Nuclear Medicine Procedure -to-Radiolabled Product Edit CMS finalized the proposal to discontinue this edit There will no longer be an edit to ensure that nuclear medicine drugs are reported with nuclear medicine procedures Therefore care must be taken to ensure these drugs are correctly reported with the appropriate charges
 39 Radiology Drugs used for contrast and diagnostic radiopharmaceuticals continue to be packaged Exception is one new pass-through drug A 9520, TC 99 m Tilimanocept, dx, up to 0. 5 millicuries (was C 1204 in 2013) Will have an off-set applied to the procedure equal to the amount included in the procedure payment that represents packaged drugs Non pass-through therapeutic radiopharmaceuticals will be reimbursed on ASP plus 6%
39 Radiology Drugs used for contrast and diagnostic radiopharmaceuticals continue to be packaged Exception is one new pass-through drug A 9520, TC 99 m Tilimanocept, dx, up to 0. 5 millicuries (was C 1204 in 2013) Will have an off-set applied to the procedure equal to the amount included in the procedure payment that represents packaged drugs Non pass-through therapeutic radiopharmaceuticals will be reimbursed on ASP plus 6%
 40 Questions and Discussion
40 Questions and Discussion
 41 Contact Us Richard Cooley Phone: Email: 518 -430 -1144 RCooley@Epoch. Health. Com Matthew Lawney Phone: Email: 845 -642 -6462 MLawney@Epoch. Health. Com Jean Russell Phone: Email: 518 -369 -4986 JRussell@Epoch. Health. Com
41 Contact Us Richard Cooley Phone: Email: 518 -430 -1144 RCooley@Epoch. Health. Com Matthew Lawney Phone: Email: 845 -642 -6462 MLawney@Epoch. Health. Com Jean Russell Phone: Email: 518 -369 -4986 JRussell@Epoch. Health. Com
 42 http: //www. Epoch. Health. com/
42 http: //www. Epoch. Health. com/
 43 CPT® Current Procedural Terminology (CPT®) Copyright 2012 American Medical Association All Rights Reserved Registered trademark of the AMA
43 CPT® Current Procedural Terminology (CPT®) Copyright 2012 American Medical Association All Rights Reserved Registered trademark of the AMA
 44 Disclaimer Information and opinions included in this presentation are provided based on our interpretation of current available regulatory resources. No representation is made as to the completeness or accuracy of the information. Please refer to your payer or specific regulatory guidelines as necessary.
44 Disclaimer Information and opinions included in this presentation are provided based on our interpretation of current available regulatory resources. No representation is made as to the completeness or accuracy of the information. Please refer to your payer or specific regulatory guidelines as necessary.


