 CDER's Office of Drug Safety: Electronic Tools for Risk Assessment and Evaluation March 31, 2006 FDA Science Board 1
CDER's Office of Drug Safety: Electronic Tools for Risk Assessment and Evaluation March 31, 2006 FDA Science Board 1
 March 31, 2006 FDA Science Board 2
March 31, 2006 FDA Science Board 2
 Background: Premarket March 31, 2006 FDA Science Board 3
Background: Premarket March 31, 2006 FDA Science Board 3
 Background: Premarket March 31, 2006 FDA Science Board 4
Background: Premarket March 31, 2006 FDA Science Board 4
 Background: Premarket March 31, 2006 FDA Science Board 5
Background: Premarket March 31, 2006 FDA Science Board 5
 March 31, 2006 FDA Science Board 6
March 31, 2006 FDA Science Board 6
 March 31, 2006 FDA Science Board 7
March 31, 2006 FDA Science Board 7
 The Adverse Event Reporting System - AERS March 31, 2006 FDA Science Board 8
The Adverse Event Reporting System - AERS March 31, 2006 FDA Science Board 8
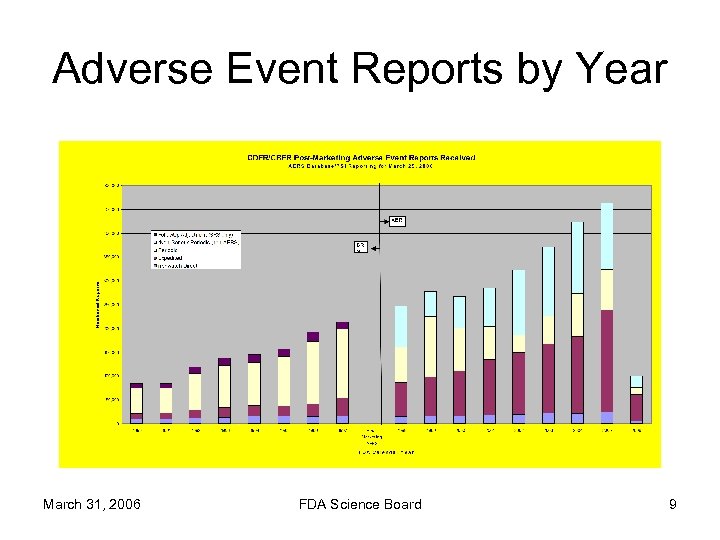 Adverse Event Reports by Year March 31, 2006 FDA Science Board 9
Adverse Event Reports by Year March 31, 2006 FDA Science Board 9
 Data Mining March 31, 2006 FDA Science Board 10
Data Mining March 31, 2006 FDA Science Board 10
 Data Mining in AERS March 31, 2006 FDA Science Board 11
Data Mining in AERS March 31, 2006 FDA Science Board 11
 Web. VDME March 31, 2006 FDA Science Board 12
Web. VDME March 31, 2006 FDA Science Board 12
 Supplements to AERS March 31, 2006 FDA Science Board 13
Supplements to AERS March 31, 2006 FDA Science Board 13
 March 31, 2006 FDA Science Board 14
March 31, 2006 FDA Science Board 14
 March 31, 2006 FDA Science Board 15
March 31, 2006 FDA Science Board 15
 March 31, 2006 FDA Science Board 16
March 31, 2006 FDA Science Board 16
 March 31, 2006 FDA Science Board 17
March 31, 2006 FDA Science Board 17
 March 31, 2006 FDA Science Board 18
March 31, 2006 FDA Science Board 18
 March 31, 2006 FDA Science Board 19
March 31, 2006 FDA Science Board 19
 March 31, 2006 FDA Science Board 20
March 31, 2006 FDA Science Board 20
 March 31, 2006 FDA Science Board 21
March 31, 2006 FDA Science Board 21
 March 31, 2006 FDA Science Board 22
March 31, 2006 FDA Science Board 22
 Epidemiology Contracts March 31, 2006 FDA Science Board 23
Epidemiology Contracts March 31, 2006 FDA Science Board 23
 Epidemiologic Databases March 31, 2006 FDA Science Board 24
Epidemiologic Databases March 31, 2006 FDA Science Board 24
 GPRD March 31, 2006 FDA Science Board 25
GPRD March 31, 2006 FDA Science Board 25
 GPRD March 31, 2006 FDA Science Board 26
GPRD March 31, 2006 FDA Science Board 26
 All Data Sources Are Valuable March 31, 2006 FDA Science Board 27
All Data Sources Are Valuable March 31, 2006 FDA Science Board 27
 Conclusions March 31, 2006 FDA Science Board 28
Conclusions March 31, 2006 FDA Science Board 28