8410fae8b50da03ae1c975763c55df32.ppt
- Количество слайдов: 27
 CDASH Initiative CDASH Project Description & Status Rhonda Facile Project Leader, CDISC
CDASH Initiative CDASH Project Description & Status Rhonda Facile Project Leader, CDISC
 CDASH Project Process • Outline – – – Background and Charter CDISC COP 001 Project Organization Project Process SDTM Review of Progress Next Steps Activities & Project Notes Accomplishments to Date Future Core Team Contact 2
CDASH Project Process • Outline – – – Background and Charter CDISC COP 001 Project Organization Project Process SDTM Review of Progress Next Steps Activities & Project Notes Accomplishments to Date Future Core Team Contact 2
 CDASH Project Process • C-path opportunity #45 • Continues ACRO’s CRF Standardization Initiative Project Charter • To develop a set of ‘content standards’ (element name, definition, metadata) for a basic set of global data collection fields that will support clinical research studies. The initial scope will be the ‘safety data domains’ to support clinical trials. • These safety domains cut across all therapeutic areas, beginning with 12 -14 domains. CDISC COP (COP-001) • Follow the CDISC COP Standards Process (COP-001) 3
CDASH Project Process • C-path opportunity #45 • Continues ACRO’s CRF Standardization Initiative Project Charter • To develop a set of ‘content standards’ (element name, definition, metadata) for a basic set of global data collection fields that will support clinical research studies. The initial scope will be the ‘safety data domains’ to support clinical trials. • These safety domains cut across all therapeutic areas, beginning with 12 -14 domains. CDISC COP (COP-001) • Follow the CDISC COP Standards Process (COP-001) 3
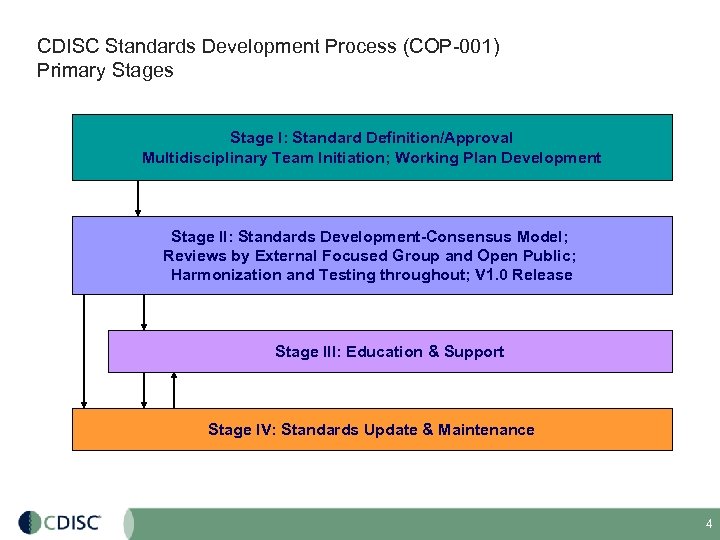 CDISC Standards Development Process (COP-001) Primary Stages Stage I: Standard Definition/Approval Multidisciplinary Team Initiation; Working Plan Development Stage II: Standards Development-Consensus Model; Reviews by External Focused Group and Open Public; Harmonization and Testing throughout; V 1. 0 Release Stage III: Education & Support Stage IV: Standards Update & Maintenance 4
CDISC Standards Development Process (COP-001) Primary Stages Stage I: Standard Definition/Approval Multidisciplinary Team Initiation; Working Plan Development Stage II: Standards Development-Consensus Model; Reviews by External Focused Group and Open Public; Harmonization and Testing throughout; V 1. 0 Release Stage III: Education & Support Stage IV: Standards Update & Maintenance 4
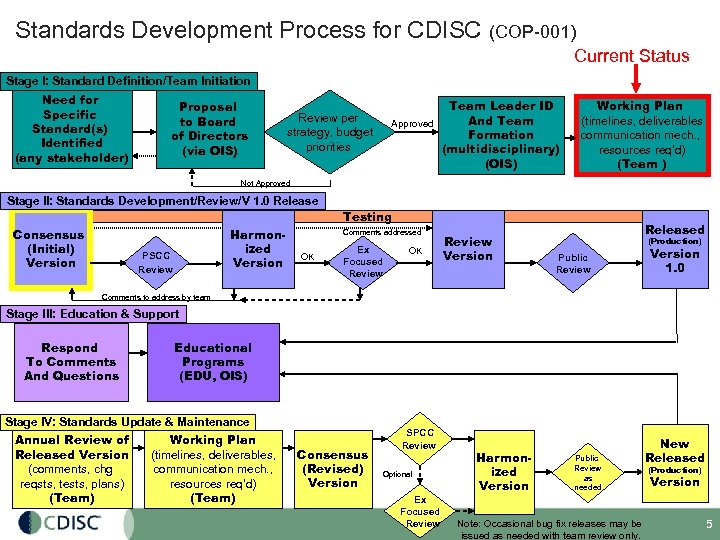 Standards Development Process for CDISC (COP-001) Current Status Stage I: Standard Definition/Team Initiation Need for Specific Standard(s) Identified (any stakeholder) Proposal to Board of Directors (via OIS) Team Leader ID And Team Approved Formation (multidisciplinary) (OIS) Review per strategy, budget priorities Working Plan (timelines, deliverables communication mech. , resources req’d) (Team ) Not Approved Stage II: Standards Development/Review/V 1. 0 Release Testing Consensus (Initial) Version Harmonized Version PSCC Review Comments addressed OK Ex Focused Review OK Review Version Released (Production) Public Review Version 1. 0 Comments to address by team Stage III: Education & Support Respond To Comments And Questions Educational Programs (EDU, OIS) Stage IV: Standards Update & Maintenance Working Plan Annual Review of Released Version (timelines, deliverables, communication mech. , (comments, chg reqsts, tests, plans) resources req’d) (Team) Consensus (Revised) Version SPCC Review Optional Ex Focused Review Harmonized Version Public Review as needed Note: Occasional bug fix releases may be issued as needed with team review only. New Released (Production) Version 5
Standards Development Process for CDISC (COP-001) Current Status Stage I: Standard Definition/Team Initiation Need for Specific Standard(s) Identified (any stakeholder) Proposal to Board of Directors (via OIS) Team Leader ID And Team Approved Formation (multidisciplinary) (OIS) Review per strategy, budget priorities Working Plan (timelines, deliverables communication mech. , resources req’d) (Team ) Not Approved Stage II: Standards Development/Review/V 1. 0 Release Testing Consensus (Initial) Version Harmonized Version PSCC Review Comments addressed OK Ex Focused Review OK Review Version Released (Production) Public Review Version 1. 0 Comments to address by team Stage III: Education & Support Respond To Comments And Questions Educational Programs (EDU, OIS) Stage IV: Standards Update & Maintenance Working Plan Annual Review of Released Version (timelines, deliverables, communication mech. , (comments, chg reqsts, tests, plans) resources req’d) (Team) Consensus (Revised) Version SPCC Review Optional Ex Focused Review Harmonized Version Public Review as needed Note: Occasional bug fix releases may be issued as needed with team review only. New Released (Production) Version 5
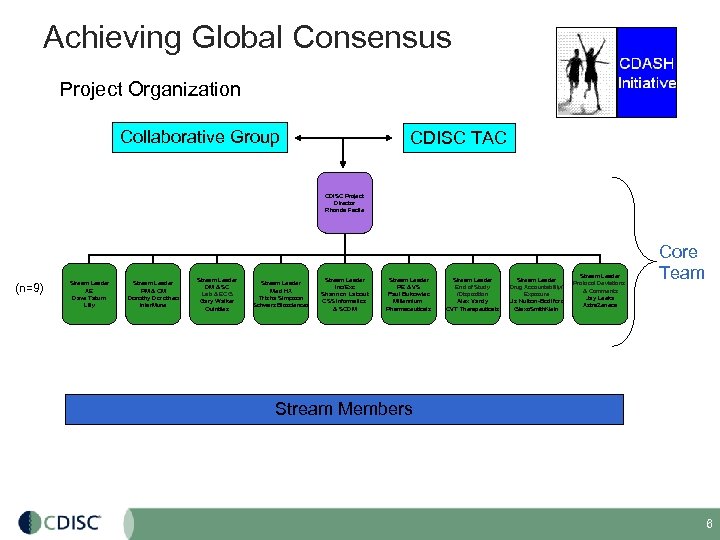 Achieving Global Consensus Project Organization Collaborative Group CDISC TAC CDISC Project Director Rhonda Facile (n=9) Stream Leader AE Dave Tatum Lilly Stream Leader PM & CM Dorothy Dorotheo Inter. Mune Stream Leader DM & SC Lab & ECG Gary Walker Quintiles Stream Leader Med HX Trisha Simpson Schwarz Biosciences Stream Leader Inc/Exc Shannon Labout CSS Informatics & SCDM Stream Leader PE & VS Paul Bukowiec Millennium Pharmaceuticals Stream Leader End of Study /Disposition Alex Vardy CVT Therapeuticals Stream Leader Drug Accountability/ Exposure Liz Nulton-Bodiford Glaxo. Smith. Klein Stream Leader Protocol Deviations & Comments Jay Leeka Astra. Zeneca Core Team Stream Members 6
Achieving Global Consensus Project Organization Collaborative Group CDISC TAC CDISC Project Director Rhonda Facile (n=9) Stream Leader AE Dave Tatum Lilly Stream Leader PM & CM Dorothy Dorotheo Inter. Mune Stream Leader DM & SC Lab & ECG Gary Walker Quintiles Stream Leader Med HX Trisha Simpson Schwarz Biosciences Stream Leader Inc/Exc Shannon Labout CSS Informatics & SCDM Stream Leader PE & VS Paul Bukowiec Millennium Pharmaceuticals Stream Leader End of Study /Disposition Alex Vardy CVT Therapeuticals Stream Leader Drug Accountability/ Exposure Liz Nulton-Bodiford Glaxo. Smith. Klein Stream Leader Protocol Deviations & Comments Jay Leeka Astra. Zeneca Core Team Stream Members 6
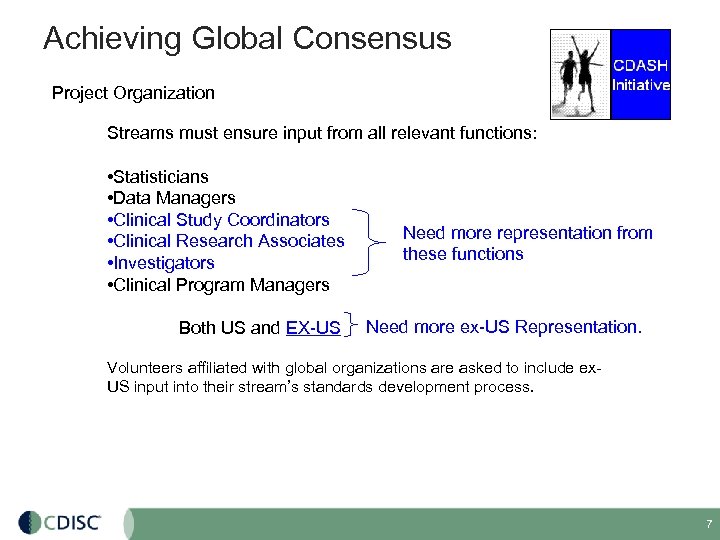 Achieving Global Consensus Project Organization Streams must ensure input from all relevant functions: • Statisticians • Data Managers • Clinical Study Coordinators • Clinical Research Associates • Investigators • Clinical Program Managers Both US and EX-US Need more representation from these functions Need more ex-US Representation. Volunteers affiliated with global organizations are asked to include ex. US input into their stream’s standards development process. 7
Achieving Global Consensus Project Organization Streams must ensure input from all relevant functions: • Statisticians • Data Managers • Clinical Study Coordinators • Clinical Research Associates • Investigators • Clinical Program Managers Both US and EX-US Need more representation from these functions Need more ex-US Representation. Volunteers affiliated with global organizations are asked to include ex. US input into their stream’s standards development process. 7
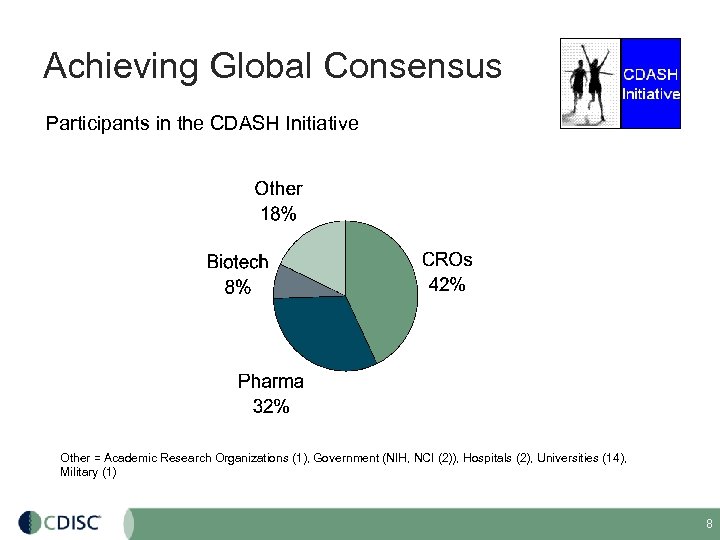 Achieving Global Consensus Participants in the CDASH Initiative Other = Academic Research Organizations (1), Government (NIH, NCI (2)), Hospitals (2), Universities (14), Military (1) 8
Achieving Global Consensus Participants in the CDASH Initiative Other = Academic Research Organizations (1), Government (NIH, NCI (2)), Hospitals (2), Universities (14), Military (1) 8
 CDASH Project Process (1) • Start with SDTM Data Variable Tables Use mandatory SDTM variables as a basis for the CDASH review process. • Refer to ACRO CRF Samples when available • SDTM Variable Tables Focus on CRF Content, not CRF Layout 9
CDASH Project Process (1) • Start with SDTM Data Variable Tables Use mandatory SDTM variables as a basis for the CDASH review process. • Refer to ACRO CRF Samples when available • SDTM Variable Tables Focus on CRF Content, not CRF Layout 9
 CDASH Project Process (2) • Collect CRF samples • Evaluate commonalities/differences of CRF samples and SDTM • Document data points included/excluded with justifications • Guiding principles: variables should – – – Start with the current SDTM Implementation guide & tables Make sure all SDTM required elements are included either directly or indirectly Be “standard” but flexible to allow customization within defined limits Limit variables to required and necessary Comply with regulatory requirements Reduce redundancies; Not duplicate information found elsewhere in CRFs Increase collection of meaningful data Facilitate use of standards by all users Be appropriate for use both pre and post approval studies Allow consistent and efficient data collection/storage/transmission and analysis 10
CDASH Project Process (2) • Collect CRF samples • Evaluate commonalities/differences of CRF samples and SDTM • Document data points included/excluded with justifications • Guiding principles: variables should – – – Start with the current SDTM Implementation guide & tables Make sure all SDTM required elements are included either directly or indirectly Be “standard” but flexible to allow customization within defined limits Limit variables to required and necessary Comply with regulatory requirements Reduce redundancies; Not duplicate information found elsewhere in CRFs Increase collection of meaningful data Facilitate use of standards by all users Be appropriate for use both pre and post approval studies Allow consistent and efficient data collection/storage/transmission and analysis 10
 CDASH Project Process Deliverables (3) • Reach agreement on BASIC (mandatory) CRF Data Elements • Map to SDTM using SDTM variable names (leveraged from the SDS Team) • Add Controlled Terminology (leveraged from the Terminology Team) • Add definitions • Write completion guidelines/instructions • Proceed to the next step in the Consensus Process as specified in COP-001 Technical Leadership Committee (TLC) 11
CDASH Project Process Deliverables (3) • Reach agreement on BASIC (mandatory) CRF Data Elements • Map to SDTM using SDTM variable names (leveraged from the SDS Team) • Add Controlled Terminology (leveraged from the Terminology Team) • Add definitions • Write completion guidelines/instructions • Proceed to the next step in the Consensus Process as specified in COP-001 Technical Leadership Committee (TLC) 11
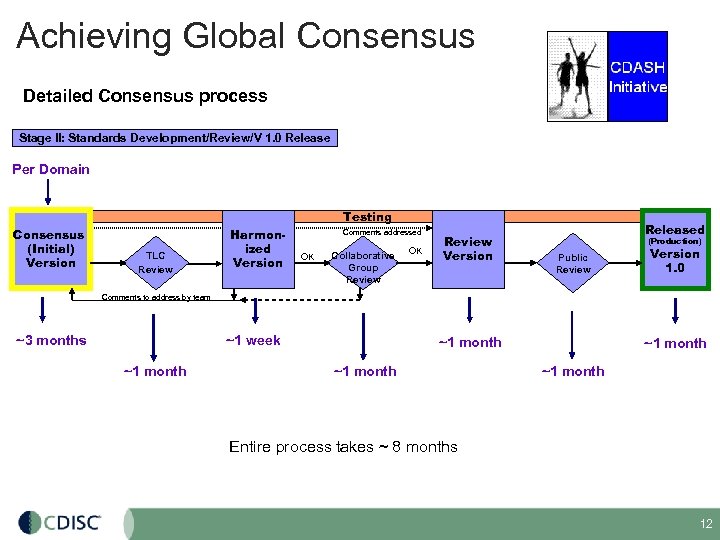 Achieving Global Consensus Detailed Consensus process Stage II: Standards Development/Review/V 1. 0 Release Per Domain Testing Consensus (Initial) Version TLC Review Harmonized Version Comments addressed OK Collaborative Group Review OK Review Version Released (Production) Public Review Version 1. 0 Comments to address by team ~1 week ~3 months ~1 month ~1 month Entire process takes ~ 8 months 12
Achieving Global Consensus Detailed Consensus process Stage II: Standards Development/Review/V 1. 0 Release Per Domain Testing Consensus (Initial) Version TLC Review Harmonized Version Comments addressed OK Collaborative Group Review OK Review Version Released (Production) Public Review Version 1. 0 Comments to address by team ~1 week ~3 months ~1 month ~1 month Entire process takes ~ 8 months 12
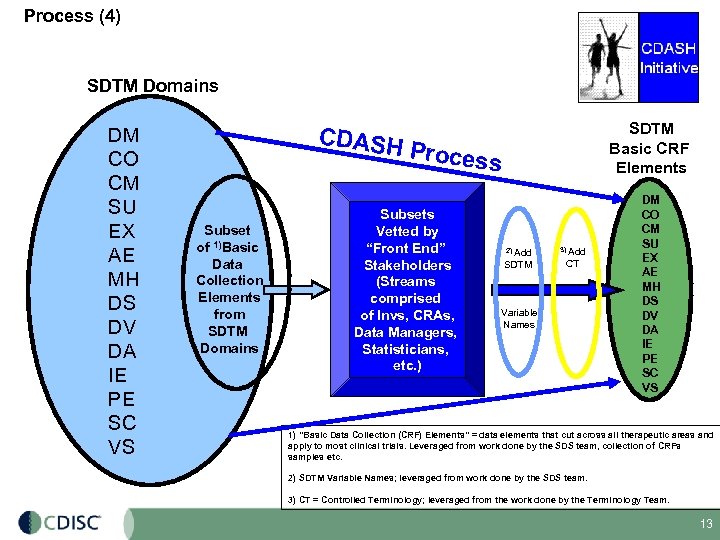 Process (4) SDTM Domains DM CO CM SU EX AE MH DS DV DA IE PE SC VS CDASH Subset of 1)Basic Data Collection Elements from SDTM Domains SDTM Basic CRF Elements Proces Subsets Vetted by “Front End” Stakeholders (Streams comprised of Invs, CRAs, Data Managers, Statisticians, etc. ) s 2) Add SDTM 3) Add CT Variable Names DM CO CM SU EX AE MH DS DV DA IE PE SC VS 1) ”Basic Data Collection (CRF) Elements” = data elements that cut across all therapeutic areas and apply to most clinical trials. Leveraged from work done by the SDS team, collection of CRFs samples etc. 2) SDTM Variable Names; leveraged from work done by the SDS team. 3) CT = Controlled Terminology; leveraged from the work done by the Terminology Team. 13
Process (4) SDTM Domains DM CO CM SU EX AE MH DS DV DA IE PE SC VS CDASH Subset of 1)Basic Data Collection Elements from SDTM Domains SDTM Basic CRF Elements Proces Subsets Vetted by “Front End” Stakeholders (Streams comprised of Invs, CRAs, Data Managers, Statisticians, etc. ) s 2) Add SDTM 3) Add CT Variable Names DM CO CM SU EX AE MH DS DV DA IE PE SC VS 1) ”Basic Data Collection (CRF) Elements” = data elements that cut across all therapeutic areas and apply to most clinical trials. Leveraged from work done by the SDS team, collection of CRFs samples etc. 2) SDTM Variable Names; leveraged from work done by the SDS team. 3) CT = Controlled Terminology; leveraged from the work done by the Terminology Team. 13
 CDASH Project Process • Progress The AE, CM, DM and SC Streams have delivered their Initial Consensus Versions (ICV). The following slides show the basic (mandatory) CRF data collection elements identified by the streams. Note: Conditional and optional data elements were also identified and included in the ICVs, however the initial remit is to focus on the basic data elements. 14
CDASH Project Process • Progress The AE, CM, DM and SC Streams have delivered their Initial Consensus Versions (ICV). The following slides show the basic (mandatory) CRF data collection elements identified by the streams. Note: Conditional and optional data elements were also identified and included in the ICVs, however the initial remit is to focus on the basic data elements. 14
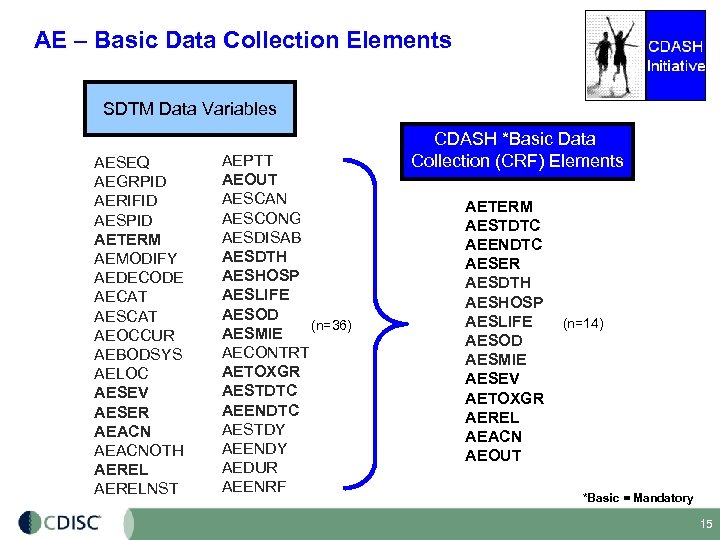 AE – Basic Data Collection Elements SDTM Data Variables AESEQ AEGRPID AERIFID AESPID AETERM AEMODIFY AEDECODE AECAT AESCAT AEOCCUR AEBODSYS AELOC AESEV AESER AEACNOTH AERELNST AEPTT AEOUT AESCAN AESCONG AESDISAB AESDTH AESHOSP AESLIFE AESOD (n=36) AESMIE AECONTRT AETOXGR AESTDTC AEENDTC AESTDY AEENDY AEDUR AEENRF CDASH *Basic Data Collection (CRF) Elements AETERM AESTDTC AEENDTC AESER AESDTH AESHOSP AESLIFE AESOD AESMIE AESEV AETOXGR AEREL AEACN AEOUT (n=14) *Basic = Mandatory 15
AE – Basic Data Collection Elements SDTM Data Variables AESEQ AEGRPID AERIFID AESPID AETERM AEMODIFY AEDECODE AECAT AESCAT AEOCCUR AEBODSYS AELOC AESEV AESER AEACNOTH AERELNST AEPTT AEOUT AESCAN AESCONG AESDISAB AESDTH AESHOSP AESLIFE AESOD (n=36) AESMIE AECONTRT AETOXGR AESTDTC AEENDTC AESTDY AEENDY AEDUR AEENRF CDASH *Basic Data Collection (CRF) Elements AETERM AESTDTC AEENDTC AESER AESDTH AESHOSP AESLIFE AESOD AESMIE AESEV AETOXGR AEREL AEACN AEOUT (n=14) *Basic = Mandatory 15
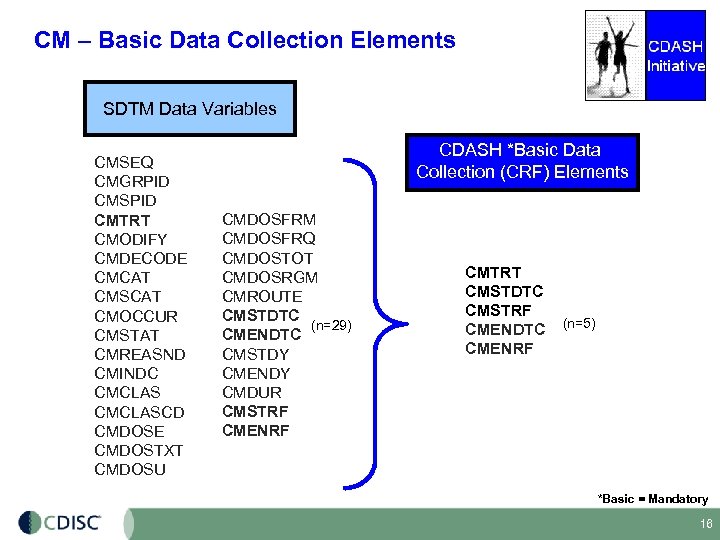 CM – Basic Data Collection Elements SDTM Data Variables CMSEQ CMGRPID CMSPID CMTRT CMODIFY CMDECODE CMCAT CMSCAT CMOCCUR CMSTAT CMREASND CMINDC CMCLASCD CMDOSE CMDOSTXT CMDOSU CDASH *Basic Data Collection (CRF) Elements CMDOSFRM CMDOSFRQ CMDOSTOT CMDOSRGM CMROUTE CMSTDTC (n=29) CMENDTC CMSTDY CMENDY CMDUR CMSTRF CMENRF CMTRT CMSTDTC CMSTRF CMENDTC CMENRF (n=5) *Basic = Mandatory 16
CM – Basic Data Collection Elements SDTM Data Variables CMSEQ CMGRPID CMSPID CMTRT CMODIFY CMDECODE CMCAT CMSCAT CMOCCUR CMSTAT CMREASND CMINDC CMCLASCD CMDOSE CMDOSTXT CMDOSU CDASH *Basic Data Collection (CRF) Elements CMDOSFRM CMDOSFRQ CMDOSTOT CMDOSRGM CMROUTE CMSTDTC (n=29) CMENDTC CMSTDY CMENDY CMDUR CMSTRF CMENRF CMTRT CMSTDTC CMSTRF CMENDTC CMENRF (n=5) *Basic = Mandatory 16
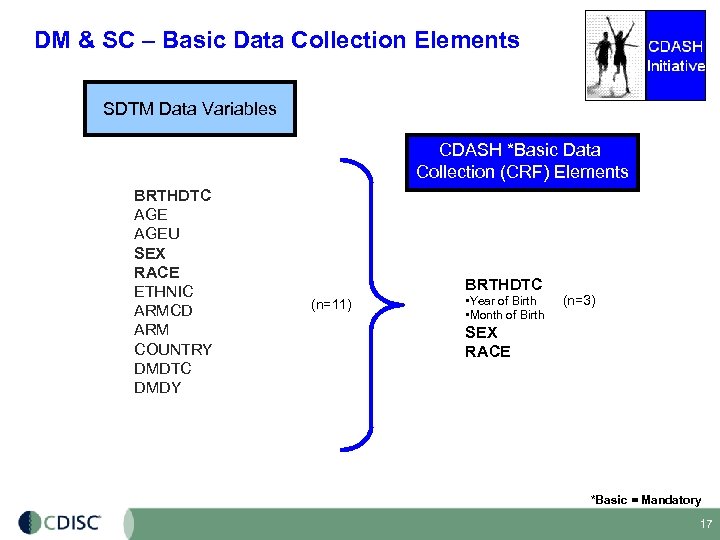 DM & SC – Basic Data Collection Elements SDTM Data Variables CDASH *Basic Data Collection (CRF) Elements BRTHDTC AGEU SEX RACE ETHNIC ARMCD ARM COUNTRY DMDTC DMDY BRTHDTC (n=11) • Year of Birth • Month of Birth (n=3) SEX RACE *Basic = Mandatory 17
DM & SC – Basic Data Collection Elements SDTM Data Variables CDASH *Basic Data Collection (CRF) Elements BRTHDTC AGEU SEX RACE ETHNIC ARMCD ARM COUNTRY DMDTC DMDY BRTHDTC (n=11) • Year of Birth • Month of Birth (n=3) SEX RACE *Basic = Mandatory 17
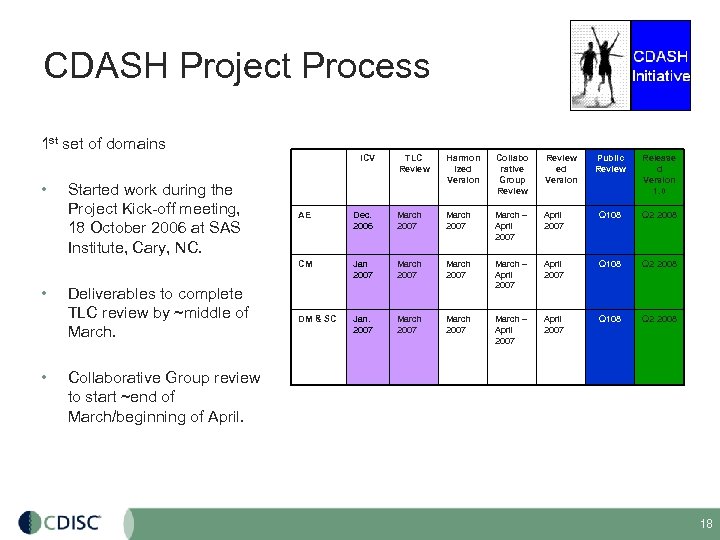 CDASH Project Process 1 st set of domains ICV • Started work during the Project Kick-off meeting, 18 October 2006 at SAS Institute, Cary, NC. TLC Review Harmon ized Version Collabo rative Group Review ed Version Public Review Release d Version 1. 0 • Deliverables to complete TLC review by ~middle of March. Dec. 2006 March 2007 March – April 2007 Q 108 Q 2 2008 CM • AE Jan 2007 March – April 2007 Q 108 Q 2 2008 DM & SC Jan. 2007 March – April 2007 Q 108 Q 2 2008 Collaborative Group review to start ~end of March/beginning of April. 18
CDASH Project Process 1 st set of domains ICV • Started work during the Project Kick-off meeting, 18 October 2006 at SAS Institute, Cary, NC. TLC Review Harmon ized Version Collabo rative Group Review ed Version Public Review Release d Version 1. 0 • Deliverables to complete TLC review by ~middle of March. Dec. 2006 March 2007 March – April 2007 Q 108 Q 2 2008 CM • AE Jan 2007 March – April 2007 Q 108 Q 2 2008 DM & SC Jan. 2007 March – April 2007 Q 108 Q 2 2008 Collaborative Group review to start ~end of March/beginning of April. 18
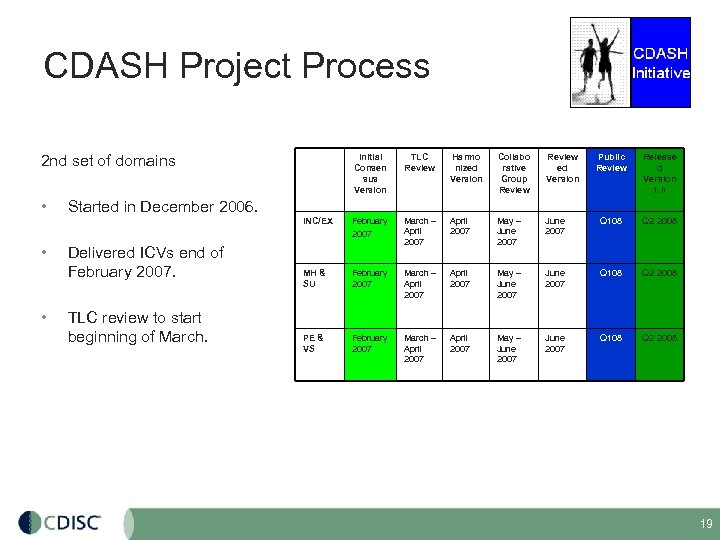 CDASH Project Process 2 nd set of domains • • TLC Review Harmo nized Version Collabo rative Group Review ed Version INC/EX • Initial Consen sus Version Public Review Release d Version 1. 0 February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 MH & SU February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 PE & VS February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 Started in December 2006. Delivered ICVs end of February 2007. TLC review to start beginning of March. 19
CDASH Project Process 2 nd set of domains • • TLC Review Harmo nized Version Collabo rative Group Review ed Version INC/EX • Initial Consen sus Version Public Review Release d Version 1. 0 February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 MH & SU February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 PE & VS February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 Started in December 2006. Delivered ICVs end of February 2007. TLC review to start beginning of March. 19
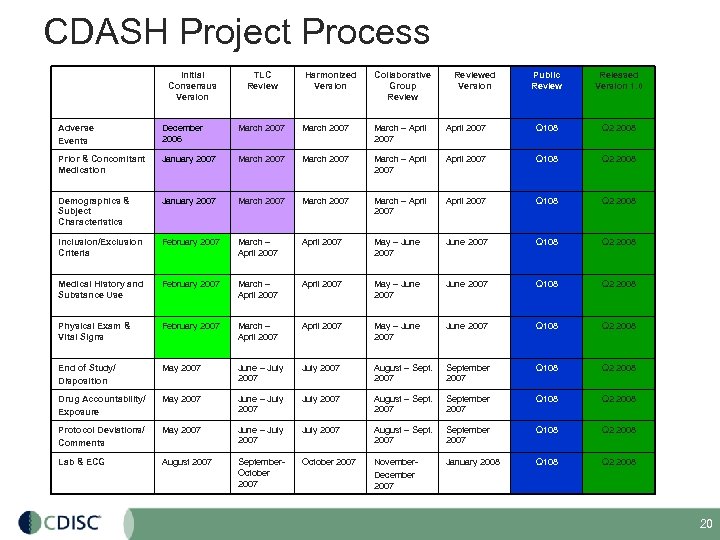 CDASH Project Process Initial Consensus Version TLC Review Harmonized Version Collaborative Group Reviewed Version Public Review Released Version 1. 0 Adverse Events December 2006 March 2007 March – April 2007 Q 108 Q 2 2008 Prior & Concomitant Medication January 2007 March – April 2007 Q 108 Q 2 2008 Demographics & Subject Characteristics January 2007 March – April 2007 Q 108 Q 2 2008 Inclusion/Exclusion Criteria February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 Medical History and Substance Use February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 Physical Exam & Vital Signs February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 End of Study/ Disposition May 2007 June – July 2007 August – Sept. 2007 September 2007 Q 108 Q 2 2008 Drug Accountability/ Exposure May 2007 June – July 2007 August – Sept. 2007 September 2007 Q 108 Q 2 2008 Protocol Deviations/ Comments May 2007 June – July 2007 August – Sept. 2007 September 2007 Q 108 Q 2 2008 Lab & ECG August 2007 September. October 2007 November. December 2007 January 2008 Q 108 Q 2 2008 20
CDASH Project Process Initial Consensus Version TLC Review Harmonized Version Collaborative Group Reviewed Version Public Review Released Version 1. 0 Adverse Events December 2006 March 2007 March – April 2007 Q 108 Q 2 2008 Prior & Concomitant Medication January 2007 March – April 2007 Q 108 Q 2 2008 Demographics & Subject Characteristics January 2007 March – April 2007 Q 108 Q 2 2008 Inclusion/Exclusion Criteria February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 Medical History and Substance Use February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 Physical Exam & Vital Signs February 2007 March – April 2007 May – June 2007 Q 108 Q 2 2008 End of Study/ Disposition May 2007 June – July 2007 August – Sept. 2007 September 2007 Q 108 Q 2 2008 Drug Accountability/ Exposure May 2007 June – July 2007 August – Sept. 2007 September 2007 Q 108 Q 2 2008 Protocol Deviations/ Comments May 2007 June – July 2007 August – Sept. 2007 September 2007 Q 108 Q 2 2008 Lab & ECG August 2007 September. October 2007 November. December 2007 January 2008 Q 108 Q 2 2008 20
 CDASH Project Process • The 3 rd set of domains (DA& EX, CM & DV, End of Study/DS) will be initiated at the beginning of March. • 4 th set of domains (Lab & ECG) to start beginning of June. 21
CDASH Project Process • The 3 rd set of domains (DA& EX, CM & DV, End of Study/DS) will be initiated at the beginning of March. • 4 th set of domains (Lab & ECG) to start beginning of June. 21
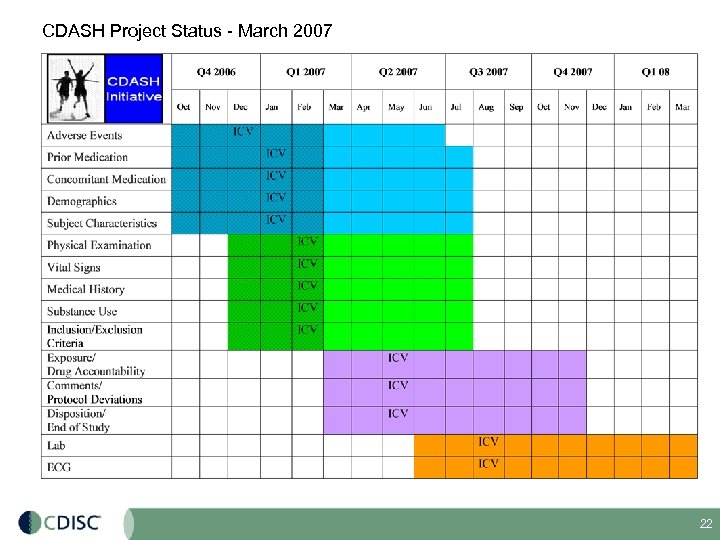 CDASH Project Status - March 2007 22
CDASH Project Status - March 2007 22
 Achieving Global Consensus CDASH Activities & Project Notes • • • Webinar - Held 22 January. Press Release – soon! Teleconferencing – continues q 2 weeks Core Team contacts – frequent, TCs q 2 weeks. CDASH web portal - up and used. To expand access to new streams. • CDISC web site – a valuable source of info. Will facilitate public review phase of project. 23
Achieving Global Consensus CDASH Activities & Project Notes • • • Webinar - Held 22 January. Press Release – soon! Teleconferencing – continues q 2 weeks Core Team contacts – frequent, TCs q 2 weeks. CDASH web portal - up and used. To expand access to new streams. • CDISC web site – a valuable source of info. Will facilitate public review phase of project. 23
 Achieving Global Consensus Accomplishments to Date: Ø Successful Kick-off meeting (October 2006) Ø List of over 260 volunteers (varying degrees of participation) Ø Initiation of 1 st set of domains Ø Delivery of ICVs for 1 st set of domains Ø Initiation of 2 nd set of domains Ø CDASH web portal – up Ø Successful CDASH Webinar held (333 Attendees – 53 International) Ø Delivery of ICVs for 2 nd set of domains Ø Initiation of 3 rd set of domains 24
Achieving Global Consensus Accomplishments to Date: Ø Successful Kick-off meeting (October 2006) Ø List of over 260 volunteers (varying degrees of participation) Ø Initiation of 1 st set of domains Ø Delivery of ICVs for 1 st set of domains Ø Initiation of 2 nd set of domains Ø CDASH web portal – up Ø Successful CDASH Webinar held (333 Attendees – 53 International) Ø Delivery of ICVs for 2 nd set of domains Ø Initiation of 3 rd set of domains 24
 CDASH Project Process Future? • • Standardize layout Standardize edit language Standardize basic therapeutic area standards? Support CDISC Forum/Help Desk New Domains to be “CDASHed” Update of CDASH developed domains SLs and Stream Members to support TLC in future reviews of new standards 25
CDASH Project Process Future? • • Standardize layout Standardize edit language Standardize basic therapeutic area standards? Support CDISC Forum/Help Desk New Domains to be “CDASHed” Update of CDASH developed domains SLs and Stream Members to support TLC in future reviews of new standards 25
 Achieving Global Consensus CDASH Core Team Contact Information • Rhonda Facile rfacile@cdisc. org • Gary Walker gary. walker@quintiles. com • Dorothy Dorotheo DDorotheo@intermune. com • David E. Tatum TATUM_DAVID_E@LILLY. COM • Paul Bukowiec Paul. Bukowiec@mpi. com • Trisha Simpson Trisha. Simpson@schwarzbiosciences. com • Shannon Labout shannon. labout@charter. net • Liz Nulton-Bodiford liz. m. nulton-bodiford@gsk. com • Jay Leeka Jay. Leeka@astrazeneca. com • Alec Vardy Alec. Vardy@cvt. com 26
Achieving Global Consensus CDASH Core Team Contact Information • Rhonda Facile rfacile@cdisc. org • Gary Walker gary. walker@quintiles. com • Dorothy Dorotheo DDorotheo@intermune. com • David E. Tatum TATUM_DAVID_E@LILLY. COM • Paul Bukowiec Paul. Bukowiec@mpi. com • Trisha Simpson Trisha. Simpson@schwarzbiosciences. com • Shannon Labout shannon. labout@charter. net • Liz Nulton-Bodiford liz. m. nulton-bodiford@gsk. com • Jay Leeka Jay. Leeka@astrazeneca. com • Alec Vardy Alec. Vardy@cvt. com 26
 Achieving Global Consensus Thanks to all the CDASH project volunteers for their time and commitment to the CDASH Project. 27
Achieving Global Consensus Thanks to all the CDASH project volunteers for their time and commitment to the CDASH Project. 27


