21096122eb46e8ec98966600cbce285a.ppt
- Количество слайдов: 15

CCD and CCR Executive Summary Jacob Reider, MD Medical Director, Allscripts

Standard A technical standard is an established norm or requirement. It is often represented as a formal document that establishes uniform technical criteria.



What is an SDO? A standards organization, standards body, standards development organization or SDO is any entity whose primary activities are developing, coordinating, promulgating, revising, amending, reissuing, interpreting, or otherwise maintaining standards that address the interests of a wide base of users outside the standards development organization.

SDOs with Healthcare Standards • • HL 7 ASTM ISO CEN (note that IHE is NOT an SDO. . IHE defines profiles that leverage existing standards)
Moving Patient Information

CCR 0. 005 - circa 1200 BC
Clinical Document Architecture “The CDA Release 2. 0 provides an exchange model for clinical documents (such as discharge summaries and progress notes) …. By leveraging the use of XML, the HL 7 Reference Information Model (RIM) and coded vocabularies, the CDA makes documents both machine-readable - ; so they are easily parsed and processed electronically - and human-readable - so they can be easily retrieved and used by the people who need them. CDA documents can be displayed using XML-aware Web browsers or wireless applications such as cell phones. While Release 2. 0 retains the simplicity of rendering and clear definition of clinical documents formulated in Release 1. 0 (2000), it provides state-of-the-art interoperability for machine-readable coded semantics. The product of 5 years of improvements, CDA R 2 body is based on the HL 7 Clinical Statement model, is fully RIM-compliant and capable of driving decision support and other sophisticated applications, while retaining the simple rendering of legally-authenticated narrative. ” Source: http: //www. hl 7. org/Library/standards_non 1. htm

HL 7 CDA • HL 7’s Clinical Document Architecture (CDA) is a standard for the storage or transfer of clinical documents in or between systems. – Documents: discharge summaries, progress notes, history and physical reports, prior lab results, etc. • CDA defines a generic structure for delivering “any document” between systems. • All messaging standards are based on the HL 7 Reference Information Model (RIM) • HL 7 Version 3 Messaging implements the RIM and provides an XML representation
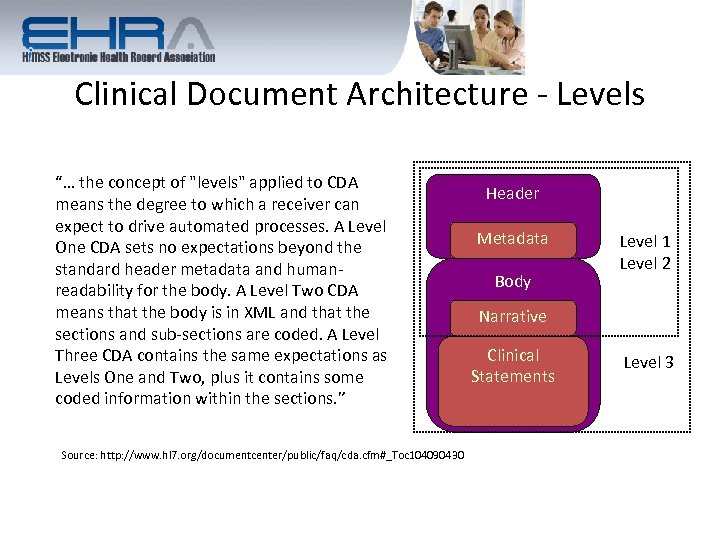
Clinical Document Architecture - Levels “… the concept of "levels" applied to CDA means the degree to which a receiver can expect to drive automated processes. A Level One CDA sets no expectations beyond the standard header metadata and humanreadability for the body. A Level Two CDA means that the body is in XML and that the sections and sub-sections are coded. A Level Three CDA contains the same expectations as Levels One and Two, plus it contains some coded information within the sections. ” Source: http: //www. hl 7. org/documentcenter/public/faq/cda. cfm#_Toc 104090430 Header Metadata Body Level 1 Level 2 Narrative Clinical Statements Level 3

CCR In a nutshell Think of the CCR standard as a way to collect summary health information from one or more sources - such as diagnoses, medications, allergies, and insurance info - and organize this in a single patient-centric XML file that permits: ü Expression of the information as a web page, a Microsoft Word document, or an Adobe pdf document for viewing on a computer screen or printed out on paper ü Secure carriage and transmission of the electronic file via physical transport media, e. g. USB thumb drive, cell phone, CD ROM, or smart card ü Secure transmission of the electronic file via a network, e. g. LAN, T 1 line, or the Internet ü Creation, editing, management, and reporting of the data using both proprietary electronic health records (EHRs) and (nearly) free desktop computing software, e. g. web forms, pdf forms, Firefox plug-in, and other familiar applications, as well as commercial PHRs

HL 7 Continuity of Care Document • HL 7 to created a template to present the ASTM CCR data set in the Clinical Document Architecture Release Two (CDA R 2) • The HL 7 CCD is likely the first “Level 3” CDA template • The HL 7 CCD template places a series of constraints on CDA R 2
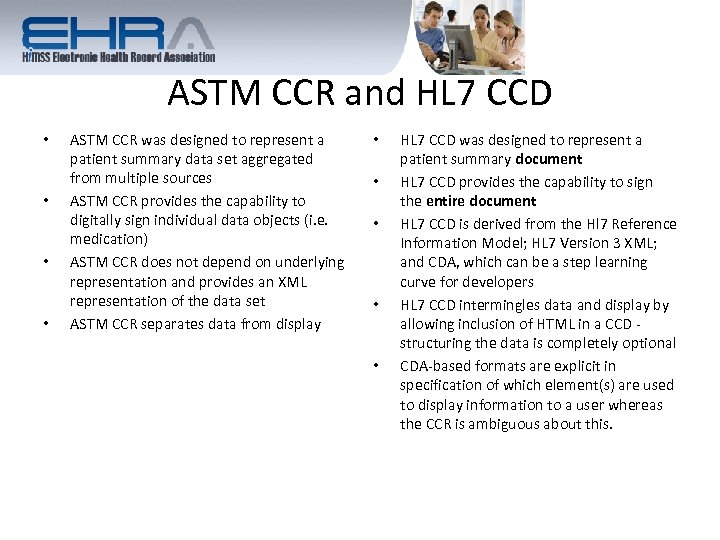
ASTM CCR and HL 7 CCD • • ASTM CCR was designed to represent a patient summary data set aggregated from multiple sources ASTM CCR provides the capability to digitally sign individual data objects (i. e. medication) ASTM CCR does not depend on underlying representation and provides an XML representation of the data set ASTM CCR separates data from display • • • HL 7 CCD was designed to represent a patient summary document HL 7 CCD provides the capability to sign the entire document HL 7 CCD is derived from the Hl 7 Reference Information Model; HL 7 Version 3 XML; and CDA, which can be a step learning curve for developers HL 7 CCD intermingles data and display by allowing inclusion of HTML in a CCD - structuring the data is completely optional CDA-based formats are explicit in specification of which element(s) are used to display information to a user whereas the CCR is ambiguous about this.

Translation? CCR CCD? Several initiatives underway to provide translation. See discussion: • http: //www. ccrstandard. com/ccrnews • http: //www. recordsforliving. com/ • Mirth may @ some point have Open Source Channels for CCR transforms. (http: //www. webreachinc. com/ )
21096122eb46e8ec98966600cbce285a.ppt