4c941797aa5457ec97d51bad3572842d.ppt
- Количество слайдов: 121

CBRNE Bomb Medic © Lt. Steven M. Albright – Paramedic / SC-EMS. com

CBRNE • • • Chemical Biological Radiological Nuclear Explosive

CBRNE Definition – The American Heritage Dictionary • Chemical • A substance with a distinct molecular composition that is produced by or used in a chemical process • Biological • Of, relating to, caused by, or affecting life or living organisms • Radiological • The use of ionizing radiation for medical diagnosis, especially the use of x-rays in medical radiography or fluoroscopy • The use of radiation for the scientific examination of material structures • Nuclear • Of, using, or possessing atomic or hydrogen bombs • Explosives • Things that go BOOM!!! (But you already know that)

Method of Delivery CBRNE as it pertains to “Bomb Medics”

High Explosives - Bursting Device Biological/Chemical Sprayer • High Explosives would most likely be utilized to deliver radiological material over a wide geographical area • Bursting devices would most likely utilize a low energy delivery system (deflagration) to distribute chemical or biological agents which are sensitive to heat and pressure • Chemical and/or Biological sprayers could also incorporate a compressed gas system or mechanical means to disperse the material

Simulated Devices

Dirty Bombs Radiological Dispersal Device

What is a Dirty Bomb? • The term “dirty bomb” is most often used to refer to a Radiological Dispersal Device (RDD) • In reality, a “dirty bomb” is any explosive device that incorporates chemical, biological or nuclear material in it’s design with the intent to cause adverse health effects by dispersal of the material upon detonation.

Dirty Bomb • Radiation Dispersal Weapon • “Weapon of Mass Disruption” • Uses conventional explosives to disperse radioactive debris • Used to create terror and economic damage but causes little physical blast damage • Considered the easiest manageable WMD • • Contain and remove persons from contaminated area Remove and bag clothing Shower decontamination Avoid internal contamination (inhalation, ingestion or injury)
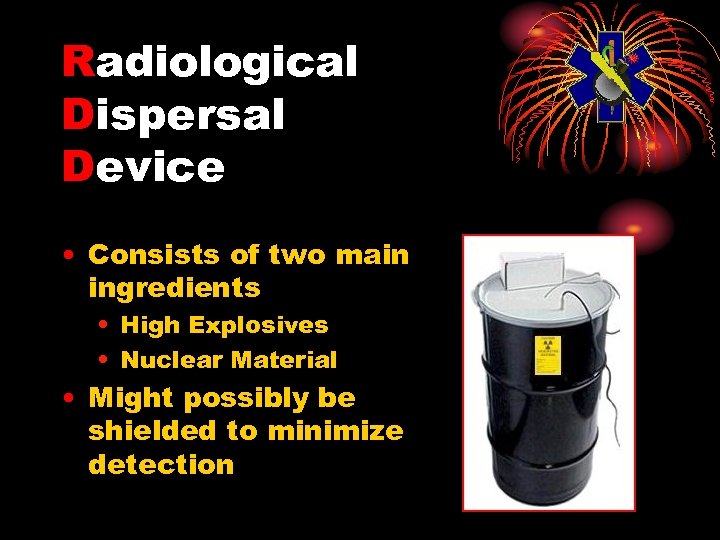
Radiological Dispersal Device • Consists of two main ingredients • High Explosives • Nuclear Material • Might possibly be shielded to minimize detection

Nuclear Material

What is Radioactivity? • Radioactivity is the property or condition of some elements that results in the spontaneous transformation, or decay, of the nucleus of an unstable atom • As the unstable atom tries to change to a stable state, it releases excess energy in the form of radiation • Substances that undergo this transformation process are called radioisotopes, or more generally, radioactive materials • Once the substances are processed into a usable form, such as pellets or powder, they are called radioactive sources
What are Isotopes? • Ninety-two naturally occurring elements make up all substances on Earth. In addition, scientists have made more than another dozen elements • Each element has unique chemical properties • Each element comes in different forms, called isotopes, which differ in their nuclear properties from the original element but have the same chemical properties • All isotopes within an element’s family contain the same number of protons, but have different numbers of neutrons
What are Isotopes? • The number of protons determines the chemical properties, and the combined number of neutrons and protons determines the nuclear properties • In general, isotopes are either stable or unstable • Unstable isotopes are called radioisotopes because they emit radiation and decay to either other unstable or stable isotopes • Radioactive sources are made from radioisotopes • Knowing the type, energy, decay rate, and amount of radiation of particular radioisotopes helps to characterize the security risk posed by a radioactive source

What is Ionizing Radiation? • Ionizing radiation, which has the ability to strip electrons from atoms and break chemical bonds, leading to possible human cell damage, comes in three types: alpha, beta, and gamma. • Alpha radiation is a stream of alpha particles, each with a helium nucleus consisting of two protons and two neutrons. • Beta radiation is the emission of high-speed electrons or their positively charged counterparts (positrons). • Gamma radiation consists of highly energetic light, and differs from alpha and beta radiation in that it is massless and uncharged. It often accompanies the emission of alpha or beta radiation from a particular radioisotope

Radioactive Material • • • Americium-241 Californium-252 Cesium-137 Cobalt-60 Iodine-129/131 • • • Iridium-192 Plutonium Radium-226 Strontium-90 Uranium

Americium-241 (ăm'-erĭsh'ē-uh m) • Americium-241 metal has a white and silvery luster, at room temperature • Used in smoke detector manufacturing • Used as a portable gamma ray source for use in radiography • Gram quantities of 241 Am emit intense gamma rays which create a serious exposure problem for anyone handling the element • Mode of Decay – alpha particles & gamma radiation • Half Life – 432. 2 years

Californium-252 (kal-uh-fawr-nee-uh m) • Californium-252 is a rare earth element, unknown color, but is suspected of having a white and silvery luster, at room temperature • Californium-252 has several medical and industrial applications, including its use in cancer therapy • It is also used to detect explosive devices in airline luggage • Gram quantities of 252 Cf emit alpha particles and is a very strong neutron emitter (one microgram spontaneously emits 170 million neutrons per minute) • Mode of Decay – alpha particles & neutron emission • Half Life – 2. 65 years

Cesium-137 (sē'zē-uh m) • Cesium-137 is one of the most common radioisotopes used in industry • Cesium-137 is produced in nuclear explosions and during the breakdown of uranium in fuel elements • Principle source of radiation in the “exclusion zone” around Chernobyl after the 1986 meltdown • Used in moisture density gauges, leveling gauges, thickness gauges, and well logging devices • Used in medical therapy to treat cancer • Mode of Decay – beta & gamma radiation • Half Life – 30. 17 years

Cobalt-60 (koh-bawlt) • Cobalt-60 is produced in linear accelerators or as a byproduct of steel exposed to neutron radiation • Cobalt-60 is a hard brittle grey metal with a bluish tint • Used in leveling gauges, thickness gauges, in “cold pasteurization” (gamma rays kill bacteria in food) and in industrial radiography • Used in medical therapy to treat cancer • Mode of Decay – beta & gamma radiation • Half Life – 5. 27 years
Iodine-129/131 (ahy-uh-dahyn) • Iodine 129 and Iodine 131 are produced by the fission of uranium in nuclear reactors or plutonium in nuclear weapon detonation • Because of it’s short half life, Iodine-131 is used extensively in nuclear medicine • Iodine's chemical properties make it easy to attach to molecules for imaging studies • Iodine-129 has little practical use, but may be used to check some radioactivity counters in diagnostic test equipment • Mode of Decay – beta & gamma radiation • Half Life (I-129) – 15. 7 million years • Half Life (I-131) – 8. 06 days

Iridium-192 (i-rid-ee-uh m) • Iridium-192 is a shiny silvery-white very dense metal that will not rust • Iridium-192 is produced from non radioactive Iridium in a nuclear reactor • Used in industrial gauges that check welding seams • Used in medical therapy, in the form of tiny seeds about the size of a grain of rice, to treat cancer • Mode of Decay – beta & gamma radiation • Half Life – 74 days

Plutonium (ploo-toh-nee-uh m) • Plutonium is a silvery-grey metal that becomes yellowish when exposed to air • Plutonium is created from uranium in nuclear reactors by bombarding special fuel rods containing uranium with neutrons • Plutonium-239 is used to make nuclear weapons or combined with uranium to produce nuclear fuel rods • Most dangerous when inhaled (limited effect on GI tract via ingestion) • Mode of Decay – alpha particles • Half Life (Pu-238) – 87. 7 years • Half Life (Pu-239) – 24, 110 years • Half Life (Pu-240) – 6, 564 years

Radium-226 (rey-dee-uh m) • Radium-226 is almost pure white, but readily oxidizes to turn almost black in color • Radium-226 was formerly used to paint watch dials • Radium-226 is a naturally occurring radioisotope, formed by the decay of uranium 238 • Gram quantities of 226 Ra emit alpha particles and gamma radiation • Mode of Decay – alpha Particles, gamma radiation and ultimately decays into radon gas • Half Life – 1600 years

Strontium-90 (stron-shee-uh m) • Strontium-90 is a shiny silvery metal that rapidly turns yellow when exposed to air • Strontium-90 is a byproduct of the nuclear fission of uranium and plutonium • Strontium-90 emits a beta particle with no gamma radiation • The heat generated by strontium-90's radioactive decay can be converted to electricity for long-lived, light-weight power supplies • Limited use in bone cancer due to it’s calcium like properties • Mode of Decay – beta radiation • Half Life – 29. 1 years

Uranium (yoo-rey-nee-uh m) • Uranium is a naturally-occurring element found at low levels in virtually all rock, soil, and water • When refined, uranium is a silvery white, weakly radioactive metal. Uranium metal has very high density, 65% more dense than lead • The main use of uranium, in the civilian sector, is to fuel commercial nuclear power plants • When it is depleted (DU), uranium is used by the military as shielding and also in parts of bullets and missiles • Mode of Decay – alpha particles • Half Life (U-235) – 700 million years • Half Life (U-238) – 4. 47 billion years

The Threat • According to "Commercial Radioactive Sources: Surveying the Security Risks, " Occasional Paper No. 11, 01 Sept 2005… the top seven radioisotopes, that would pose inherent radiological security risks, are americium-241, californium-252, cesium-137, cobalt-60, iridium-192, plutonium-238, strontium-90 and radium-226 • Radium-226, because it is naturally occurring, is not subject to the licensing rules governing the other reactor-produced radioisotopes. • These radioisotopes (except for certain restrictions on plutonium, americium, and californium) can be exported to almost all countries (except for Iran, Iraq and NK) • OP No. 11 can be viewed at www. cns. miis. edu

Suitcase Nukes
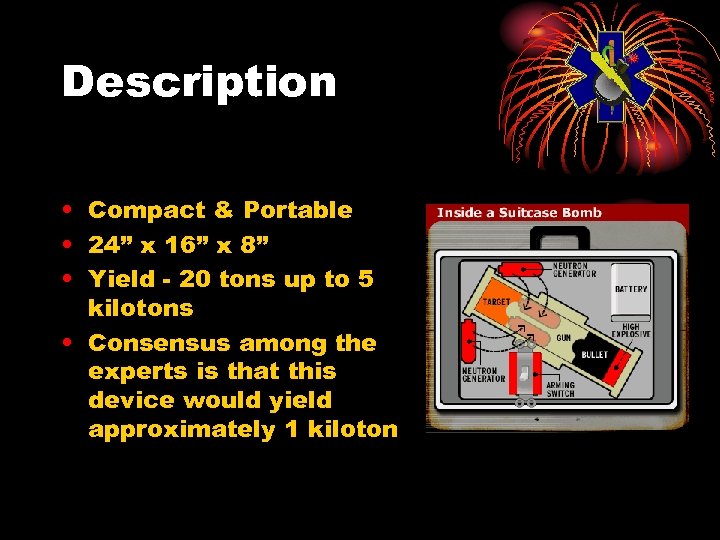
Description • Compact & Portable • 24” x 16” x 8” • Yield - 20 tons up to 5 kilotons • Consensus among the experts is that this device would yield approximately 1 kiloton

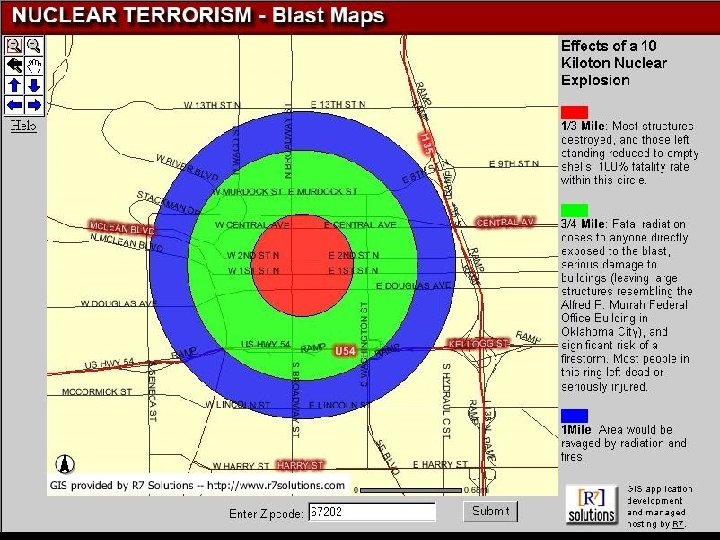
One Kiloton Yield
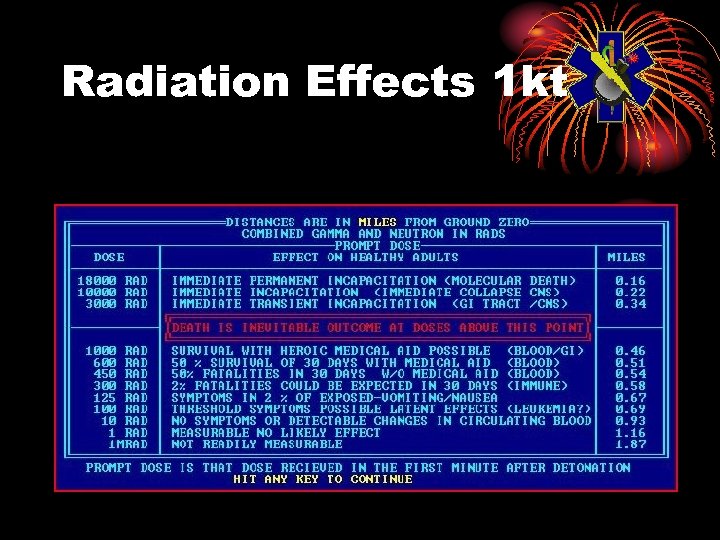
Radiation Effects 1 kt

The Flash • Last several millionths of a second • Causes temporary blindness from indirect exposure • Causes permanent blindness (Choriretinal Burns) if looking directly at the flash
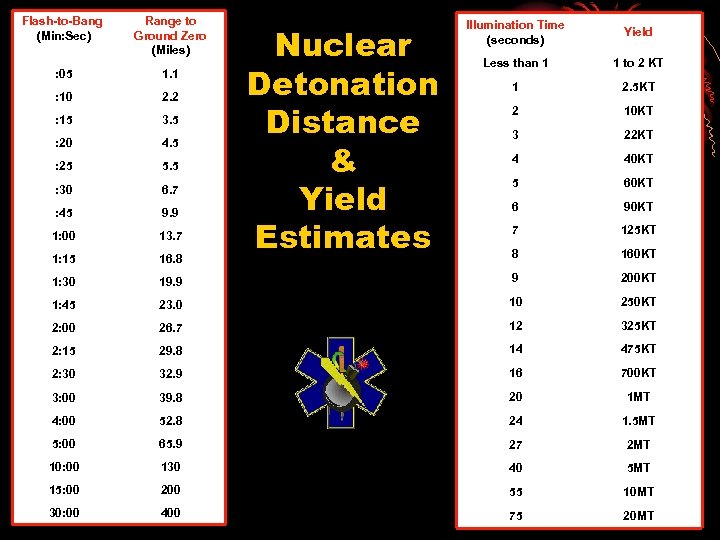
Flash-to-Bang (Min: Sec) Range to Ground Zero (Miles) : 05 1. 1 : 10 2. 2 : 15 3. 5 : 20 4. 5 : 25 5. 5 : 30 6. 7 : 45 9. 9 1: 00 13. 7 1: 15 16. 8 1: 30 Illumination Time (seconds) Yield Less than 1 1 to 2 KT 1 2. 5 KT 2 10 KT 3 22 KT 4 40 KT 5 60 KT 6 90 KT 7 125 KT 8 160 KT 19. 9 9 200 KT 1: 45 23. 0 10 250 KT 2: 00 26. 7 12 325 KT 2: 15 29. 8 14 475 KT 2: 30 32. 9 16 700 KT 3: 00 39. 8 20 1 MT 4: 00 52. 8 24 1. 5 MT 5: 00 65. 9 27 2 MT 10: 00 130 40 5 MT 15: 00 200 55 10 MT 30: 00 400 75 20 MT Nuclear Detonation Distance & Yield Estimates

Initial Radiation • Total radiation released during the first 60 -100 seconds of detonation • Represents 10% of weapons energy • Source of neutron radiation • Consists of a neutron particle • 10 times more destructive to biologicals than gamma rays

Fireball • Reaches temperatures of millions of degrees Fahrenheit • Converts all matter into gaseous form • Lasts no longer than 30 -90 seconds • A 100 KT Blast • Ground burst – visible for 400+ miles • Air burst – visible 700 miles away

Thermal Radiation • Lasts less than one minute and travels at the speed of light • Represents 35% of the weapons energy • Consists of two pulses: • Short duration UV pulse (eye damage) • Visible and infrared (ignites combustibles material, skin burns, eye injury)

Blast Effect • Represents 50% of weapon’s energy • Consists of 3 phases for ground bursts: • Positive Overpressure • Blast winds • Negative Overpressure
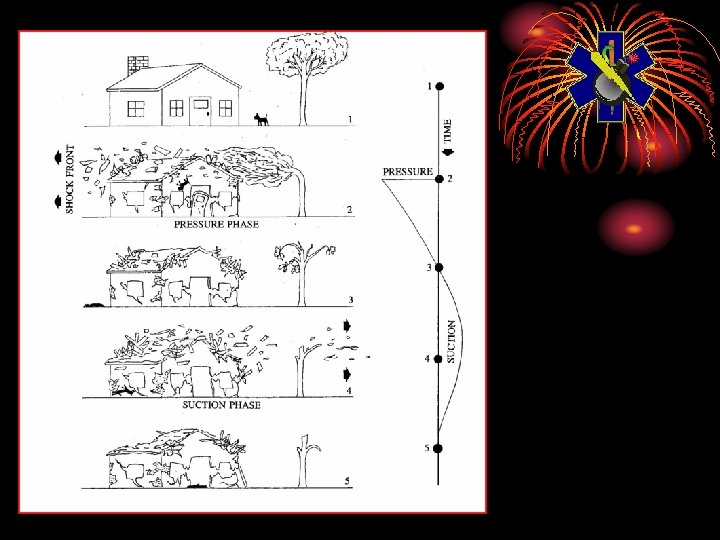
Blast Effect

Ionizing Radiation • • Alpha Particles Beta Particles Neutron Particles Gamma Rays

Ionizing Radiation • Alpha Particles • Source: Plutonium, Uranium, & other alpha emitting radioactive elements • Shielding: Paper, Skin • Hazard: Internal contamination only

Ionizing Radiation • Beta Particles (electrons) • Source: Radioisotope decay • Shielding: Clothing • Hazard: Internal and skin contamination

Ionizing Radiation • Gamma Rays • Source: Radioisotope decay following Beta decay • Shielding: Concrete, lead • Hazard: Whole body irradiation contamination only

Ionizing Radiation • Neutron Particles • Source: Nuclear detonations & Nuclear power plants • Shielding: High hydrogen content (water) • Hazard: Whole body irradiation

The Golden Rule • Time • Distance • Shielding/Shelter

Time Radioactive Decay The 7 -10 Rule For every seven multiples of time, there is a 10 fold decrease in radiation • If the initial radiation shows 1000 R/hr • At 7 hours it will show 100 R/hr • At 49 hours, it will show 10 R/hr • At 343 hours (14 days), it will show 1 R/hr

Distance What is a safe Distance? • The farther away people are from a radiation source, the less their exposure. • How close to a source of radiation can you be without getting a high exposure? • It depends on the energy of the radiation and the size (or activity) of the source. Distance is a prime concern when dealing with gamma rays, because they can travel long distances. Alpha and beta particles don't have enough energy to travel very far. • As a rule, if you double the distance, you reduce the exposure by a factor of four. Halving the distance, increases the exposure by a factor of four.
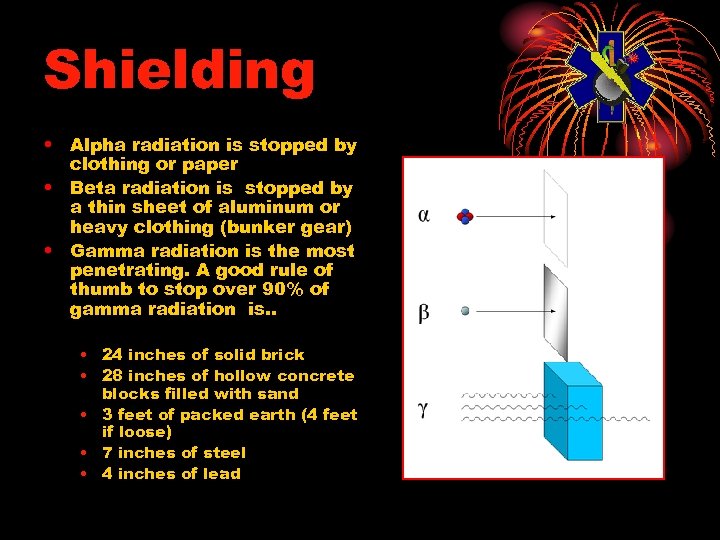
Shielding • Alpha radiation is stopped by clothing or paper • Beta radiation is stopped by a thin sheet of aluminum or heavy clothing (bunker gear) • Gamma radiation is the most penetrating. A good rule of thumb to stop over 90% of gamma radiation is. . • 24 inches of solid brick • 28 inches of hollow concrete blocks filled with sand • 3 feet of packed earth (4 feet if loose) • 7 inches of steel • 4 inches of lead

Units of Measure
![Units of Measure • Rad [R] (Radiation Absorbed Dose) • 1 Rad = 100 Units of Measure • Rad [R] (Radiation Absorbed Dose) • 1 Rad = 100](https://present5.com/presentation/4c941797aa5457ec97d51bad3572842d/image-50.jpg)
Units of Measure • Rad [R] (Radiation Absorbed Dose) • 1 Rad = 100 ergs of energy deposited in 1 gram of material. • Rem (Roentgen Equivalent in Man) • Rems = Rads times a biological factor for different types of radiation (Man = 1) • Gray [Gy] is the System International (SI) unit • 1 Gy = 100 Rads (has replaced the Rad) • Sievert [Sv] is the SI unit in terms of Rems • 1 Sv = 100 Rems (has replaced the Rem)

Radiation Monitors • There are several brands of radiation monitors on the market and each brand has several models designed for specific functions • The ideal radiation monitor for the bomb medic would be a pocket or key chain device that can be carried on your person at all times!!

Exposure & Treatment

Normal Exposures • • • Sleeping next to human - 0. 1 m. R Flying in aircraft - 0. 5 m. R 3 -mile Island accident - 1. 5 m. R Exposure to smoke detectors - 3. 5 m. R/yr Working in capitol building - 20 m. R Cosmic rays & terrestrial sources - 25 m. R/yr Medical diagnostics - 93 m. R/yr Radon - 200 m. R/yr Smoking tobacco - 280 m. R/pack yr Radiation worker - 5000 m. R/yr Decrease in sperm count - 15 R Cancer Rx - 5000 R
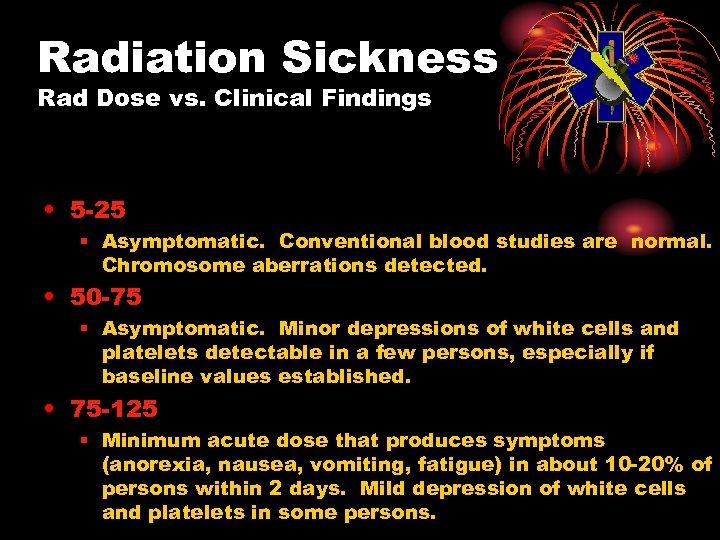
Radiation Sickness Rad Dose vs. Clinical Findings • 5 -25 § Asymptomatic. Conventional blood studies are normal. Chromosome aberrations detected. • 50 -75 § Asymptomatic. Minor depressions of white cells and platelets detectable in a few persons, especially if baseline values established. • 75 -125 § Minimum acute dose that produces symptoms (anorexia, nausea, vomiting, fatigue) in about 10 -20% of persons within 2 days. Mild depression of white cells and platelets in some persons.
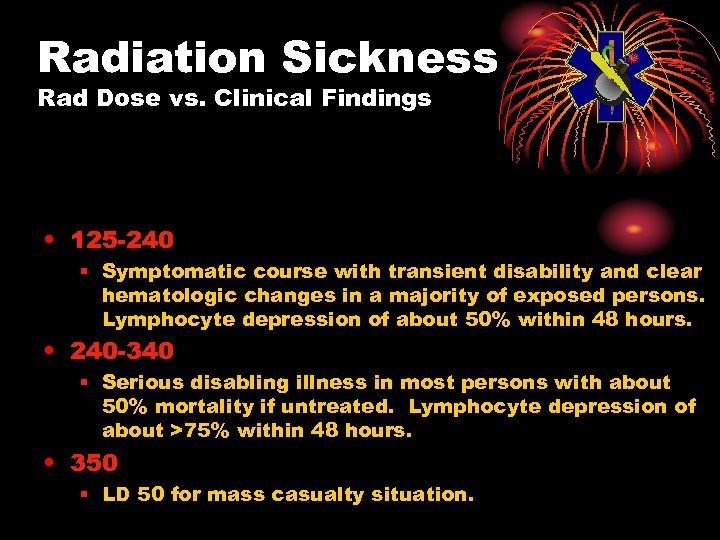
Radiation Sickness Rad Dose vs. Clinical Findings • 125 -240 § Symptomatic course with transient disability and clear hematologic changes in a majority of exposed persons. Lymphocyte depression of about 50% within 48 hours. • 240 -340 § Serious disabling illness in most persons with about 50% mortality if untreated. Lymphocyte depression of about >75% within 48 hours. • 350 § LD 50 for mass casualty situation.
Radiation Sickness Rad Dose vs. Clinical Findings • 450 § LD 50 for isolated individual exposure. • 500+ § Accelerated version of acute radiation syndrome with GI complications within 2 weeks, bleeding and death in most exposed persons. • 5000+ § Fulminating course with cardiovascular, GI, and CNS complications resulting in death within 24 -72 hours.
Acute Radiation Syndrome • Must affect a particular system • Must be penetrating (alpha and beta not in this category) • Most of body must be exposed for Acute Radiation Syndrome • 900 to 1000 Rads result in mortality • Dose estimate based upon onset of nausea and vomiting from time of exposure
Acute Radiation Syndrome Threshold for ARS 25 Rads to 100 Rads • Bone marrow depression affects Hematopoietic system (white cell and thrombocytes) • chances for sepsis • Epithelial system in skin, gastrointestinal, and pulmonary system • starts to slough off without production of new cells • Cardiovascular system & CNS affects
Acute Radiation Syndrome Phases of ARS • Prodromal Stage - nausea and vomiting (the sooner the N/V starts, the more acute) • Latent Stage - (period of well being) • Stem cells inhibited or destroyed • Epithelial cell as bone marrow will not be produced • Manifest Illness - (normal cells live out their lives without any reproduction) • High radiation dose and trauma interact synergistically to increase mortality
Acute Radiation Syndrome Skin Effects • • Epilation (hair loss) Erythema (redness) Pigmentation Dry desquamation (shedding of skin) • Treatment: • Treat patients symptomatically • Prevent and manage infections • Electrolytes • Ulcerations do not heal well. • Lesions do not appear from days to weeks • If lesions or burns are noted, they are thermal or traumatic in nature, not a result of radioactivity

Radiation Exposure Treatment Guidelines

“Gas Mask” Respirators • The first line of defense in the treatment & prevention of any potential exposure whether it is radiological, biological, or chemical is respiratory protection • Respirators should be fit tested with an RFT test kit to ensure proper fit with NO AIR LEAKS!! • Masks should be fitted with an M 95 cartridge to protect against most CBRN inhalation hazards • Consider masks that provide for hydration

“DECON” The second line of defense is DECONTAMINATION • First – Remove all personnel from the area of contamination whether it be Chemical, Biological or Radiological in nature • Second – Remove and bag all clothing. While the bomb disposal suit might provide some protection from particulate matter it will not protect from particles emitting gamma radiation and may not provide protection from chemical and/or biological infiltration • Third - Shower with soap and water • Fourth - Shower with soap and water • Fifth - Shower with soap and water

Acute Radiation Syndrome Decontamination • External Contamination - removal of clothing at scene removes 80% - 90% of contaminants • Handle foreign objects with care until proven non-radioactive • Survey patient and collect samples • Radioactive material is usually in the form of dust particles on the body surface/clothing • Radiation dose rate from contamination is usually low - but will continue to expose until removed
Acute Radiation Syndrome Decontamination Priorities • Wounds (irrigate and gently scrub then debride) • Thermal burns (gently rinse and change dressing frequently to remove additional contamination) • Skin - soap and water, cut hair if necessary DO NOT SHAVE, promote sweating (cover with plastic), use survey meter • Cease decontamination when level of radiation is less than twice the background radiation • Internal contamination (inhalation, ingestion, wounds) generally does not cause early signs or symptoms and rarely causes ARS (but does increases the risk of cancer)
DTPA Diethylenetriaminepentaacetate • DTPA is a calcium or zinc salt that is administered to adults suspected of being internally contaminated (inhalation or ingestion) with Americium, Plutonium, Californium and Curium • DTPA is given via injection, slow drip or inhalation mist. It works by chelating (bonding) to the radioactive material and is excreted through the urine • Contraindications are renal dysfunction or bone marrow depression • Side effects include headache, metallic taste in mouth, chest pain and nausea

Prussian blue • Prussian blue is used to treat Cesium exposure (mainly 137 Cesium) and radioactive thallium • Prussian blue works in the intestines by blocking re-absorption and is excreted through the bowel • Prussian blue also reduces the half life of Cesium from 110 days to 30 days • The dosage is 500 mg and is administered orally three times a day for thirty days

Potassium Iodide • Potassium Iodide is a salt of stable iodine • Potassium Iodide (KI) and Potassium Iodate are used to treat radioactive Iodine exposure • Radioactive Iodine is quickly absorbed by the thyroid gland. KI acts to block this absorption • A single dose of KI protects for 24 hours • • Adult dosage – 130 mg Children 3 -18 (<150 lbs) – 65 mg Infants – 1 month to 3 years – 32 mg Newborn – 16 mg

Biological Dispersal Devices

The Sky’s the Limit • There are no hard and fast rules for what type of package you will find that incorporates biological material • The device will most likely be a “Low Energy Explosive” that does not consume the contaminate during detonation • The most likely biological contaminant would be bacillus anthracis due to it’s normal resting state as a spore and it’s lethality

Anthrax

Anthrax Summary & Clinical Findings • Signs & Symptoms • Incubation period 1 -6 days • Fever, Malaise, Cough and Mild Chest Discomfort • Followed by severe respiratory distress, dyspnea, diaphorisis, stridor and cyanosis • Mediastinal widening is noted on x-ray with inhalation exposure • Shock and Death occurs within 24 -36 hours after onset of severe symptoms

Anthrax Treatment Guidelines

“Gas Mask” Respirators • The first line of defense in the treatment & prevention of any potential exposure whether it is radiological, biological, or chemical is respiratory protection • Respirators should be fit tested with an RFT test kit to ensure proper fit with NO AIR LEAKS!! • Masks should be fitted with an M 95 cartridge to protect against most CBRN inhalation hazards • Consider masks that provide for hydration

“DECON” The second line of defense is DECONTAMINATION • First – Remove all personnel from the area of contamination whether it be Chemical, Biological or Radiological in nature • Second – Remove and bag all clothing. While the bomb disposal suit might provide some protection from particulate matter it will not protect from particles emitting gamma radiation and may not provide protection from chemical and/or biological infiltration • Third - Shower with soap and water • Fourth - Shower with soap and water • Fifth - Shower with soap and water

Recommended Post exposure Prophylaxis to Prevent Inhalational Anthrax Initial Therapy Duration Adults Ciprofloxacin 60 days (including pregnant 500 mg PO BID women and OR immunocompromised) Doxycycline 100 mg PO BID Children Ciprofloxacin* 60 days 10– 15 mg/kg PO Q 12 hrs Change to OR amoxicillin Doxycycline: if susceptible >8 yrs and >45 kg: 100 mg PO BID >8 yrs and <45 kg: 2. 2 mg/kg PO BID <8 yrs: 2. 2 mg/kg PO BID *Ciprofloxacin not to exceed 1 gram daily in children Patient information sheets at www. bt. cdc. gov
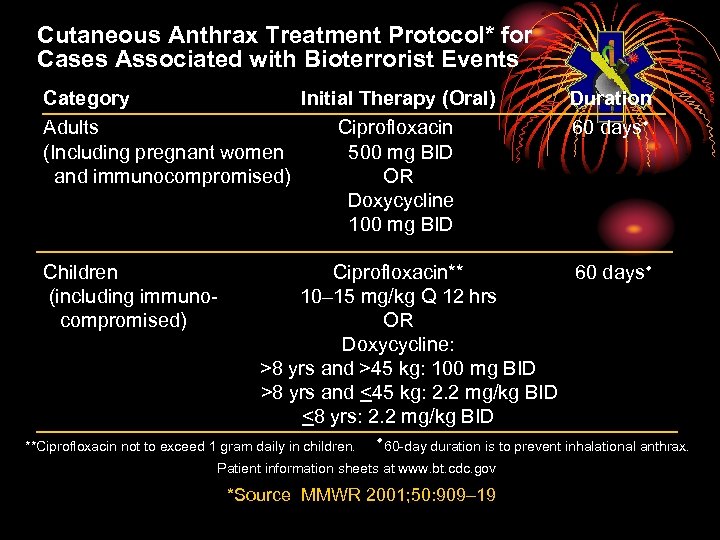
Cutaneous Anthrax Treatment Protocol* for Cases Associated with Bioterrorist Events Category Initial Therapy (Oral) Adults Ciprofloxacin (Including pregnant women 500 mg BID and immunocompromised) OR Doxycycline 100 mg BID Children (including immunocompromised) Duration 60 daysw Ciprofloxacin** 60 daysw 10– 15 mg/kg Q 12 hrs OR Doxycycline: >8 yrs and >45 kg: 100 mg BID >8 yrs and <45 kg: 2. 2 mg/kg BID <8 yrs: 2. 2 mg/kg BID **Ciprofloxacin not to exceed 1 gram daily in children. w 60 -day duration is to prevent inhalational anthrax. Patient information sheets at www. bt. cdc. gov *Source MMWR 2001; 50: 909– 19

Inhalational Anthrax Treatment Protocol* for Cases Associated with Bioterrorist Events (Adult) Category Initial therapy (intravenous) Adults Ciprofloxacin (Including pregnant 400 mg Q 12 hrs women** and OR immunocompromised) Doxycycline 100 mg Q 12 hrs AND One or two additional antimicrobials Duration Switch to oral therapy when clinically appropriate: Ciprofloxacin 500 mg BID OR Doxycycline 100 mg BID Continue for 60 days (IV and PO combined) **High death rate from infection outweighs risk of antimicrobials Patient information sheets at www. bt. cdc. gov *Source MMWR 2001; 50: 909– 19

Inhalational Anthrax Treatment Protocol* for Cases Associated with Bioterrorist Events Category Children (including immunocompromised Initial therapy (intravenous) Duration Ciprofloxacin 10– 15 mg/kg Q 12 hrs OR Doxycycline >8 yrs and >45 kg: 100 mg Q 12 hrs >8 yrs and <45 kg: 2. 2 mg/kg Q 12 hrs <8 yrs: 2. 2 mg/kg Q 12 hrs AND One or two additional antimicrobials Switch to oral therapy when clinically appropriate: Ciprofloxacin 10– 15 mg/kg Q 12 hrs OR Doxycycline >8 yrs and >45 kg: 100 mg BID >8 yrs and <45 kg: 2. 2 mg/kg BID <8 yrs: 2. 2 mg/kg BID **Ciprofloxacin not to exceed 1 gram daily w. Continue for 60 days (IV and po combined) Patient information sheets at www. bt. cdc. gov *Source MMWR 2001; 50: 909– 19

Chemical Dispersal Devices

The Sky’s the Limit • There are no hard and fast rules for what type of package you will find that incorporates chemical material • The device will most likely be a “Low Energy Explosive” that does not consume the contaminate during detonation • For this section, we will concentrate on the organophosphate group of chemicals due to the rapid onset of symptoms and possible lethality if not treated immediately

Organophosphates Nerve Agents & Pesticides

Nerve Agents Overview • Nerve agents are a group of highly toxic organic esters of phosphoric acid derivatives and are among the deadliest of chemical agents • Classification • G - Agents • Tabun (GA), Sarin (GB), Soman (GD), and GF • V – Agents • VX • Nerve agents are colorless to light brown liquids • The effects of organophosphate nerve agents in general are due to their ability to inhibit acetylcholinesterase throughout the body • RAPID TREATMENT AND EVACUATION IS A MUST

Methyl-Parathion Overview • Methyl-Parathion is a highly toxic commercial pesticide • In it’s concentrated form of 45. 5% it is also known as Folidol M , Metacide , Metafos, Metilparation , and Metron • Acute signs and symptoms may be seen with as little as 20 mg/kg exposure via inhalation or ingestion • Methyl parathion is available in dust, emulsifiable concentrate, ULV liquid, and wettable powder formulations • The effects of organophosphate agents in general are mainly due to their ability to inhibit acetylcholinesterase throughout the body • RAPID TREATMENT AND EVACUATION IS A MUST

Organophosphates Physiology of Exposure

Physiology: Normal • Electrical impulse goes down nerve • The impulse causes release of neurotransmitter, acetylcholine • ACh stimulates receptor site on organ • This causes the organ to act • ACh is then destroyed by ACh. E • Resulting in no more organ activity
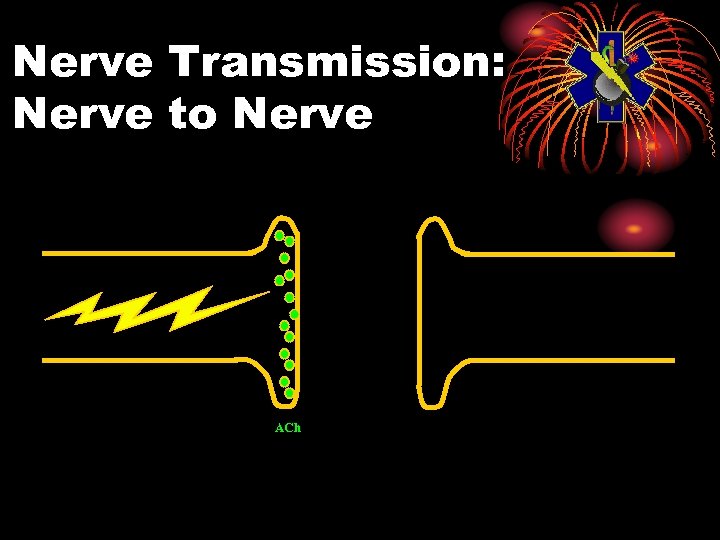
Nerve Transmission: Nerve to Nerve ACh
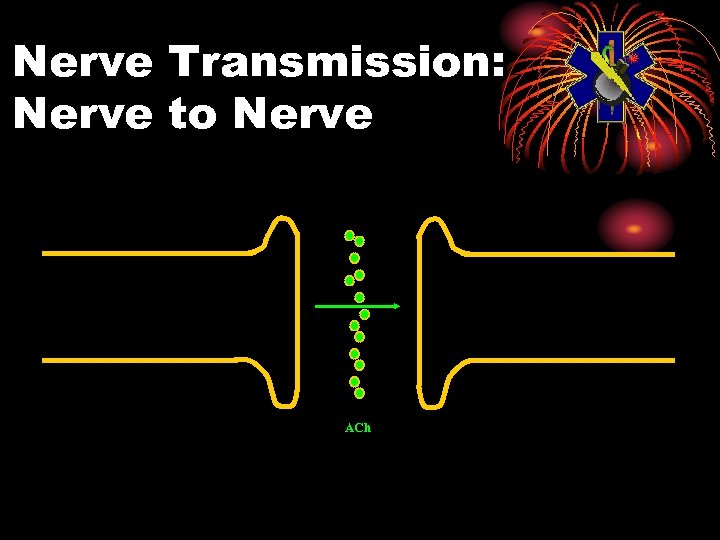
Nerve Transmission: Nerve to Nerve ACh
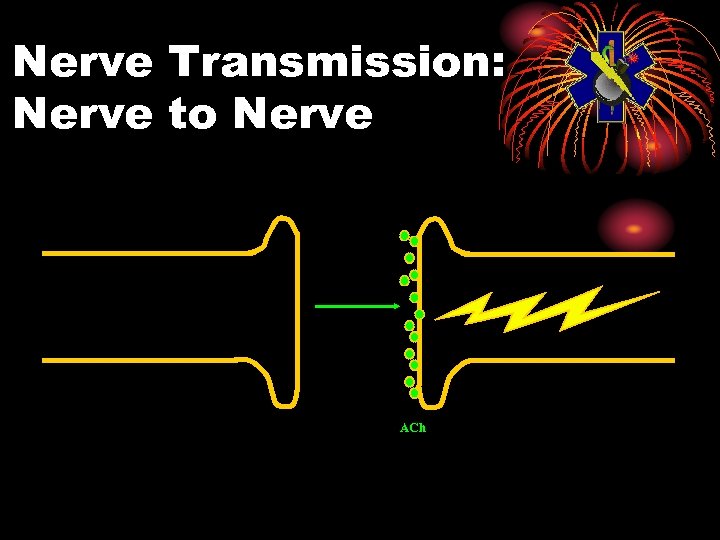
Nerve Transmission: Nerve to Nerve ACh
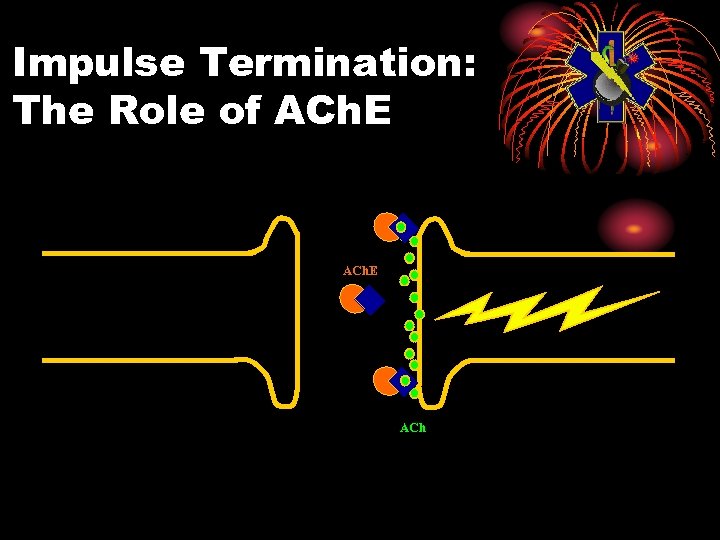
Impulse Termination: The Role of ACh. E ACh
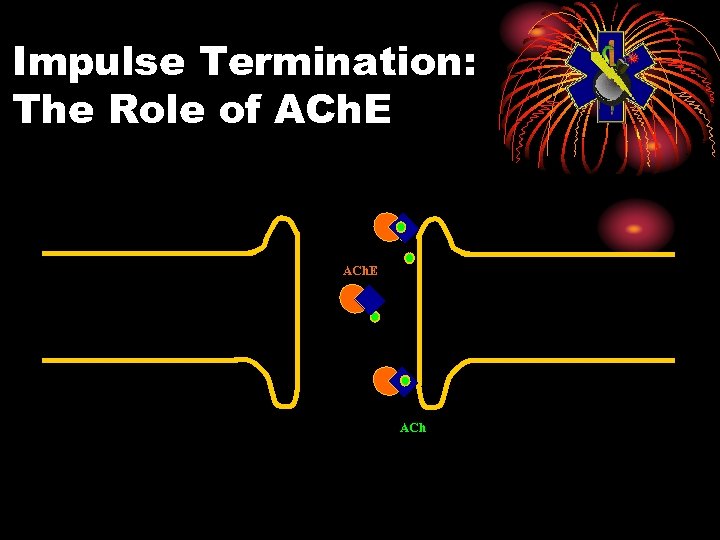
Impulse Termination: The Role of ACh. E ACh
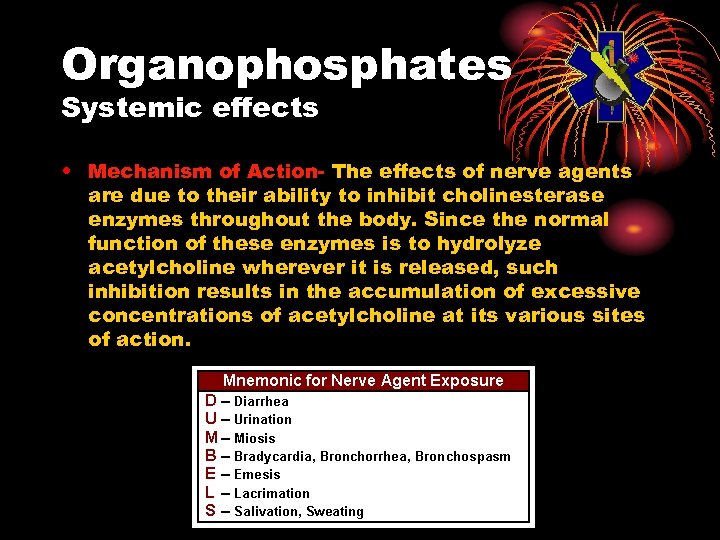
Organophosphates Systemic effects • Mechanism of Action- The effects of nerve agents are due to their ability to inhibit cholinesterase enzymes throughout the body. Since the normal function of these enzymes is to hydrolyze acetylcholine wherever it is released, such inhibition results in the accumulation of excessive concentrations of acetylcholine at its various sites of action.

PHYSIOLOGY: NERVE AGENT • Enzyme (ACh. E) is inhibited • Does not destroy ACh • Excess ACh continues to stimulate organ • Organ overstimulation
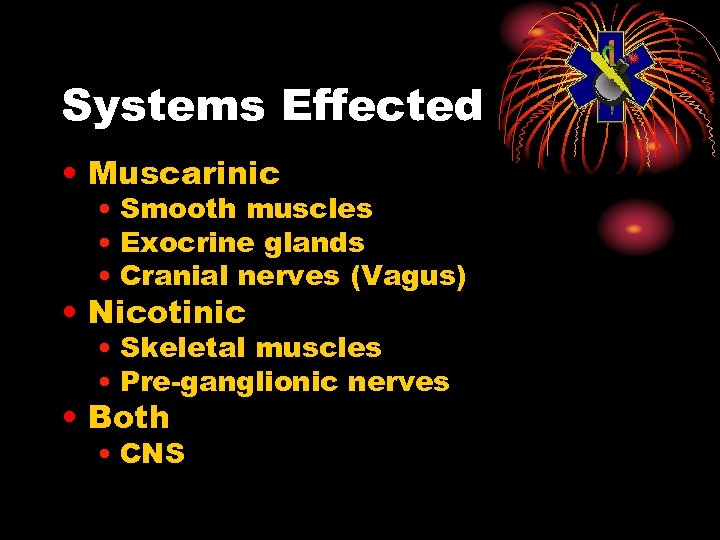
Systems Effected • Muscarinic • Smooth muscles • Exocrine glands • Cranial nerves (Vagus) • Nicotinic • Skeletal muscles • Pre-ganglionic nerves • Both • CNS

Muscarinic Receptors • Muscarinic • Smooth muscles • Airways - constrict • GI tract - constrict • Pupils - constrict • Glands • Eyes, nose, mouth, sweat, airways, GI • Heart, bradycardia (Vagal)

Nicotinic Receptors • Skeletal muscles • Fasciculations, twitching, fatigue, flaccid paralysis • Pre-ganglionic • Tachycardia, hypertension
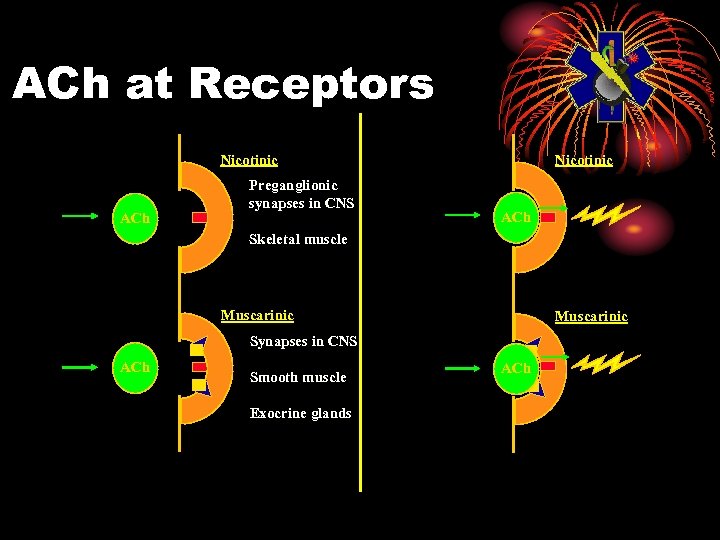
ACh at Receptors Nicotinic ACh Preganglionic synapses in CNS Nicotinic ACh Skeletal muscle Muscarinic Synapses in CNS ACh Smooth muscle Exocrine glands ACh
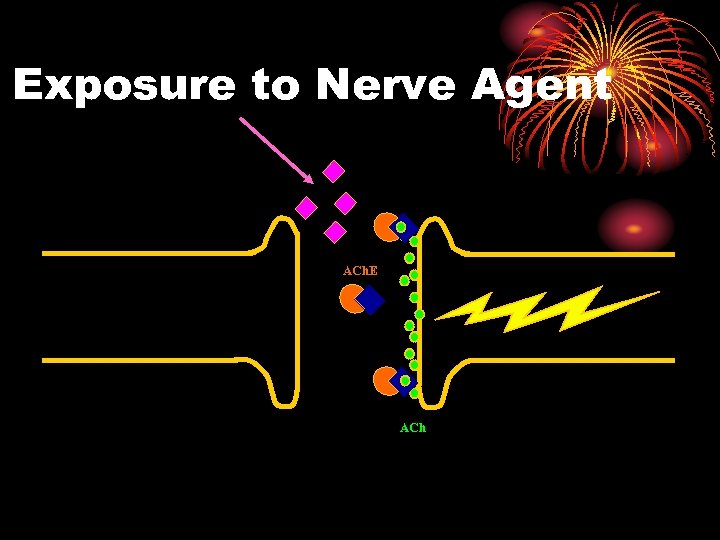
Exposure to Nerve Agent ACh. E ACh
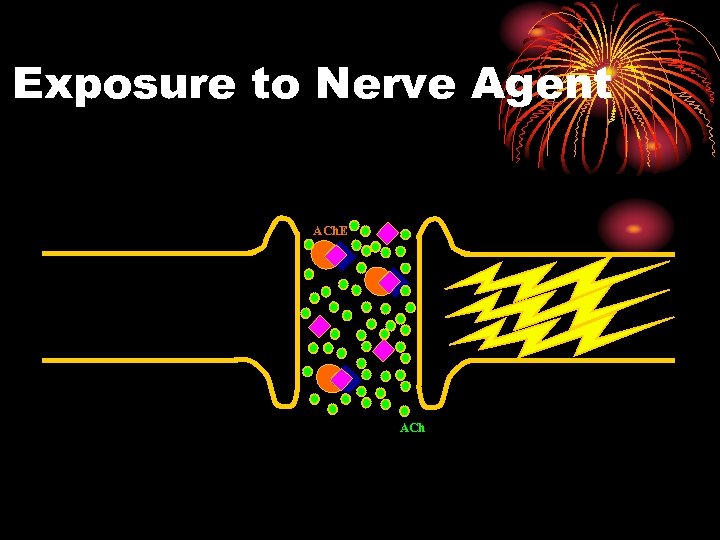
Exposure to Nerve Agent ACh. E ACh

Organophosphates Treatment / Antidote

“Gas Mask” Respirators • The first line of defense in the treatment & prevention of any potential exposure whether it is radiological, biological, or chemical is respiratory protection • Respirators should be fit tested with an RFT test kit to ensure proper fit with NO AIR LEAKS!! • Masks should be fitted with an M 95 cartridge to protect against most CBRN inhalation hazards • Consider masks that provide for hydration

“DECON” The second line of defense is DECONTAMINATION • First – Remove all personnel from the area of contamination whether it be Chemical, Biological or Radiological in nature • Second – Remove and bag all clothing. While the bomb disposal suit might provide some protection from particulate matter it will not protect from particles emitting gamma radiation and may not provide protection from chemical and/or biological infiltration • Third - Shower with soap and water • Fourth - Shower with soap and water • Fifth - Shower with soap and water

ANTIDOTES • Too much acetylcholine? ØBlock excess acetylcholine • Enzyme inhibited? ØReactivate enzyme

ATROPINE • Cholinergic blocking drug • Mark I Kit contains 2 mg/0. 7 cc • Blocks excess acetylcholine • Clinical effects at muscarinic sites • Dries secretions • Reduces smooth muscle constriction
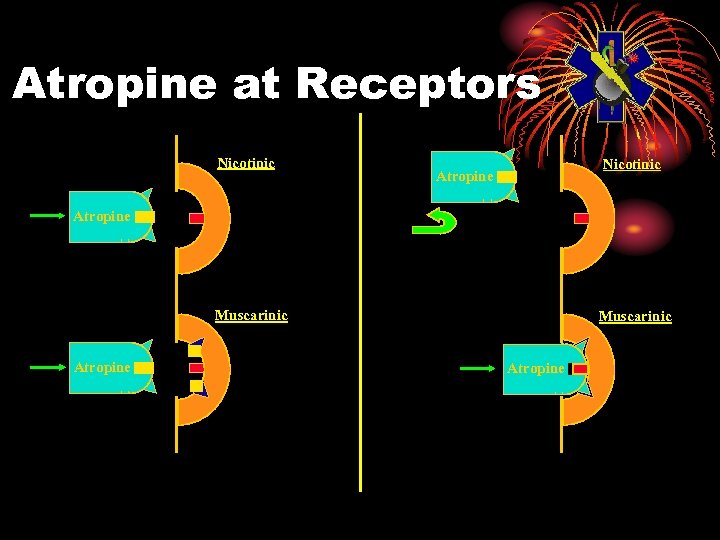
Atropine at Receptors Nicotinic Atropine Muscarinic Atropine

ATROPINE • Starting dose 2 mg to 6 mg • If needed, 2 mg every 5 to 10 minutes until • Secretions dry up • Ventilation is improved • Usual dose: (severe casualty) 15 to 20 mg • 100’s of mgs are used in insecticide poisoning

ATROPINE • Not for • Skeletal muscle effects • Miosis, unless used topically • Use will cause blurred vision for up to 24 hours
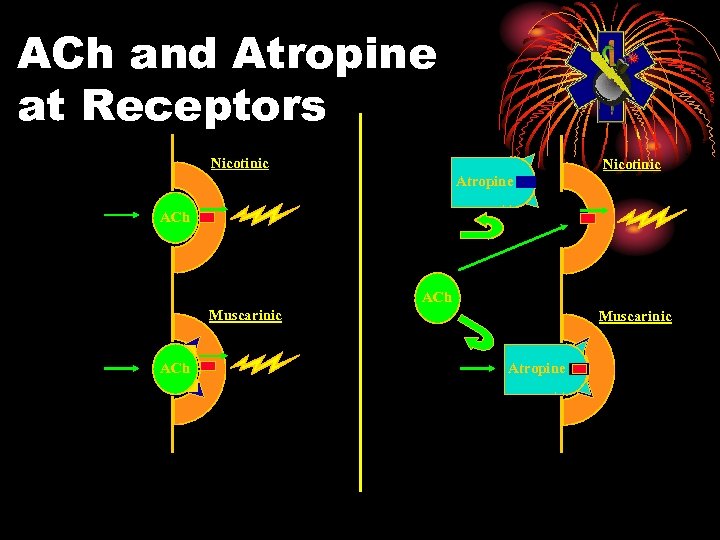
ACh and Atropine at Receptors Nicotinic Atropine ACh Muscarinic Atropine
Pralidoxime Chloride (2 -PAM Cl) Oximes • Effects at nicotinic sites • Increase skeletal muscle strength • No clinical effects at muscarinic receptor sites
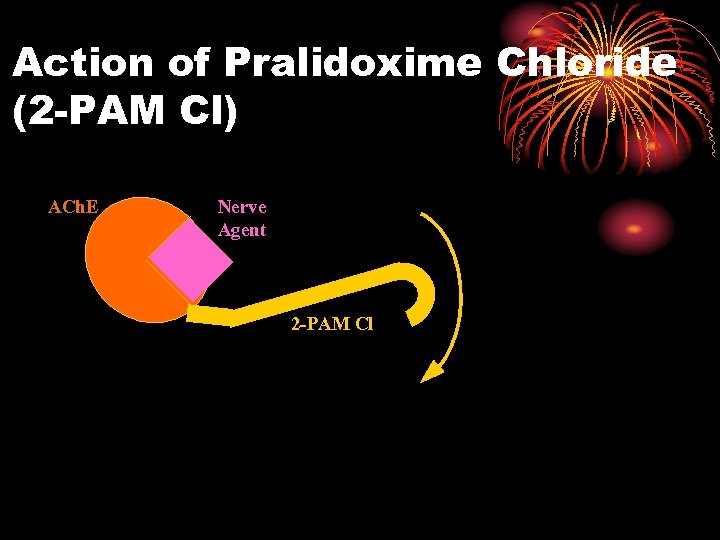
Action of Pralidoxime Chloride (2 -PAM Cl) ACh. E Nerve Agent 2 -PAM Cl
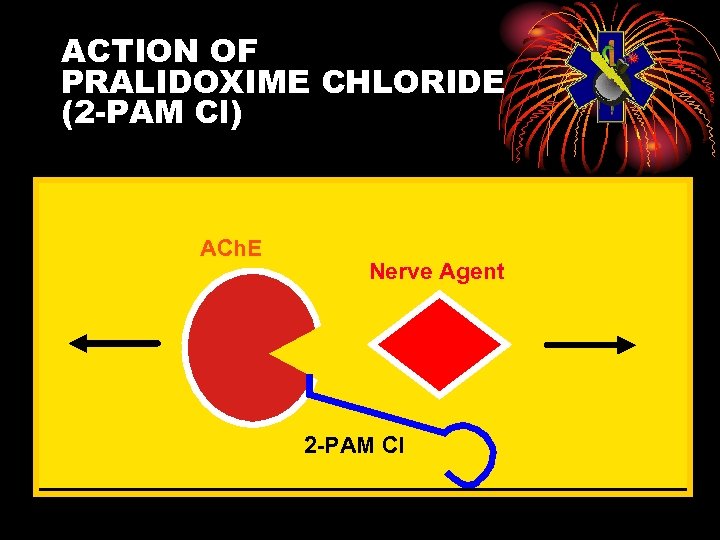
ACTION OF PRALIDOXIME CHLORIDE (2 -PAM Cl) ACh. E Nerve Agent 2 -PAM Cl
Pralidoxime Chloride (2 -PAM Cl) Oximes • Remove agent from enzyme, unless aging has occurred • Aging: agent-enzyme complex changes • Oximes cannot reactivate enzyme • Aging times: GD 2 min GB 3 to 4 hours Others longer
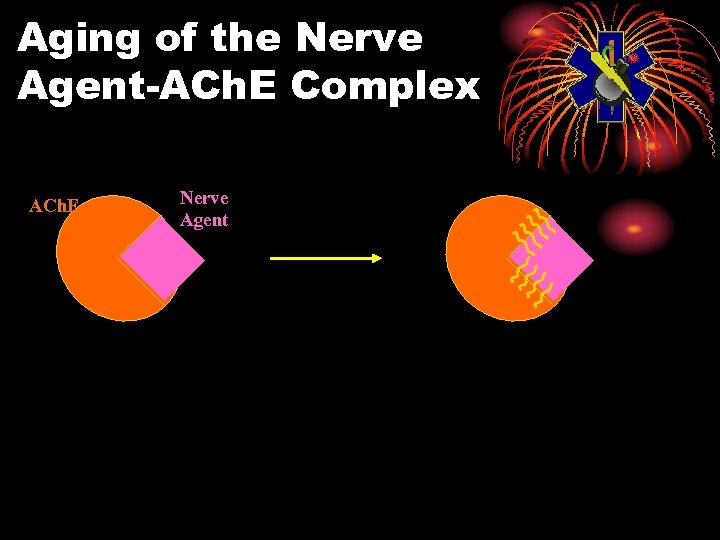
Aging of the Nerve Agent-ACh. E Complex ACh. E Nerve Agent
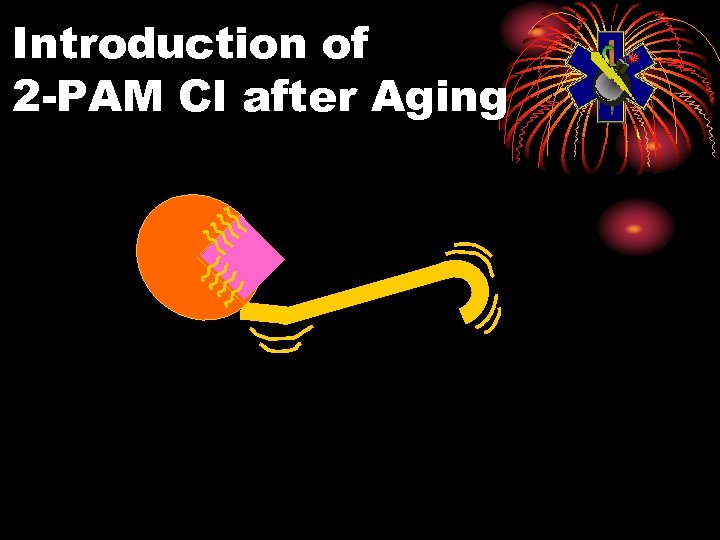
Introduction of 2 -PAM Cl after Aging

2 -PAMCL DOSE • MARK I Kit: contains 600 mg • One to three Combopens; repeat in one hour • IV: One gram slowly (20 to 30 min) • Repeat in one hour

Organophosphates Treatment Guidelines • Rapidly move from area of contamination • Donning the protective mask and hood at the first indication of a nerve agent exposure • Administering the MARK I kit as soon as any signs or symptoms are noted • Administering Diazepam to MODERATE to SEVERELY poisoned personnel

Organophosphates Treatment Guidelines • Removing or neutralizing any liquid contamination immediately • Removing airway secretions if they are obstructing the airway • Airway suction may be needed • Establishing a patent airway (for example, with a cricothyroidotomy or endotracheal tube) and administering assisted ventilation, if required

Lab Exercise Mark 1 PPT & Administration

If nothing else, Remember… • • Respiratory Protection Radiation Detection Mark 1 Kit Decon

References • • • Centers for Disease Control - cdc. gov USAMRIID - usamriid. army. mil Wikipedia - wikipedia. com Environmental Protection Agency - epa. gov Center for Nonproliferation Studies - cns. miis. edu Nuclear Threat Initiative- nti. org NWMD - Glenn Williams MD Nuclear War Survival Skills - Cresson H. Kearny FEMA - fema. gov The Nuclear Weapon Archive - nuclearweaponarchive. org Law Enforcement Targets - letargets. com Radiobiological Research Institute - afrri. usuhs. mil

CBRNE Bomb Medic Presentation compiled and presented by: Lt. Steven M. Albright – Paramedic / SC-EMS. com
4c941797aa5457ec97d51bad3572842d.ppt