770ed875f36d8f26f5e60fd1c146dfdb.ppt
- Количество слайдов: 20
 Carr Uni. Fuge Single-Use Separation System
Carr Uni. Fuge Single-Use Separation System
 Customers Across the Globe United States Germany Canada France Mexico Austria Brazil Italy U. K. Japan Sweden Belgium Australia Denmark Switzerland The Netherlands
Customers Across the Globe United States Germany Canada France Mexico Austria Brazil Italy U. K. Japan Sweden Belgium Australia Denmark Switzerland The Netherlands
 New Product Update Uni. Fuge Single-Use Cell Harvesting Technology CONFIDENTIAL
New Product Update Uni. Fuge Single-Use Cell Harvesting Technology CONFIDENTIAL
 Design Objectives n Robust, single use cell harvesting centrifuge n Scalable platform n Harvest capacity of 10 L to 2, 500 L bioreactor n Pilot ≈ 3 L/min with 1. 5 L cell volume collection n Production ≈ 20 L/min with 40 L cell volume collection n Gamma irradiated single use liner n Fully automated processing n High recovery of cells with minimal cell lysis CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘A’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. n Process rates allow for single shift harvesting
Design Objectives n Robust, single use cell harvesting centrifuge n Scalable platform n Harvest capacity of 10 L to 2, 500 L bioreactor n Pilot ≈ 3 L/min with 1. 5 L cell volume collection n Production ≈ 20 L/min with 40 L cell volume collection n Gamma irradiated single use liner n Fully automated processing n High recovery of cells with minimal cell lysis CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘A’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. n Process rates allow for single shift harvesting
 Uni. Fuge® Concept, Prototype & Testing n Dec 2007 - Concept n Mar. 2009 – Alpha unit mammalian cell test n Oct. 2009 – Beta unit field trials begin Alpha Prototype Beta Prototype
Uni. Fuge® Concept, Prototype & Testing n Dec 2007 - Concept n Mar. 2009 – Alpha unit mammalian cell test n Oct. 2009 – Beta unit field trials begin Alpha Prototype Beta Prototype
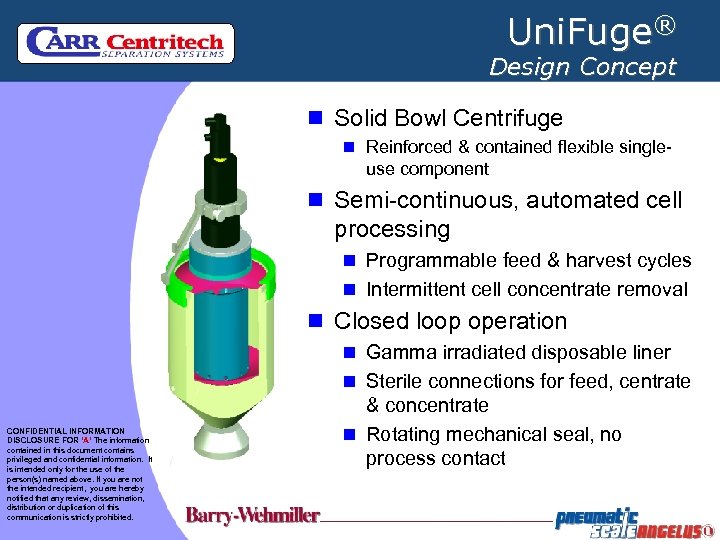 Uni. Fuge® Design Concept n Solid Bowl Centrifuge n Reinforced & contained flexible single- use component n Semi-continuous, automated cell processing n Programmable feed & harvest cycles n Intermittent cell concentrate removal n Closed loop operation n Gamma irradiated disposable liner n Sterile connections for feed, centrate CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘A’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. & concentrate n Rotating mechanical seal, no process contact
Uni. Fuge® Design Concept n Solid Bowl Centrifuge n Reinforced & contained flexible single- use component n Semi-continuous, automated cell processing n Programmable feed & harvest cycles n Intermittent cell concentrate removal n Closed loop operation n Gamma irradiated disposable liner n Sterile connections for feed, centrate CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘A’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. & concentrate n Rotating mechanical seal, no process contact
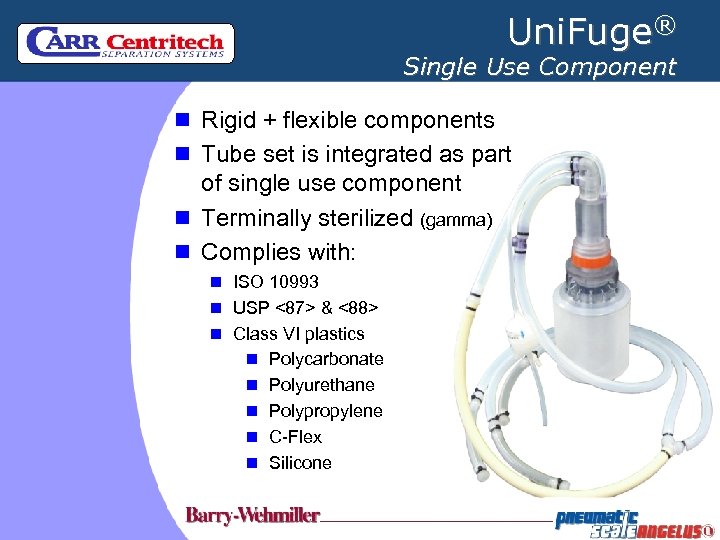 Uni. Fuge® Single Use Component n Rigid + flexible components n Tube set is integrated as part of single use component n Terminally sterilized (gamma) n Complies with: n ISO 10993 n USP <87> & <88> n Class VI plastics n Polycarbonate n Polyurethane n Polypropylene n C-Flex n Silicone
Uni. Fuge® Single Use Component n Rigid + flexible components n Tube set is integrated as part of single use component n Terminally sterilized (gamma) n Complies with: n ISO 10993 n USP <87> & <88> n Class VI plastics n Polycarbonate n Polyurethane n Polypropylene n C-Flex n Silicone
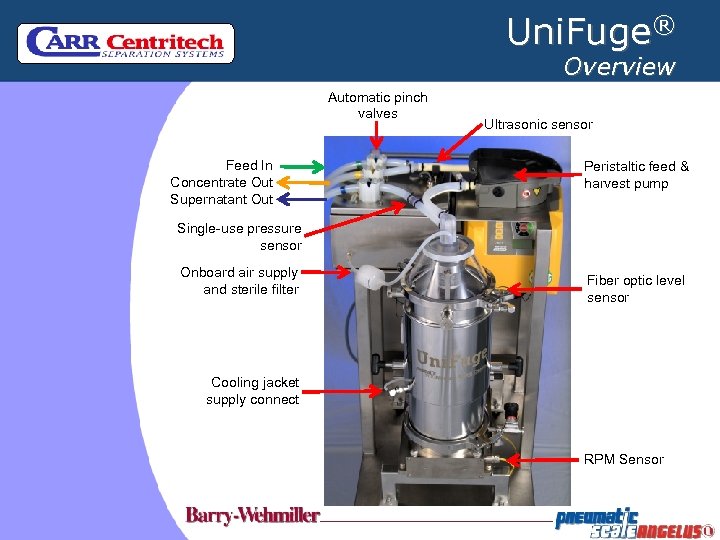 Uni. Fuge® Overview Automatic pinch valves Feed In Concentrate Out Supernatant Out Ultrasonic sensor Peristaltic feed & harvest pump Single-use pressure sensor Onboard air supply and sterile filter Fiber optic level sensor Cooling jacket supply connect RPM Sensor
Uni. Fuge® Overview Automatic pinch valves Feed In Concentrate Out Supernatant Out Ultrasonic sensor Peristaltic feed & harvest pump Single-use pressure sensor Onboard air supply and sterile filter Fiber optic level sensor Cooling jacket supply connect RPM Sensor
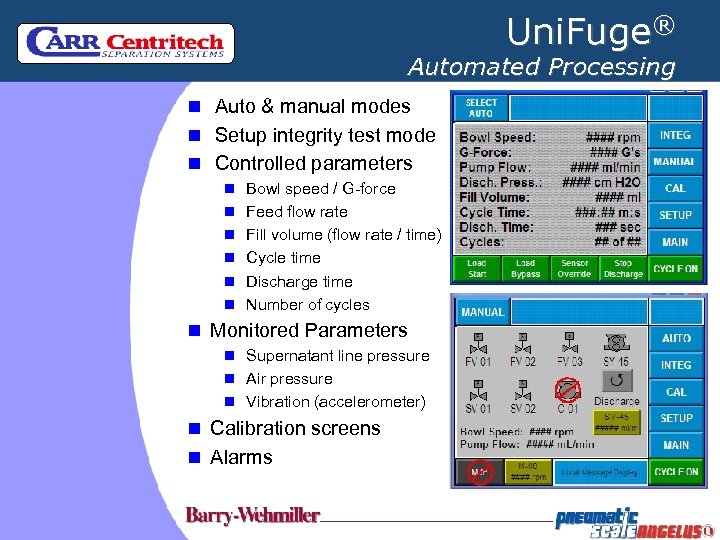 Uni. Fuge® Automated Processing n Auto & manual modes n Setup integrity test mode n Controlled parameters n Bowl speed / G-force n Feed flow rate n Fill volume (flow rate / time) n Cycle time n Discharge time n Number of cycles n Monitored Parameters n Supernatant line pressure n Air pressure n Vibration (accelerometer) n Calibration screens n Alarms
Uni. Fuge® Automated Processing n Auto & manual modes n Setup integrity test mode n Controlled parameters n Bowl speed / G-force n Feed flow rate n Fill volume (flow rate / time) n Cycle time n Discharge time n Number of cycles n Monitored Parameters n Supernatant line pressure n Air pressure n Vibration (accelerometer) n Calibration screens n Alarms
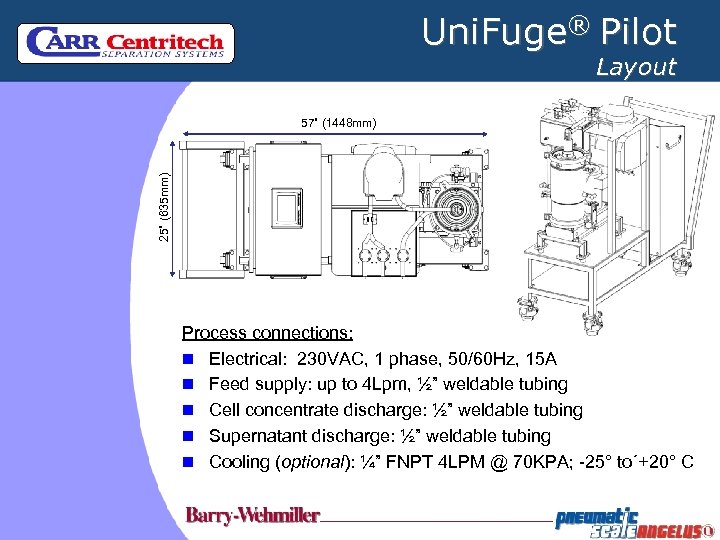 Uni. Fuge® Pilot Layout 25” (635 mm) 57” (1448 mm) Process connections: n Electrical: 230 VAC, 1 phase, 50/60 Hz, 15 A n Feed supply: up to 4 Lpm, ½” weldable tubing n Cell concentrate discharge: ½” weldable tubing n Supernatant discharge: ½” weldable tubing n Cooling (optional): ¼” FNPT 4 LPM @ 70 KPA; -25° to´+20° C
Uni. Fuge® Pilot Layout 25” (635 mm) 57” (1448 mm) Process connections: n Electrical: 230 VAC, 1 phase, 50/60 Hz, 15 A n Feed supply: up to 4 Lpm, ½” weldable tubing n Cell concentrate discharge: ½” weldable tubing n Supernatant discharge: ½” weldable tubing n Cooling (optional): ¼” FNPT 4 LPM @ 70 KPA; -25° to´+20° C
 Uni. Fuge® Pilot Prototype
Uni. Fuge® Pilot Prototype
 Uni. Fuge® Pilot Prototype
Uni. Fuge® Pilot Prototype
 Uni. Fuge® Development Tests Alpha Unit 3/4/09 Beta Unit 8/26/09 n Objective: separation performance n 20 L CHO Cells performance, lysis, viability n 20 L SF 9 Cells n Feed: 1. 15 x 106 cells/ml @ 95% viability n Feed: 6 x 106 cells/ml @ 73% viability n Three tested conditions n 1, 2 & 3 L/min n 2, 000 x g CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. n Three tested conditions n 1, 2 & 3 L/min n 2, 000 x g n Cell Capture Results n 1 L/min: 99. 99% n 2 L/min: 99. 8% n 3 L/min: 98. 6% n Cell Capture Results n 1 L/min: 99. 99% n 2 L/min: 99. 9% n 3 L/min: 98. 8%
Uni. Fuge® Development Tests Alpha Unit 3/4/09 Beta Unit 8/26/09 n Objective: separation performance n 20 L CHO Cells performance, lysis, viability n 20 L SF 9 Cells n Feed: 1. 15 x 106 cells/ml @ 95% viability n Feed: 6 x 106 cells/ml @ 73% viability n Three tested conditions n 1, 2 & 3 L/min n 2, 000 x g CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. n Three tested conditions n 1, 2 & 3 L/min n 2, 000 x g n Cell Capture Results n 1 L/min: 99. 99% n 2 L/min: 99. 8% n 3 L/min: 98. 6% n Cell Capture Results n 1 L/min: 99. 99% n 2 L/min: 99. 9% n 3 L/min: 98. 8%
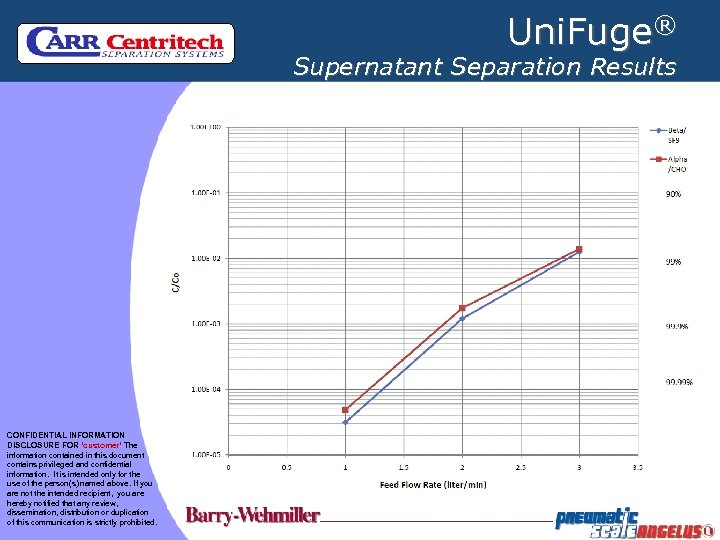 Uni. Fuge® Supernatant Separation Results CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited.
Uni. Fuge® Supernatant Separation Results CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited.
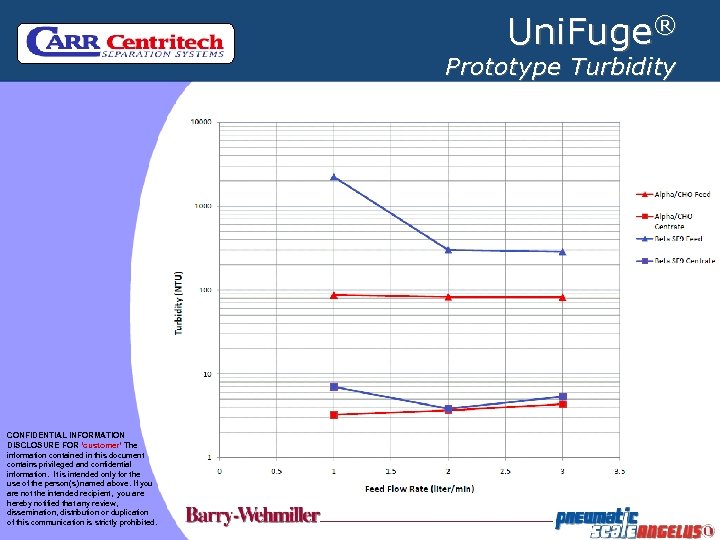 Uni. Fuge® Prototype Turbidity CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited.
Uni. Fuge® Prototype Turbidity CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited.
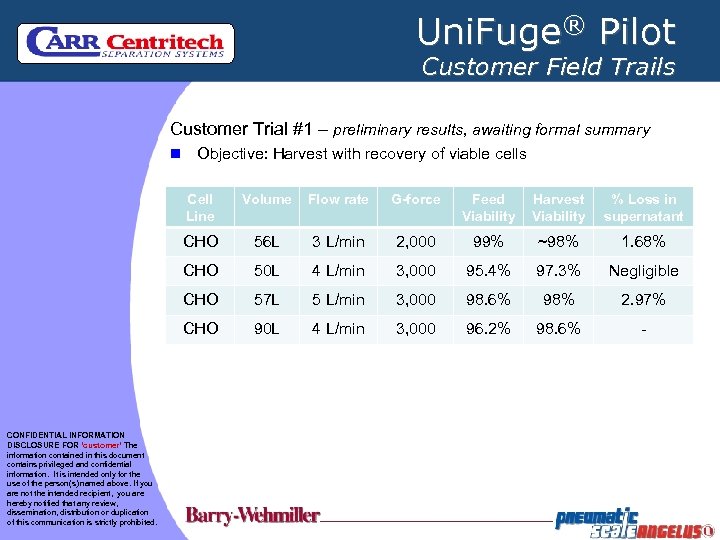 Uni. Fuge® Pilot Customer Field Trails Customer Trial #1 – preliminary results, awaiting formal summary n Objective: Harvest with recovery of viable cells Cell Line Flow rate G-force Feed Viability Harvest Viability % Loss in supernatant CHO 56 L 3 L/min 2, 000 99% ~98% 1. 68% CHO 50 L 4 L/min 3, 000 95. 4% 97. 3% Negligible CHO 57 L 5 L/min 3, 000 98. 6% 98% 2. 97% CHO CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. Volume 90 L 4 L/min 3, 000 96. 2% 98. 6% -
Uni. Fuge® Pilot Customer Field Trails Customer Trial #1 – preliminary results, awaiting formal summary n Objective: Harvest with recovery of viable cells Cell Line Flow rate G-force Feed Viability Harvest Viability % Loss in supernatant CHO 56 L 3 L/min 2, 000 99% ~98% 1. 68% CHO 50 L 4 L/min 3, 000 95. 4% 97. 3% Negligible CHO 57 L 5 L/min 3, 000 98. 6% 98% 2. 97% CHO CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. Volume 90 L 4 L/min 3, 000 96. 2% 98. 6% -
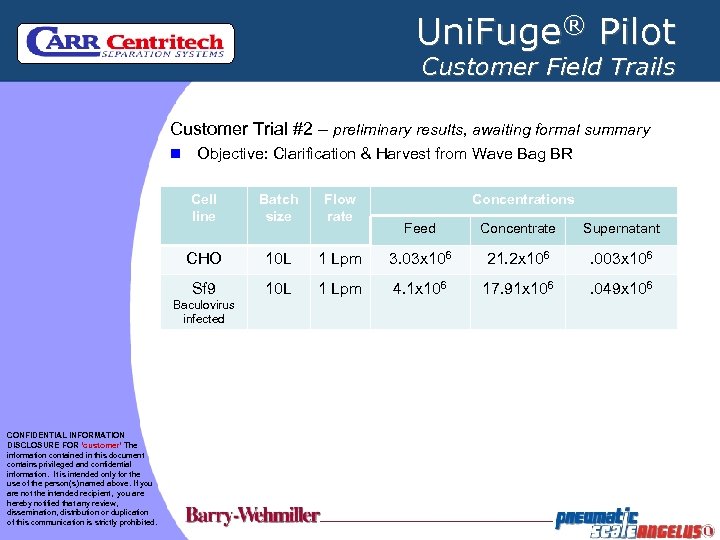 Uni. Fuge® Pilot Customer Field Trails Customer Trial #2 – preliminary results, awaiting formal summary n Objective: Clarification & Harvest from Wave Bag BR Cell line Batch size Flow rate CHO 10 L Sf 9 10 L Baculovirus infected CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. Concentrations Feed Concentrate Supernatant 1 Lpm 3. 03 x 106 21. 2 x 106 . 003 x 106 1 Lpm 4. 1 x 106 17. 91 x 106 . 049 x 106
Uni. Fuge® Pilot Customer Field Trails Customer Trial #2 – preliminary results, awaiting formal summary n Objective: Clarification & Harvest from Wave Bag BR Cell line Batch size Flow rate CHO 10 L Sf 9 10 L Baculovirus infected CONFIDENTIAL INFORMATION DISCLOSURE FOR ‘customer’ The information contained in this document contains privileged and confidential information. It is intended only for the use of the person(s) named above. If you are not the intended recipient, you are hereby notified that any review, dissemination, distribution or duplication of this communication is strictly prohibited. Concentrations Feed Concentrate Supernatant 1 Lpm 3. 03 x 106 21. 2 x 106 . 003 x 106 1 Lpm 4. 1 x 106 17. 91 x 106 . 049 x 106
 Uni. Fuge® Summary • Benefits – Centrifugation • Improved separation performance means less downstream processing • Better suited for high titers – no blinding or fouling of filters – Single Use Technology • Lower cost due to less cleaning/sterilization - No CIP/SIP required • Less product losses due to contamination – Programmable automatic processing • Variable separation force • Optimize feed flow rates • Programmable harvest cycle time – Capable of recovering and concentrating viable cells – Reduced downstream filtration – Scalability, Pilot -> Production
Uni. Fuge® Summary • Benefits – Centrifugation • Improved separation performance means less downstream processing • Better suited for high titers – no blinding or fouling of filters – Single Use Technology • Lower cost due to less cleaning/sterilization - No CIP/SIP required • Less product losses due to contamination – Programmable automatic processing • Variable separation force • Optimize feed flow rates • Programmable harvest cycle time – Capable of recovering and concentrating viable cells – Reduced downstream filtration – Scalability, Pilot -> Production
 Uni. Fuge® Next Steps n Unit(s) available for field testing in US and Europe n Typical field trial period is for one (1) week and includes on site technical support n Each customer will purchase the disposable liner(s) needed for testing n The beta test single-use components will not be gamma irradiated n Unifuge Pilot is available for sale, estimated delivery is 12 weeks
Uni. Fuge® Next Steps n Unit(s) available for field testing in US and Europe n Typical field trial period is for one (1) week and includes on site technical support n Each customer will purchase the disposable liner(s) needed for testing n The beta test single-use components will not be gamma irradiated n Unifuge Pilot is available for sale, estimated delivery is 12 weeks


