 Carnot Thermodynamics Professor Lee Carkner Lecture 12
Carnot Thermodynamics Professor Lee Carkner Lecture 12
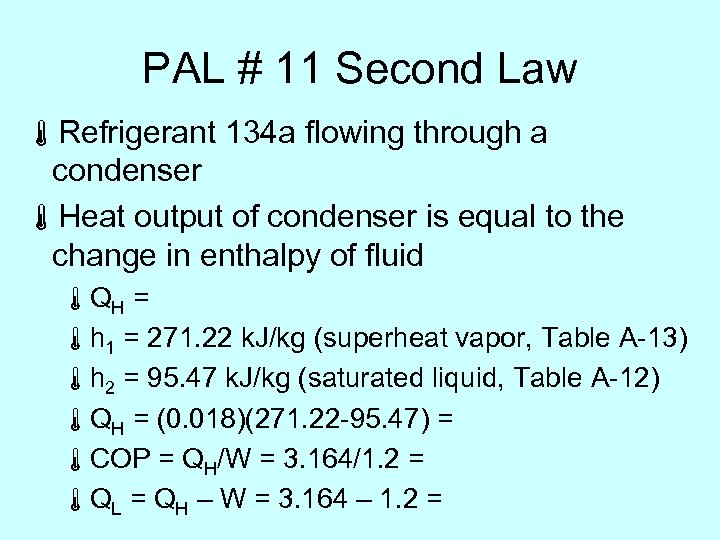 PAL # 11 Second Law áRefrigerant 134 a flowing through a condenser áHeat output of condenser is equal to the change in enthalpy of fluid áQH = áh 1 = 271. 22 k. J/kg (superheat vapor, Table A-13) áh 2 = 95. 47 k. J/kg (saturated liquid, Table A-12) áQH = (0. 018)(271. 22 -95. 47) = áCOP = QH/W = 3. 164/1. 2 = áQL = QH – W = 3. 164 – 1. 2 =
PAL # 11 Second Law áRefrigerant 134 a flowing through a condenser áHeat output of condenser is equal to the change in enthalpy of fluid áQH = áh 1 = 271. 22 k. J/kg (superheat vapor, Table A-13) áh 2 = 95. 47 k. J/kg (saturated liquid, Table A-12) áQH = (0. 018)(271. 22 -95. 47) = áCOP = QH/W = 3. 164/1. 2 = áQL = QH – W = 3. 164 – 1. 2 =
 Reversible áA reversible process: á áhas a net heat and work exchange for all systems as zero á áis theoretical limits for a process á
Reversible áA reversible process: á áhas a net heat and work exchange for all systems as zero á áis theoretical limits for a process á
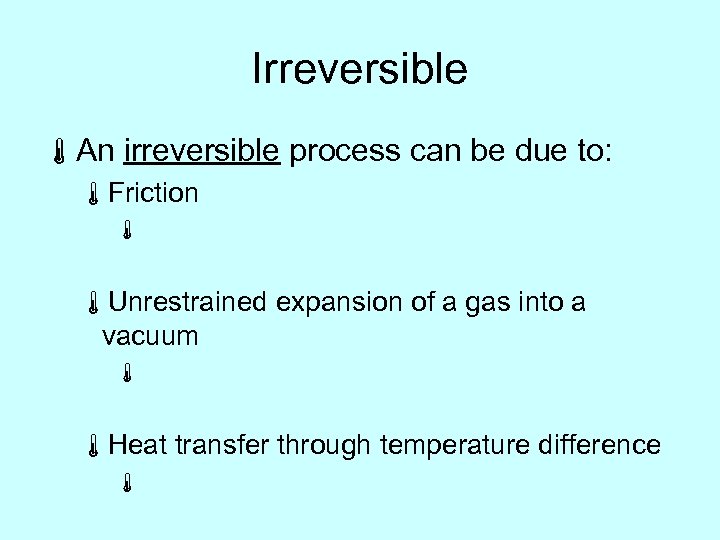 Irreversible áAn irreversible process can be due to: áFriction á áUnrestrained expansion of a gas into a vacuum á áHeat transfer through temperature difference á
Irreversible áAn irreversible process can be due to: áFriction á áUnrestrained expansion of a gas into a vacuum á áHeat transfer through temperature difference á
 Achieving Reversibility á áHeat transfer through a very small temperature differential d. T becomes reversible as d. T approaches zero áExample Isothermal Work: á ád. T always very small
Achieving Reversibility á áHeat transfer through a very small temperature differential d. T becomes reversible as d. T approaches zero áExample Isothermal Work: á ád. T always very small
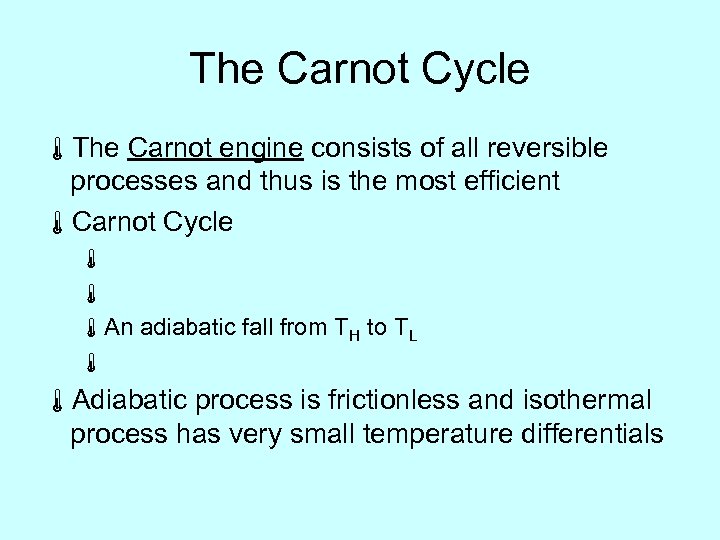 The Carnot Cycle áThe Carnot engine consists of all reversible processes and thus is the most efficient áCarnot Cycle á á áAn adiabatic fall from TH to TL á áAdiabatic process is frictionless and isothermal process has very small temperature differentials
The Carnot Cycle áThe Carnot engine consists of all reversible processes and thus is the most efficient áCarnot Cycle á á áAn adiabatic fall from TH to TL á áAdiabatic process is frictionless and isothermal process has very small temperature differentials
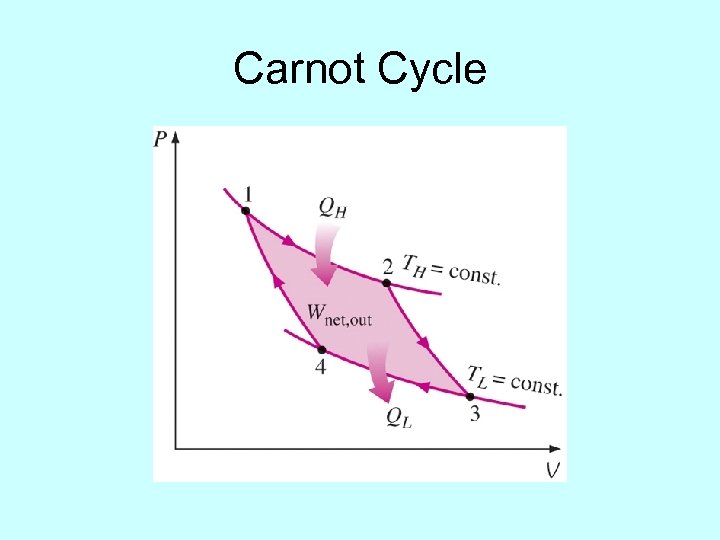 Carnot Cycle
Carnot Cycle
 Carnot Principles á áAll Carnot engines operating between two heat reservoirs have the same efficiency áWhile we cannot build a real Carnot engine, it gives us the upper limit for the efficiency of a real engine
Carnot Principles á áAll Carnot engines operating between two heat reservoirs have the same efficiency áWhile we cannot build a real Carnot engine, it gives us the upper limit for the efficiency of a real engine
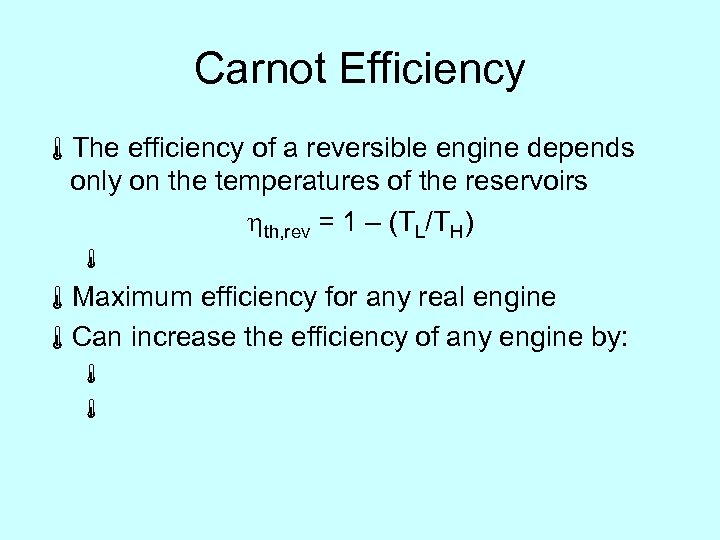 Carnot Efficiency áThe efficiency of a reversible engine depends only on the temperatures of the reservoirs hth, rev = 1 – (TL/TH) á áMaximum efficiency for any real engine áCan increase the efficiency of any engine by: á á
Carnot Efficiency áThe efficiency of a reversible engine depends only on the temperatures of the reservoirs hth, rev = 1 – (TL/TH) á áMaximum efficiency for any real engine áCan increase the efficiency of any engine by: á á
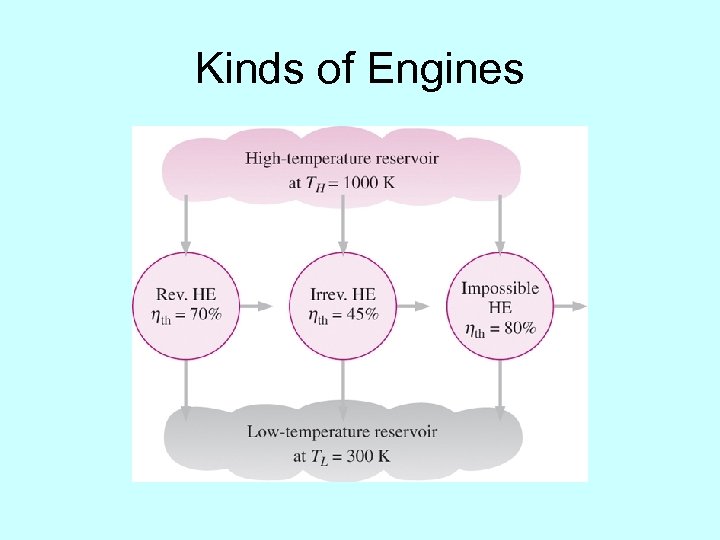 Kinds of Engines
Kinds of Engines
 Quality of Energy á áSince work is what we want, we can say that high temperature sources have higher quality energy than low temperature sources á áQuality is different from quantity á
Quality of Energy á áSince work is what we want, we can say that high temperature sources have higher quality energy than low temperature sources á áQuality is different from quantity á
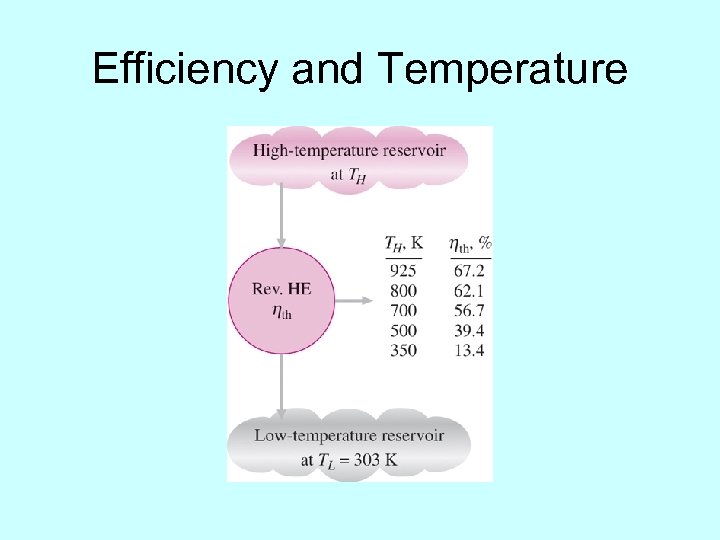 Efficiency and Temperature
Efficiency and Temperature
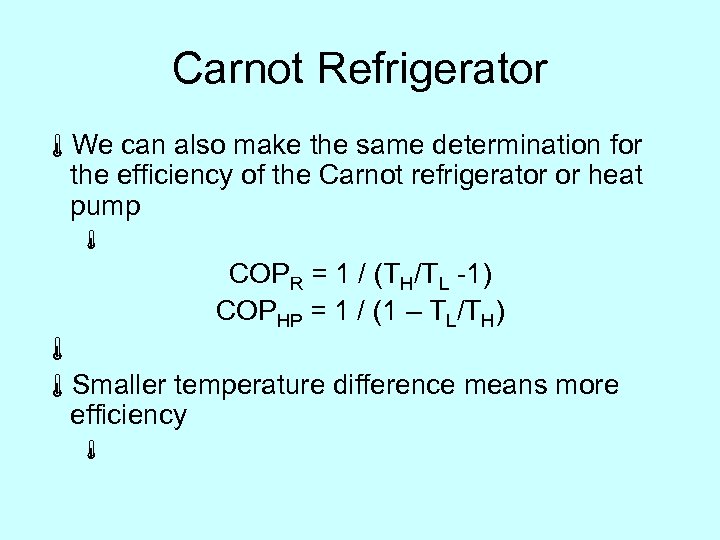 Carnot Refrigerator áWe can also make the same determination for the efficiency of the Carnot refrigerator or heat pump á COPR = 1 / (TH/TL -1) COPHP = 1 / (1 – TL/TH) á áSmaller temperature difference means more efficiency á
Carnot Refrigerator áWe can also make the same determination for the efficiency of the Carnot refrigerator or heat pump á COPR = 1 / (TH/TL -1) COPHP = 1 / (1 – TL/TH) á áSmaller temperature difference means more efficiency á
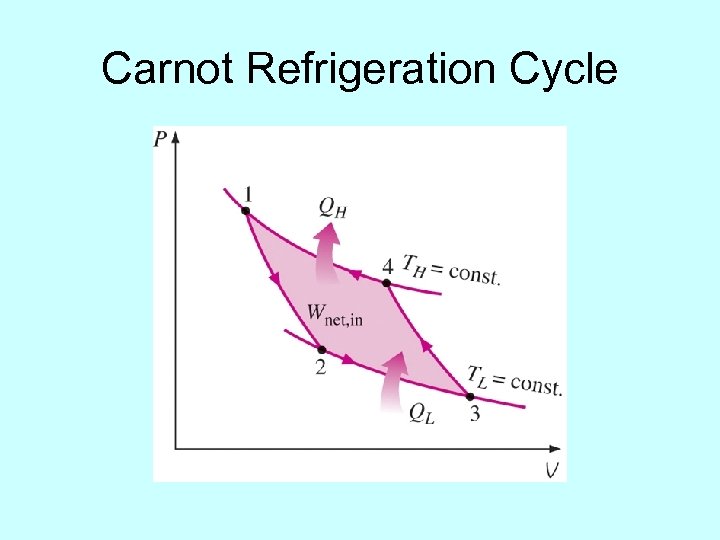 Carnot Refrigeration Cycle
Carnot Refrigeration Cycle
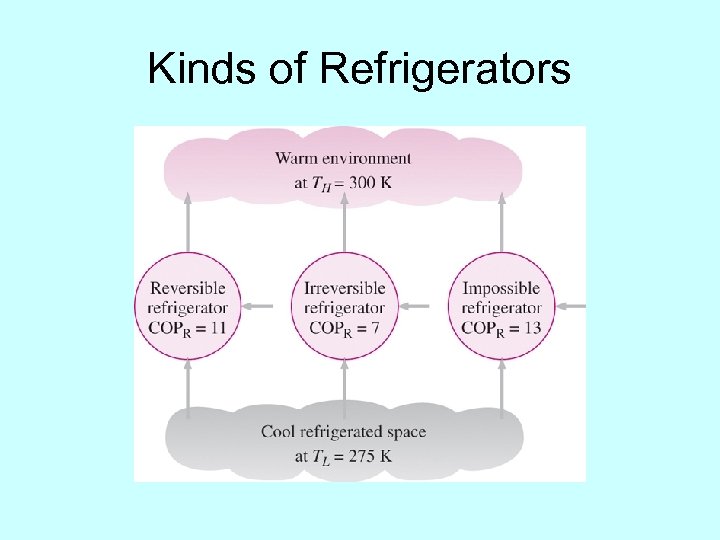 Kinds of Refrigerators
Kinds of Refrigerators
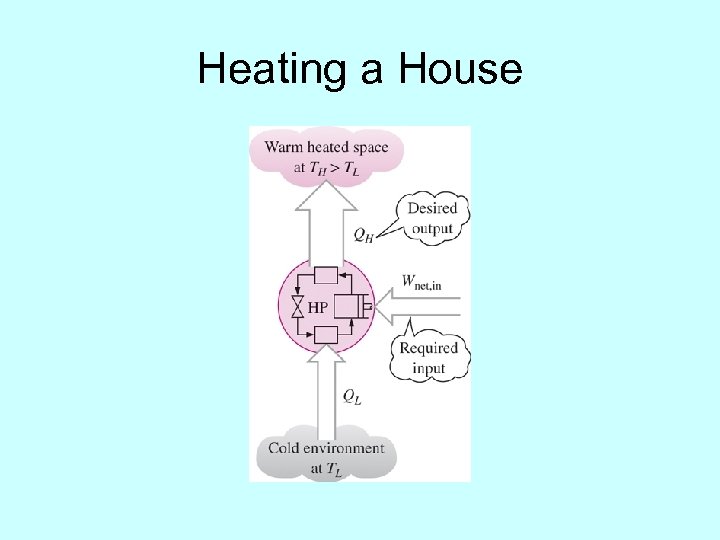 Heating a House
Heating a House
 Thermodynamic Temperature Scale á áThe efficiency of any engine depends on the ratio of the heats áThus we can determine the temperature of two reservoirs by measuring the heat flow in and out of an ideal engine operating between them
Thermodynamic Temperature Scale á áThe efficiency of any engine depends on the ratio of the heats áThus we can determine the temperature of two reservoirs by measuring the heat flow in and out of an ideal engine operating between them
 Kelvin Scale á áIf assign a magnitude to the degree size we get a complete temperature scale, independent of any substance in a thermometer á áNote that we don’t actually use an engine to find T
Kelvin Scale á áIf assign a magnitude to the degree size we get a complete temperature scale, independent of any substance in a thermometer á áNote that we don’t actually use an engine to find T
 Perpetual Motion á á 1 st kind: áMachine that creates energy á á 2 nd kind: áMachine that converts heat completely into work á á 3 rd kind: áMachine with no dissipation á
Perpetual Motion á á 1 st kind: áMachine that creates energy á á 2 nd kind: áMachine that converts heat completely into work á á 3 rd kind: áMachine with no dissipation á
 Next Time áRead: 7. 1 -7. 6 áHomework: Ch 6, P: 131, 138, Ch 7, P: 29, 37
Next Time áRead: 7. 1 -7. 6 áHomework: Ch 6, P: 131, 138, Ch 7, P: 29, 37