2f0dfec9ae1e4712d2035ce5ce46b124.ppt
- Количество слайдов: 69
 Care and Maintenance of GP Contact Lens Lecture 5 L 4 Version: 2012 -May-10 Published in Australia by The International Association of Contact Lens Educators Second. Edition 2011
Care and Maintenance of GP Contact Lens Lecture 5 L 4 Version: 2012 -May-10 Published in Australia by The International Association of Contact Lens Educators Second. Edition 2011
 COPYRIGHT NOTICE The IACLE Contact Lens Course (all formats) is the sole property of the International Association of Contact Lens Educators (IACLE) and is protected, without limitations, by copyright. By accessing this material, you agree to the following terms and conditions: You may only access and use the IACLE Contact Lens Course for personal or educational purposes. Any dissemination or sale of the IACLE Contact Lens Course, either in whole or in part, or use of the materials for other than educational and personal purposes, is strictly prohibited without the express written consent of IACLE. Except as declared below, you may not reproduce, republish, post, transmit, or distribute any material included in the IACLE Contact Lens Course. You may print materials for personal or educational purposes only. All copyright information, including the IACLE logo, must remain on the material. Appropriate reference must be provided to any use of the content of the IACLE Contact Lens Course, including text, images, &/or illustrations.
COPYRIGHT NOTICE The IACLE Contact Lens Course (all formats) is the sole property of the International Association of Contact Lens Educators (IACLE) and is protected, without limitations, by copyright. By accessing this material, you agree to the following terms and conditions: You may only access and use the IACLE Contact Lens Course for personal or educational purposes. Any dissemination or sale of the IACLE Contact Lens Course, either in whole or in part, or use of the materials for other than educational and personal purposes, is strictly prohibited without the express written consent of IACLE. Except as declared below, you may not reproduce, republish, post, transmit, or distribute any material included in the IACLE Contact Lens Course. You may print materials for personal or educational purposes only. All copyright information, including the IACLE logo, must remain on the material. Appropriate reference must be provided to any use of the content of the IACLE Contact Lens Course, including text, images, &/or illustrations.
 IACLE CONTACT LENS COURSE Published in Australia by The International Association of Contact Lens Educators Revised Edition 2011 The International Association of Contact Lens Educators 2000 -2011 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission, in writing, of: The International Association of Contact Lens Educators IACLE Secretariat, PO Box 656 KENSINGTON NSW 1465 Australia Email: iacle@iacle. org
IACLE CONTACT LENS COURSE Published in Australia by The International Association of Contact Lens Educators Revised Edition 2011 The International Association of Contact Lens Educators 2000 -2011 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission, in writing, of: The International Association of Contact Lens Educators IACLE Secretariat, PO Box 656 KENSINGTON NSW 1465 Australia Email: iacle@iacle. org
 SPONSORS Development and delivery of contact lens education by IACLE is supported through educational grants and in-kind contributions Industry Supporters Major In-Kind Supporters
SPONSORS Development and delivery of contact lens education by IACLE is supported through educational grants and in-kind contributions Industry Supporters Major In-Kind Supporters
 CONTRIBUTORS Care and Maintenance of GP Contact Lens Gina Sorbara, OD, MS, FAAO Rashmi Mahajan, BOptom Lewis Williams, AQIT(Optom), MOptom, Ph. D For a complete list of acknowledgements please see our website: www. iacle. org
CONTRIBUTORS Care and Maintenance of GP Contact Lens Gina Sorbara, OD, MS, FAAO Rashmi Mahajan, BOptom Lewis Williams, AQIT(Optom), MOptom, Ph. D For a complete list of acknowledgements please see our website: www. iacle. org
 CONTACT LENS DEPOSITS LENS MATERIAL: GP CLs • GP CLs are different: – charged sites water or deposit binding – no net overall charge – matrix porosity • Some contaminants soluble in GP materials – can form deposits on or in CLs • GP CL deposits take time to appear & more slowly than on other CLs – lipid deposits can occur on & in GP CLs – normally, deposits of lipid-calcium complexes do not occur on GP CLs
CONTACT LENS DEPOSITS LENS MATERIAL: GP CLs • GP CLs are different: – charged sites water or deposit binding – no net overall charge – matrix porosity • Some contaminants soluble in GP materials – can form deposits on or in CLs • GP CL deposits take time to appear & more slowly than on other CLs – lipid deposits can occur on & in GP CLs – normally, deposits of lipid-calcium complexes do not occur on GP CLs
 CONTACT LENS DEPOSITS LENS MATERIAL: GP CLs • GP CLs still susceptible to tear components presence of p. DMS in siloxane acrylate (SA) materials relatively hydrophobic surfaces with lipophilic tendencies hydrophilic monomers (e. g. methacrylic acid) added to CL wettability • –ve charged surfaces • protein deposition follows – – • Later, fluorine (F) added fluoro-siloxane acrylate CLs (FSAs): • • O 2 permeability, wettability, spoilage rates. still susceptible to lipid deposits
CONTACT LENS DEPOSITS LENS MATERIAL: GP CLs • GP CLs still susceptible to tear components presence of p. DMS in siloxane acrylate (SA) materials relatively hydrophobic surfaces with lipophilic tendencies hydrophilic monomers (e. g. methacrylic acid) added to CL wettability • –ve charged surfaces • protein deposition follows – – • Later, fluorine (F) added fluoro-siloxane acrylate CLs (FSAs): • • O 2 permeability, wettability, spoilage rates. still susceptible to lipid deposits
![CONTACT LENS CARE FDA HYDROPHOBIC LENS GROUPS (Stone [2007]) • Group 1 No silicon CONTACT LENS CARE FDA HYDROPHOBIC LENS GROUPS (Stone [2007]) • Group 1 No silicon](https://present5.com/presentation/2f0dfec9ae1e4712d2035ce5ce46b124/image-8.jpg) CONTACT LENS CARE FDA HYDROPHOBIC LENS GROUPS (Stone [2007]) • Group 1 No silicon or fluorine content • Group 2 Contains silicon but no fluorine focon stem • Group 3 Contains silicon & fluorine • Group 4 Contains fluorine but no silicon 5 L 1 -8
CONTACT LENS CARE FDA HYDROPHOBIC LENS GROUPS (Stone [2007]) • Group 1 No silicon or fluorine content • Group 2 Contains silicon but no fluorine focon stem • Group 3 Contains silicon & fluorine • Group 4 Contains fluorine but no silicon 5 L 1 -8
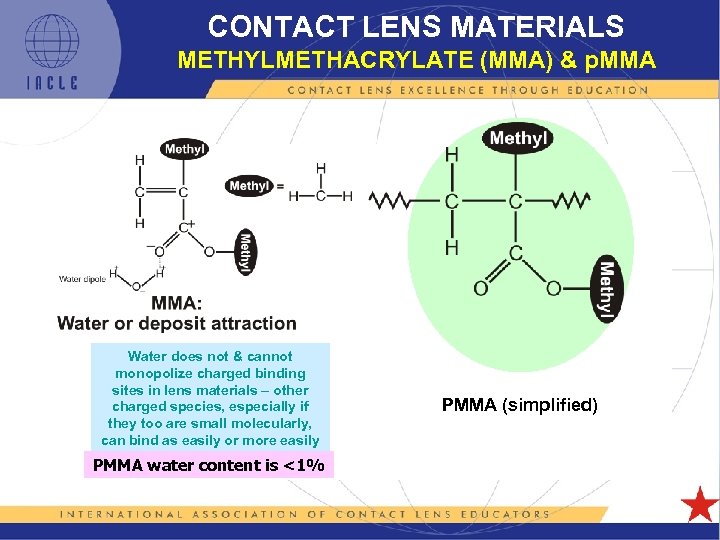 CONTACT LENS MATERIALS METHYLMETHACRYLATE (MMA) & p. MMA Water does not & cannot monopolize charged binding sites in lens materials – other charged species, especially if they too are small molecularly, can bind as easily or more easily PMMA water content is <1% PMMA (simplified)
CONTACT LENS MATERIALS METHYLMETHACRYLATE (MMA) & p. MMA Water does not & cannot monopolize charged binding sites in lens materials – other charged species, especially if they too are small molecularly, can bind as easily or more easily PMMA water content is <1% PMMA (simplified)
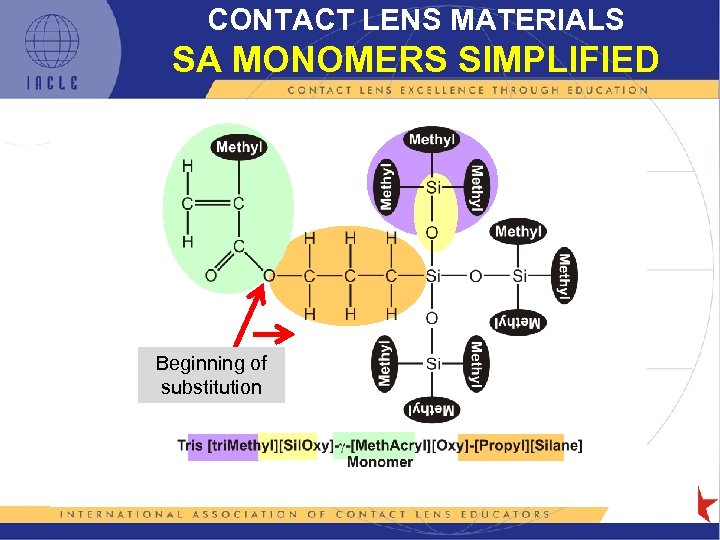 CONTACT LENS MATERIALS SA MONOMERS SIMPLIFIED Beginning of substitution
CONTACT LENS MATERIALS SA MONOMERS SIMPLIFIED Beginning of substitution
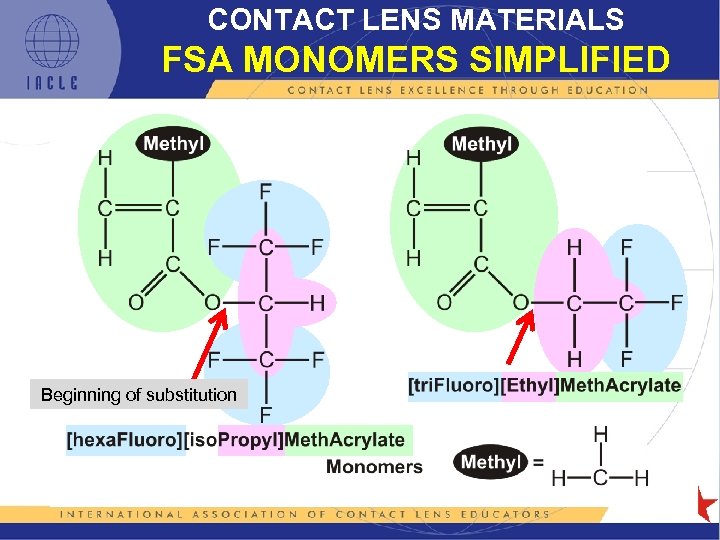 CONTACT LENS MATERIALS FSA MONOMERS SIMPLIFIED Beginning of substitution
CONTACT LENS MATERIALS FSA MONOMERS SIMPLIFIED Beginning of substitution
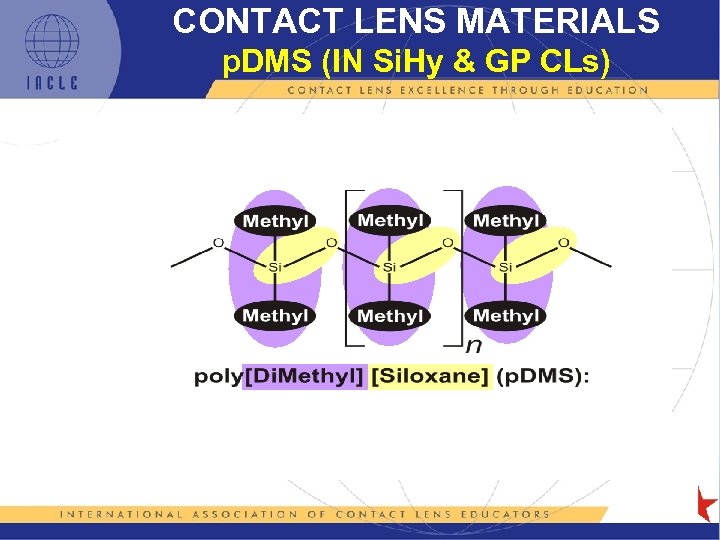 CONTACT LENS MATERIALS p. DMS (IN Si. Hy & GP CLs)
CONTACT LENS MATERIALS p. DMS (IN Si. Hy & GP CLs)
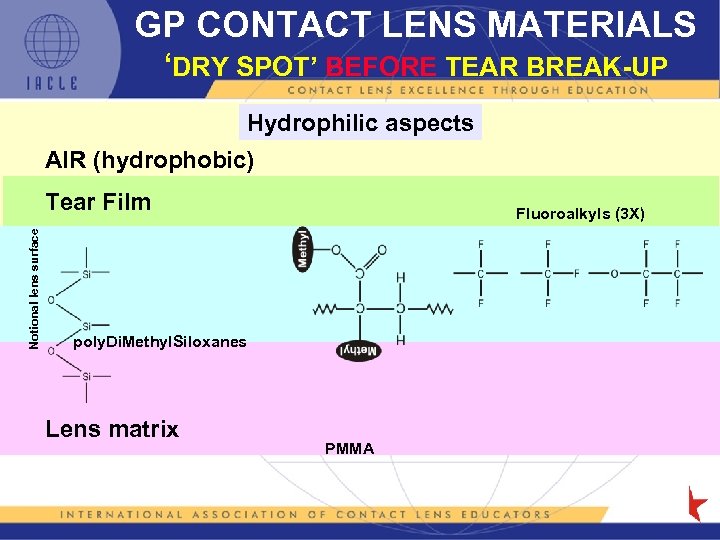 GP CONTACT LENS MATERIALS ‘DRY SPOT’ BEFORE TEAR BREAK-UP Hydrophilic aspects AIR (hydrophobic) Notional lens surface Tear Film Fluoroalkyls (3 X) poly. Di. Methyl. Siloxanes Lens matrix PMMA
GP CONTACT LENS MATERIALS ‘DRY SPOT’ BEFORE TEAR BREAK-UP Hydrophilic aspects AIR (hydrophobic) Notional lens surface Tear Film Fluoroalkyls (3 X) poly. Di. Methyl. Siloxanes Lens matrix PMMA
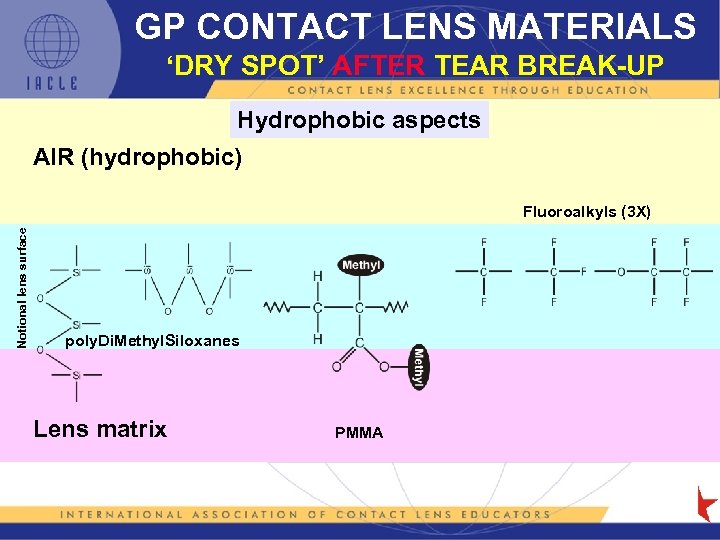 GP CONTACT LENS MATERIALS ‘DRY SPOT’ AFTER TEAR BREAK-UP Hydrophobic aspects AIR (hydrophobic) Notional lens surface Fluoroalkyls (3 X) poly. Di. Methyl. Siloxanes Lens matrix PMMA
GP CONTACT LENS MATERIALS ‘DRY SPOT’ AFTER TEAR BREAK-UP Hydrophobic aspects AIR (hydrophobic) Notional lens surface Fluoroalkyls (3 X) poly. Di. Methyl. Siloxanes Lens matrix PMMA
 CHARACTERISTICS OF GP CL MATERIALS Hydrophobicity: • Siloxane, e. g. p. DMS, SA, or FSA polymers O 2 but are hydrophobic & lipophilic – CL wettability lubricity lid traction lens movement comfort & ↕ vision – if CL surface-hydrophobic & lipophilic surfaces lipids, proteins, & other tear film components & contaminants (drying may deposition rates further) – treated wettability but in the long-term, treatment may not endure prolonged & repeated lens cleaning by rubbing
CHARACTERISTICS OF GP CL MATERIALS Hydrophobicity: • Siloxane, e. g. p. DMS, SA, or FSA polymers O 2 but are hydrophobic & lipophilic – CL wettability lubricity lid traction lens movement comfort & ↕ vision – if CL surface-hydrophobic & lipophilic surfaces lipids, proteins, & other tear film components & contaminants (drying may deposition rates further) – treated wettability but in the long-term, treatment may not endure prolonged & repeated lens cleaning by rubbing
 CHARACTERISTICS OF GP CL LENS MATERIALS continued. . . Pore Size • GP CL’s small pore size (<10 nm – see notes) most deposits cannot penetrate lens matrix & therefore tend to remain on surface • GP multipurpose solutions (GP MPSs) contain antimicrobial agents at higher concentrations than in SCL or Si. Hy CL care regimens. This is possible because GPs are typically non-ionic, have small pore sizes, & negligible water content. Disinfectants do not penetrate the lens matrix & adsorbed preservatives can be easily rinsed off the lens surface (Luensmann, 2009)
CHARACTERISTICS OF GP CL LENS MATERIALS continued. . . Pore Size • GP CL’s small pore size (<10 nm – see notes) most deposits cannot penetrate lens matrix & therefore tend to remain on surface • GP multipurpose solutions (GP MPSs) contain antimicrobial agents at higher concentrations than in SCL or Si. Hy CL care regimens. This is possible because GPs are typically non-ionic, have small pore sizes, & negligible water content. Disinfectants do not penetrate the lens matrix & adsorbed preservatives can be easily rinsed off the lens surface (Luensmann, 2009)
 CHARACTERISTICS OF GP CL LENS MATERIALS continued. . . Surface Chemistry • Surface chemistry of GP CLs is complex. Can be influenced further by environment, e. g. hydrophilic (normal tear film) or hydrophobic (dry spots deposits/surface contamination) – exposure to inappropriate LCPs or excessive polishing during manufacture ( localized heating) can wettability & other properties permanently – FSA materials can be plasma treated to their wettability • surface treatment may not last the life of the CL • surface failure may define CL ‘end-of-life’
CHARACTERISTICS OF GP CL LENS MATERIALS continued. . . Surface Chemistry • Surface chemistry of GP CLs is complex. Can be influenced further by environment, e. g. hydrophilic (normal tear film) or hydrophobic (dry spots deposits/surface contamination) – exposure to inappropriate LCPs or excessive polishing during manufacture ( localized heating) can wettability & other properties permanently – FSA materials can be plasma treated to their wettability • surface treatment may not last the life of the CL • surface failure may define CL ‘end-of-life’
 GP CL MATERIALS • Most modern GP CLs are FSA polymers although SA CLs are still in use – GP-specific LCPs are required – unlike single rôle SCL disinfecting solutions, GP disinfecting solutions disinfection & wettability – however, fundamentally, GP lens care goals are the same as for other CLs • Generally, GP LCPs should not be used on SCL or Si. Hy CLs
GP CL MATERIALS • Most modern GP CLs are FSA polymers although SA CLs are still in use – GP-specific LCPs are required – unlike single rôle SCL disinfecting solutions, GP disinfecting solutions disinfection & wettability – however, fundamentally, GP lens care goals are the same as for other CLs • Generally, GP LCPs should not be used on SCL or Si. Hy CLs
 GP CL CARE & MAINTENANCE BENEFITS • Although GP CLs are less deposit-prone than many other types of CLs, proper lens care is still the key to successful wear because it: – further deposit accumulation – microbial contamination ( organism adherence) – lens wettability – comfort & vision during CL wear – lens life
GP CL CARE & MAINTENANCE BENEFITS • Although GP CLs are less deposit-prone than many other types of CLs, proper lens care is still the key to successful wear because it: – further deposit accumulation – microbial contamination ( organism adherence) – lens wettability – comfort & vision during CL wear – lens life
 GP CLs ADVANTAGES • MK rates in GP CL wearers is lower than for Hy & Si. Hy CLs – – GPs: 1 -1. 5 per 10, 000 Hy CLs: 3. 5 per 10, 000 (20 per 10, 000 in EW) DW GP CLs have lowest risk Si. Hy CLs have rates similar to Hy CLs • CLPC rate • binding of Pseudomonas aeruginosa & Acanthamoeba spp.
GP CLs ADVANTAGES • MK rates in GP CL wearers is lower than for Hy & Si. Hy CLs – – GPs: 1 -1. 5 per 10, 000 Hy CLs: 3. 5 per 10, 000 (20 per 10, 000 in EW) DW GP CLs have lowest risk Si. Hy CLs have rates similar to Hy CLs • CLPC rate • binding of Pseudomonas aeruginosa & Acanthamoeba spp.
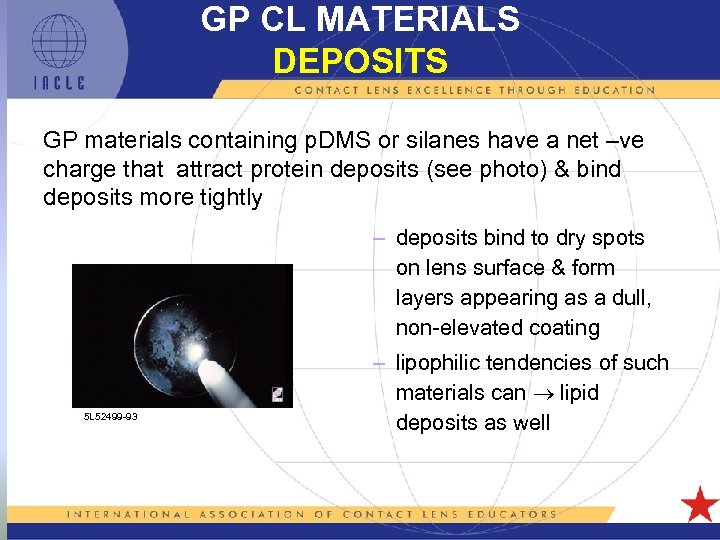 GP CL MATERIALS DEPOSITS GP materials containing p. DMS or silanes have a net –ve charge that attract protein deposits (see photo) & bind deposits more tightly – deposits bind to dry spots on lens surface & form layers appearing as a dull, non-elevated coating 5 L 52499 -93 – lipophilic tendencies of such materials can lipid deposits as well
GP CL MATERIALS DEPOSITS GP materials containing p. DMS or silanes have a net –ve charge that attract protein deposits (see photo) & bind deposits more tightly – deposits bind to dry spots on lens surface & form layers appearing as a dull, non-elevated coating 5 L 52499 -93 – lipophilic tendencies of such materials can lipid deposits as well
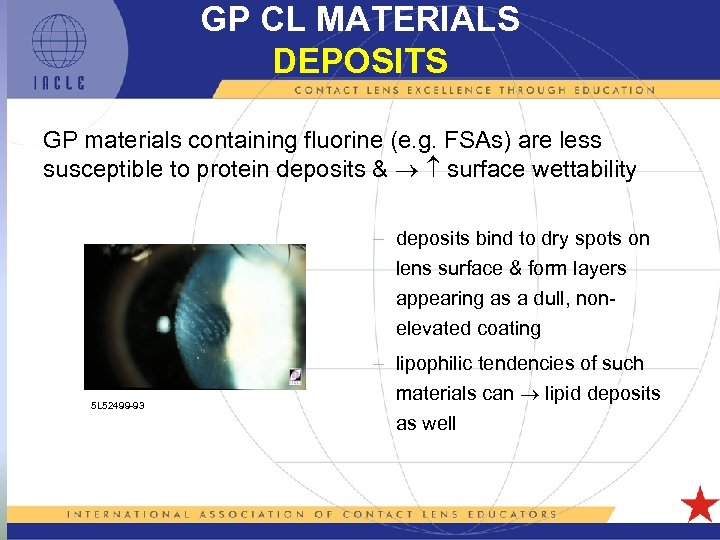 GP CL MATERIALS DEPOSITS GP materials containing fluorine (e. g. FSAs) are less susceptible to protein deposits & surface wettability – deposits bind to dry spots on lens surface & form layers appearing as a dull, nonelevated coating 5 L 52499 -93 – lipophilic tendencies of such materials can lipid deposits as well
GP CL MATERIALS DEPOSITS GP materials containing fluorine (e. g. FSAs) are less susceptible to protein deposits & surface wettability – deposits bind to dry spots on lens surface & form layers appearing as a dull, nonelevated coating 5 L 52499 -93 – lipophilic tendencies of such materials can lipid deposits as well
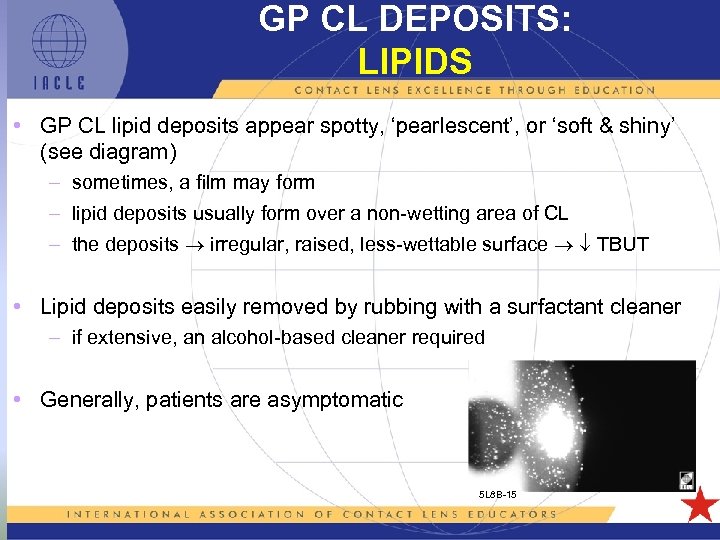 GP CL DEPOSITS: LIPIDS • GP CL lipid deposits appear spotty, ‘pearlescent’, or ‘soft & shiny’ (see diagram) – sometimes, a film may form – lipid deposits usually form over a non-wetting area of CL – the deposits irregular, raised, less-wettable surface TBUT • Lipid deposits easily removed by rubbing with a surfactant cleaner – if extensive, an alcohol-based cleaner required • Generally, patients are asymptomatic 5 L 8 B-15
GP CL DEPOSITS: LIPIDS • GP CL lipid deposits appear spotty, ‘pearlescent’, or ‘soft & shiny’ (see diagram) – sometimes, a film may form – lipid deposits usually form over a non-wetting area of CL – the deposits irregular, raised, less-wettable surface TBUT • Lipid deposits easily removed by rubbing with a surfactant cleaner – if extensive, an alcohol-based cleaner required • Generally, patients are asymptomatic 5 L 8 B-15
 GP CL DEPOSITS: LIPIDS • Incubated, unworn CLs show SA CLs accumulate 2 -3 X the lipid of SCLs (Si. Hy CLs not tested in this study) • Lipids bind to surface of GP CLs hydrophobicity protein deposition over the lipid layer – this lipid-protein coating alters subsequent lipid-protein deposition • It has been shown that lipid (but GPs still < Hy CLs) was apparent on worn GP CLs with or without surface treatment – the amount of lipid appeared to be independent of: • the material used • the presence or absence of surface treatment – some differences in lipid layer patterns were noted between materials
GP CL DEPOSITS: LIPIDS • Incubated, unworn CLs show SA CLs accumulate 2 -3 X the lipid of SCLs (Si. Hy CLs not tested in this study) • Lipids bind to surface of GP CLs hydrophobicity protein deposition over the lipid layer – this lipid-protein coating alters subsequent lipid-protein deposition • It has been shown that lipid (but GPs still < Hy CLs) was apparent on worn GP CLs with or without surface treatment – the amount of lipid appeared to be independent of: • the material used • the presence or absence of surface treatment – some differences in lipid layer patterns were noted between materials
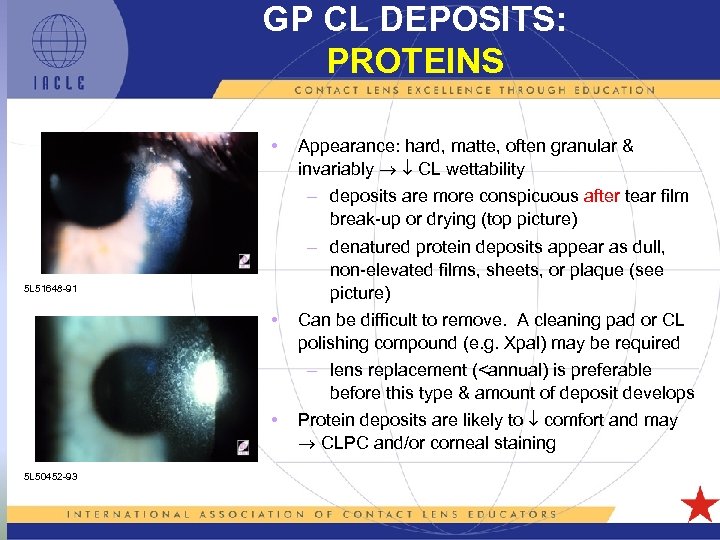 GP CL DEPOSITS: PROTEINS • 5 L 51648 -91 • • 5 L 50452 -93 Appearance: hard, matte, often granular & invariably CL wettability – deposits are more conspicuous after tear film break-up or drying (top picture) – denatured protein deposits appear as dull, non-elevated films, sheets, or plaque (see picture) Can be difficult to remove. A cleaning pad or CL polishing compound (e. g. Xpal) may be required – lens replacement (
GP CL DEPOSITS: PROTEINS • 5 L 51648 -91 • • 5 L 50452 -93 Appearance: hard, matte, often granular & invariably CL wettability – deposits are more conspicuous after tear film break-up or drying (top picture) – denatured protein deposits appear as dull, non-elevated films, sheets, or plaque (see picture) Can be difficult to remove. A cleaning pad or CL polishing compound (e. g. Xpal) may be required – lens replacement (
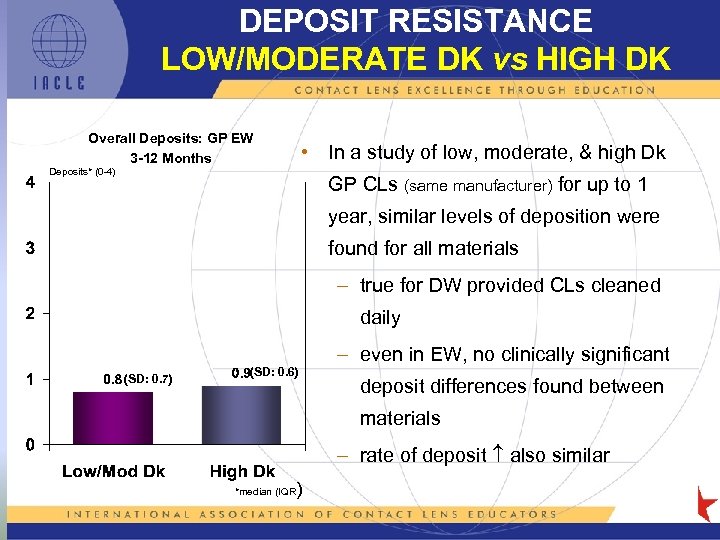 DEPOSIT RESISTANCE LOW/MODERATE DK vs HIGH DK Overall Deposits: GP EW 3 -12 Months • In a study of low, moderate, & high Dk Deposits* (0 -4) GP CLs (same manufacturer) for up to 1 year, similar levels of deposition were found for all materials – true for DW provided CLs cleaned daily (SD: 0. 7) (SD: 0. 6) – even in EW, no clinically significant deposit differences found between materials – rate of deposit also similar ) *median (IQR
DEPOSIT RESISTANCE LOW/MODERATE DK vs HIGH DK Overall Deposits: GP EW 3 -12 Months • In a study of low, moderate, & high Dk Deposits* (0 -4) GP CLs (same manufacturer) for up to 1 year, similar levels of deposition were found for all materials – true for DW provided CLs cleaned daily (SD: 0. 7) (SD: 0. 6) – even in EW, no clinically significant deposit differences found between materials – rate of deposit also similar ) *median (IQR
 GP CL CARE PRODUCT CATEGORIES • Cleaners : – there are two types of ‘cleaner’: • daily surfactant • protein removers (supplied in tablet or liquid form) • Disinfecting/Soaking and Wetting Solutions: – sometimes also referred to as conditioning solutions – can be classified as multipurpose (MPS) because they fulfill the functions of disinfection, soaking, & wetting • Lubricants: – these are in-eye products intended for instillation while the CLs are being worn. They re-wet, cushion, & rehydrate the CL in situ
GP CL CARE PRODUCT CATEGORIES • Cleaners : – there are two types of ‘cleaner’: • daily surfactant • protein removers (supplied in tablet or liquid form) • Disinfecting/Soaking and Wetting Solutions: – sometimes also referred to as conditioning solutions – can be classified as multipurpose (MPS) because they fulfill the functions of disinfection, soaking, & wetting • Lubricants: – these are in-eye products intended for instillation while the CLs are being worn. They re-wet, cushion, & rehydrate the CL in situ
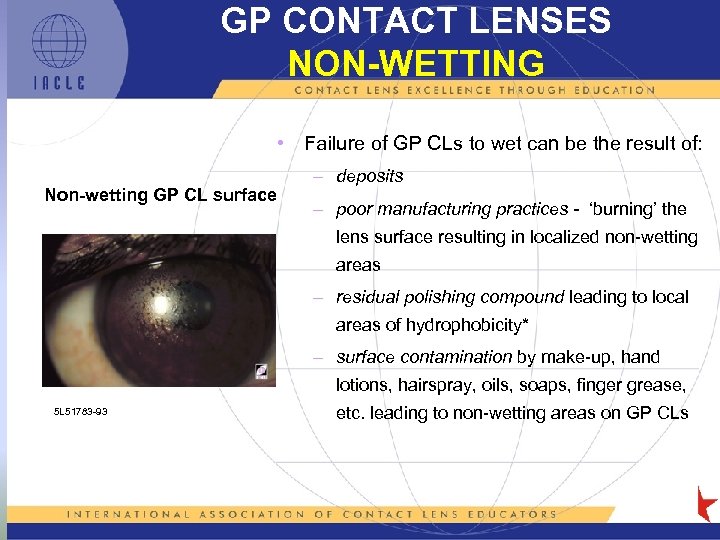 GP CONTACT LENSES NON-WETTING • Failure of GP CLs to wet can be the result of: Non-wetting GP CL surface – deposits – poor manufacturing practices - ‘burning’ the lens surface resulting in localized non-wetting areas – residual polishing compound leading to local areas of hydrophobicity* – surface contamination by make-up, hand lotions, hairspray, oils, soaps, finger grease, 5 L 51783 -93 etc. leading to non-wetting areas on GP CLs
GP CONTACT LENSES NON-WETTING • Failure of GP CLs to wet can be the result of: Non-wetting GP CL surface – deposits – poor manufacturing practices - ‘burning’ the lens surface resulting in localized non-wetting areas – residual polishing compound leading to local areas of hydrophobicity* – surface contamination by make-up, hand lotions, hairspray, oils, soaps, finger grease, 5 L 51783 -93 etc. leading to non-wetting areas on GP CLs
 GP CL CARE CLEANING • Surfactant cleaning: – surfactant cleaners for GP CLs similar to SCL counterparts – alcohol-based cleaners (increasingly uncommon) well suited to GP CLs that acquire lipid deposits. • alcohol-based cleaners must be rinsed from lens surface promptly & thoroughly as they can alter lens parameters if allowed to remain on GP CLs – some cleaners contain ‘abrasive’ particulate matter that are more effective against adherent deposits than normal surfactant cleaners • they have been shown to be safe in normal use
GP CL CARE CLEANING • Surfactant cleaning: – surfactant cleaners for GP CLs similar to SCL counterparts – alcohol-based cleaners (increasingly uncommon) well suited to GP CLs that acquire lipid deposits. • alcohol-based cleaners must be rinsed from lens surface promptly & thoroughly as they can alter lens parameters if allowed to remain on GP CLs – some cleaners contain ‘abrasive’ particulate matter that are more effective against adherent deposits than normal surfactant cleaners • they have been shown to be safe in normal use
 RGP LENS CARE CLEANING • Enzymatic cleaners are available in tablet (most commonly) or liquid form & contain either an enzyme or a chemical agent – they are recommended for weekly (sometimes daily) protein removal in deposit-prone wearers* • Surface polishing may be necessary for lenses >12 months old 5 L 51552 -91 • A cleaning pad may be effective in removing some deposits from GP lenses (opposite)
RGP LENS CARE CLEANING • Enzymatic cleaners are available in tablet (most commonly) or liquid form & contain either an enzyme or a chemical agent – they are recommended for weekly (sometimes daily) protein removal in deposit-prone wearers* • Surface polishing may be necessary for lenses >12 months old 5 L 51552 -91 • A cleaning pad may be effective in removing some deposits from GP lenses (opposite)
 GP CLs CLEANING TECHNIQUE • GP CLs more susceptible to abrasions & less rigid than PMMA –need careful handling –place CL in the taut palm of the hand • avoid holding the CL between thumb & forefinger due to risk of distortion or lens fracture 5 L 50170 -93 – • • • rub lens with forefinger for 10 seconds avoid aggressive pressure & overly vigorous rubbing avoid prolonged cleaning with ‘abrasive’ cleaners front surface’s first curve junction on high plus CLs is more difficult to clean due to ‘bridging’ by finger or palm. A ‘ring’ deposit can result
GP CLs CLEANING TECHNIQUE • GP CLs more susceptible to abrasions & less rigid than PMMA –need careful handling –place CL in the taut palm of the hand • avoid holding the CL between thumb & forefinger due to risk of distortion or lens fracture 5 L 50170 -93 – • • • rub lens with forefinger for 10 seconds avoid aggressive pressure & overly vigorous rubbing avoid prolonged cleaning with ‘abrasive’ cleaners front surface’s first curve junction on high plus CLs is more difficult to clean due to ‘bridging’ by finger or palm. A ‘ring’ deposit can result
 GP LENSES CLEANING TECHNIQUE – rinse CL with sterile saline or GP CL MPS • final lens rinse should be with manufacturer’s recommended soaking/storage/conditioning solution 5 L 50833 -94 Caution: If aerosol saline used for rinsing, propellant forms small bubbles in dispensed saline. These may cause ‘dimple veiling’ (opposite) if the lens is applied to the eye immediately. Dimple veiling can blurred vision
GP LENSES CLEANING TECHNIQUE – rinse CL with sterile saline or GP CL MPS • final lens rinse should be with manufacturer’s recommended soaking/storage/conditioning solution 5 L 50833 -94 Caution: If aerosol saline used for rinsing, propellant forms small bubbles in dispensed saline. These may cause ‘dimple veiling’ (opposite) if the lens is applied to the eye immediately. Dimple veiling can blurred vision
 GP LENSES RUB or NO RUB Fortunately for GP CL wearers, the misguided push to abandon CL rubbing as the initial step in lens care was much less aggressive & less successful among GP CL practitioners & wearers. This means that less ‘undoing’ of this falsehood should be required
GP LENSES RUB or NO RUB Fortunately for GP CL wearers, the misguided push to abandon CL rubbing as the initial step in lens care was much less aggressive & less successful among GP CL practitioners & wearers. This means that less ‘undoing’ of this falsehood should be required
 GP LENS CARE DISINFECTION • GP CL surfaces are just as susceptible to bacterial colonization as SCLs. Bacteria can also attach to deposits • As most GP CLs are thermoplastics (heat-deformable), thermal disinfection not used • Soaking time usually four hours to overnight – preservatives used include: thimerosal, phenylmercuric nitrate, BAK (benzalkonium chloride), CHX (chlorhexidine), a PHX (polihexanide), or PQ-1 (polyquaternium-1) • GP care system simplicity & efficacy has resulted in the demise of the usage of peroxide on GP CLs*
GP LENS CARE DISINFECTION • GP CL surfaces are just as susceptible to bacterial colonization as SCLs. Bacteria can also attach to deposits • As most GP CLs are thermoplastics (heat-deformable), thermal disinfection not used • Soaking time usually four hours to overnight – preservatives used include: thimerosal, phenylmercuric nitrate, BAK (benzalkonium chloride), CHX (chlorhexidine), a PHX (polihexanide), or PQ-1 (polyquaternium-1) • GP care system simplicity & efficacy has resulted in the demise of the usage of peroxide on GP CLs*
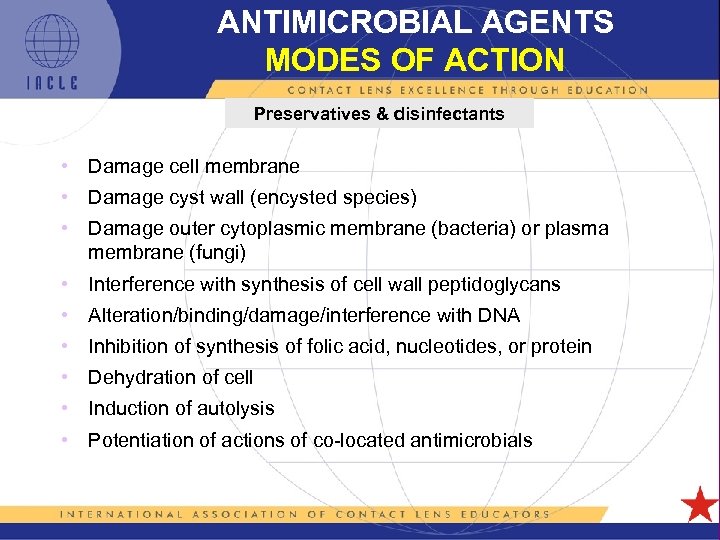 ANTIMICROBIAL AGENTS MODES OF ACTION Preservatives & disinfectants • Damage cell membrane • Damage cyst wall (encysted species) • Damage outer cytoplasmic membrane (bacteria) or plasma membrane (fungi) • Interference with synthesis of cell wall peptidoglycans • Alteration/binding/damage/interference with DNA • Inhibition of synthesis of folic acid, nucleotides, or protein • Dehydration of cell • Induction of autolysis • Potentiation of actions of co-located antimicrobials 5 L 1 -35
ANTIMICROBIAL AGENTS MODES OF ACTION Preservatives & disinfectants • Damage cell membrane • Damage cyst wall (encysted species) • Damage outer cytoplasmic membrane (bacteria) or plasma membrane (fungi) • Interference with synthesis of cell wall peptidoglycans • Alteration/binding/damage/interference with DNA • Inhibition of synthesis of folic acid, nucleotides, or protein • Dehydration of cell • Induction of autolysis • Potentiation of actions of co-located antimicrobials 5 L 1 -35
 GP LENS CARE WETTING • Wetting solutions CL wetting characteristics: – hydrophobic surface hydrophilic – uniform tear spreading vision ( optical quality) – comfort on insertion ( solution viscosity) • however, excessive viscosity temporary in vision quality ( ‘tear film’ thickness & regularity) • Wetting agents: PVA (polyvinyl alcohol), PVP (polyvinyl pyrrolidone), & Polysorbate • Saliva must never be used to wet a CL because of its microflora & fauna • Although less common now, single-purpose GP wetting solutions have been/are available
GP LENS CARE WETTING • Wetting solutions CL wetting characteristics: – hydrophobic surface hydrophilic – uniform tear spreading vision ( optical quality) – comfort on insertion ( solution viscosity) • however, excessive viscosity temporary in vision quality ( ‘tear film’ thickness & regularity) • Wetting agents: PVA (polyvinyl alcohol), PVP (polyvinyl pyrrolidone), & Polysorbate • Saliva must never be used to wet a CL because of its microflora & fauna • Although less common now, single-purpose GP wetting solutions have been/are available
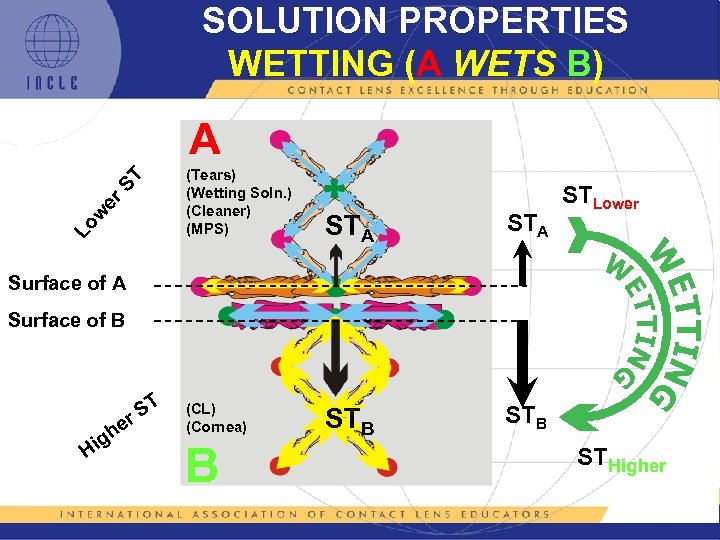 SOLUTION PROPERTIES WETTING (A WETS B) Lo w er ST A (Tears) (Wetting Soln. ) (Cleaner) (MPS) STA STB STLower STB Surface of A Surface of B Hi g he ST r (CL) (Cornea) B STHigher
SOLUTION PROPERTIES WETTING (A WETS B) Lo w er ST A (Tears) (Wetting Soln. ) (Cleaner) (MPS) STA STB STLower STB Surface of A Surface of B Hi g he ST r (CL) (Cornea) B STHigher
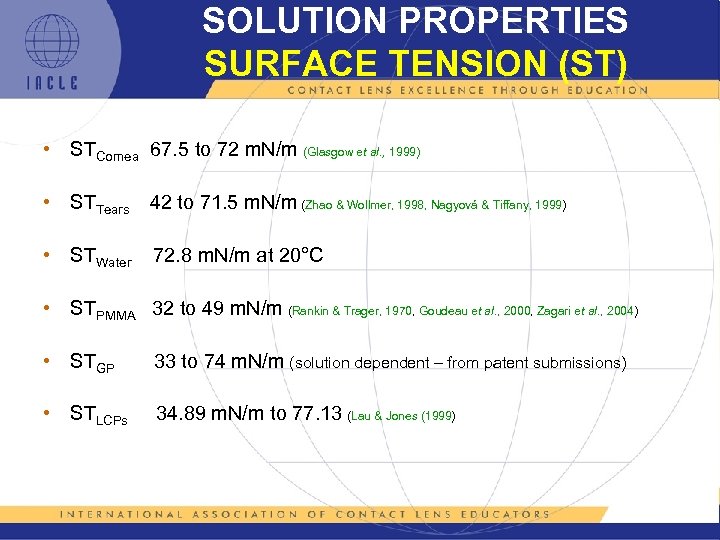 SOLUTION PROPERTIES SURFACE TENSION (ST) • STCornea 67. 5 to 72 m. N/m (Glasgow et al. , 1999) • STTears 42 to 71. 5 m. N/m (Zhao & Wollmer, 1998, Nagyová & Tiffany, 1999) • STWater 72. 8 m. N/m at 20°C • STPMMA 32 to 49 m. N/m (Rankin & Trager, 1970, Goudeau et al. , 2000, Zagari et al. , 2004) • STGP 33 to 74 m. N/m (solution dependent – from patent submissions) • STLCPs 34. 89 m. N/m to 77. 13 (Lau & Jones (1999)
SOLUTION PROPERTIES SURFACE TENSION (ST) • STCornea 67. 5 to 72 m. N/m (Glasgow et al. , 1999) • STTears 42 to 71. 5 m. N/m (Zhao & Wollmer, 1998, Nagyová & Tiffany, 1999) • STWater 72. 8 m. N/m at 20°C • STPMMA 32 to 49 m. N/m (Rankin & Trager, 1970, Goudeau et al. , 2000, Zagari et al. , 2004) • STGP 33 to 74 m. N/m (solution dependent – from patent submissions) • STLCPs 34. 89 m. N/m to 77. 13 (Lau & Jones (1999)
 GP LENS CARE WETTING & SOAKING GP CL Soaking/ Wetting/ Conditioning • Purpose – disinfection: wet storage microbial control of CL storage conditions & initial ‘on-eye’ comfort – wetting: dry storage of GP CLs wetting, CL hydration & flatter BOZR • maximum in situ lens hydration may also be affected – storage : better for regular GP CL wearers to wet-store their CLs* • Solutions that combine the disinfecting, wetting, & soaking functions are often called conditioning solutions
GP LENS CARE WETTING & SOAKING GP CL Soaking/ Wetting/ Conditioning • Purpose – disinfection: wet storage microbial control of CL storage conditions & initial ‘on-eye’ comfort – wetting: dry storage of GP CLs wetting, CL hydration & flatter BOZR • maximum in situ lens hydration may also be affected – storage : better for regular GP CL wearers to wet-store their CLs* • Solutions that combine the disinfecting, wetting, & soaking functions are often called conditioning solutions
 GP LENS CARE WETTING & SOAKING • Composition of GP CL wetting & soaking (conditioning) solutions: – antimicrobial agent(s): disinfect CLs & preserve the solution after initial opening – wetting agent: lens wettability – viscosity-increasing agent : ‘thicken’ formulation & stay-time of solution on CLs after insertion – buffer system: maintain p. H within limits – osmolality adjusting agents: added to solution osmolality to isotonicity with the wearer’s tears* (or near isotonicity) – p. H-adjusting agent(s): set initial solution p. H
GP LENS CARE WETTING & SOAKING • Composition of GP CL wetting & soaking (conditioning) solutions: – antimicrobial agent(s): disinfect CLs & preserve the solution after initial opening – wetting agent: lens wettability – viscosity-increasing agent : ‘thicken’ formulation & stay-time of solution on CLs after insertion – buffer system: maintain p. H within limits – osmolality adjusting agents: added to solution osmolality to isotonicity with the wearer’s tears* (or near isotonicity) – p. H-adjusting agent(s): set initial solution p. H
 GP LENS CARE RINSING • Rinsing GP CLs after soaking in wetting/soaking (conditioning) solution usually unnecessary – insert lens directly into eye • If vision blurry/variable on lens insertion, change to a less viscous solution • If stinging on insertion reported, change to a solution with different chemistry and/or p. H characteristics • Rinsing with sterile saline before insertion likely to CL wetting & initial comfort
GP LENS CARE RINSING • Rinsing GP CLs after soaking in wetting/soaking (conditioning) solution usually unnecessary – insert lens directly into eye • If vision blurry/variable on lens insertion, change to a less viscous solution • If stinging on insertion reported, change to a solution with different chemistry and/or p. H characteristics • Rinsing with sterile saline before insertion likely to CL wetting & initial comfort
 GP LENS CARE MULTI-PURPOSE SOLUTIONS • GP CL MPSs combine cleaning, disinfection, & soaking functions into a single bottle – convenient for user – sometimes referred to as one-bottle systems (OBS)
GP LENS CARE MULTI-PURPOSE SOLUTIONS • GP CL MPSs combine cleaning, disinfection, & soaking functions into a single bottle – convenient for user – sometimes referred to as one-bottle systems (OBS)
 GP LENS CARE LUBRICATING DROPS • Lubricating drops in situ wetting of CLs – contain surfactants & agent(s) viscosity – functions include: • comfort • CL cleaning • & CL wettability • friction between lids, CL, & cornea 5 L 31692 -91 • volume of viscous fluid on the anterior eye • removing debris from the PLTF by CL movement
GP LENS CARE LUBRICATING DROPS • Lubricating drops in situ wetting of CLs – contain surfactants & agent(s) viscosity – functions include: • comfort • CL cleaning • & CL wettability • friction between lids, CL, & cornea 5 L 31692 -91 • volume of viscous fluid on the anterior eye • removing debris from the PLTF by CL movement
 GP LENS CARE LUBRICATING DROPS Lubricating drops usually contain: • Preservative (but preservative-free lubricants do exist, usually in unitdose form) – e. g. benzyl alcohol, EDTA, BAK, PQ-1, CHX, PAPB (a PHX), sodium perborate, or stabilized oxychloro complex • Viscosity-increasing agent, e. g. methyl cellulose, hydroxypropyl methyl cellulose (hypromellose), or hydroxyethyl cellulose • Wetting agent, e. g. polyvinyl alcohol (PVA) • p. H-adjusting agents, e. g. trace amounts of Na. OH &/or HCl • Buffer system to maintain p. H, e. g. borate or citrate system • Osmolality adjusting agent(s), e. g. sodium chloride
GP LENS CARE LUBRICATING DROPS Lubricating drops usually contain: • Preservative (but preservative-free lubricants do exist, usually in unitdose form) – e. g. benzyl alcohol, EDTA, BAK, PQ-1, CHX, PAPB (a PHX), sodium perborate, or stabilized oxychloro complex • Viscosity-increasing agent, e. g. methyl cellulose, hydroxypropyl methyl cellulose (hypromellose), or hydroxyethyl cellulose • Wetting agent, e. g. polyvinyl alcohol (PVA) • p. H-adjusting agents, e. g. trace amounts of Na. OH &/or HCl • Buffer system to maintain p. H, e. g. borate or citrate system • Osmolality adjusting agent(s), e. g. sodium chloride
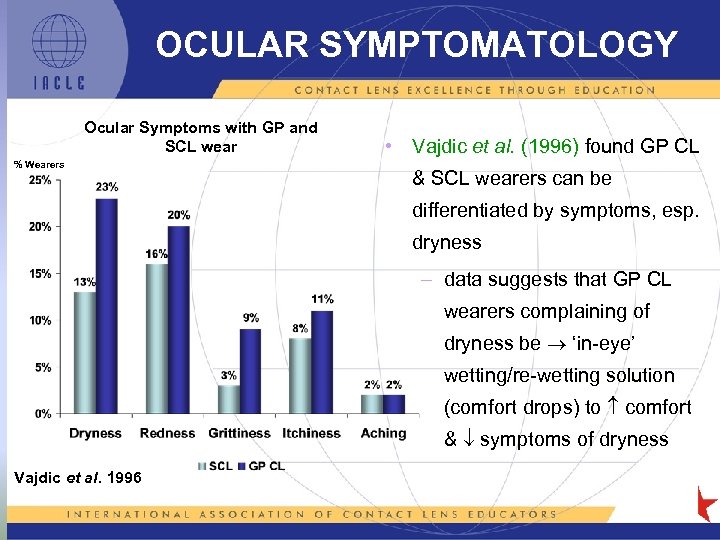 OCULAR SYMPTOMATOLOGY Ocular Symptoms with GP and SCL wear % Wearers • Vajdic et al. (1996) found GP CL & SCL wearers can be differentiated by symptoms, esp. dryness – data suggests that GP CL wearers complaining of dryness be ‘in-eye’ wetting/re-wetting solution (comfort drops) to comfort & symptoms of dryness Vajdic et al. 1996
OCULAR SYMPTOMATOLOGY Ocular Symptoms with GP and SCL wear % Wearers • Vajdic et al. (1996) found GP CL & SCL wearers can be differentiated by symptoms, esp. dryness – data suggests that GP CL wearers complaining of dryness be ‘in-eye’ wetting/re-wetting solution (comfort drops) to comfort & symptoms of dryness Vajdic et al. 1996
 GP LENS CARE COMPLICATIONS • Adverse reactions to GP LCPs are uncommon: – preservatives not normally absorbed by GP CLs*. – solution toxicity possible, but small volume on GP CL the ‘dose’ size • Punctate staining can occur • Viscous soaking solutions can corneal adherence staytime or solution residency – wearers may complain of blurry/fluctuating vision & a sticky sensation some time after lens insertion. – a dehydrated-solution residue may be left on the eyelashes/lid margins.
GP LENS CARE COMPLICATIONS • Adverse reactions to GP LCPs are uncommon: – preservatives not normally absorbed by GP CLs*. – solution toxicity possible, but small volume on GP CL the ‘dose’ size • Punctate staining can occur • Viscous soaking solutions can corneal adherence staytime or solution residency – wearers may complain of blurry/fluctuating vision & a sticky sensation some time after lens insertion. – a dehydrated-solution residue may be left on the eyelashes/lid margins.
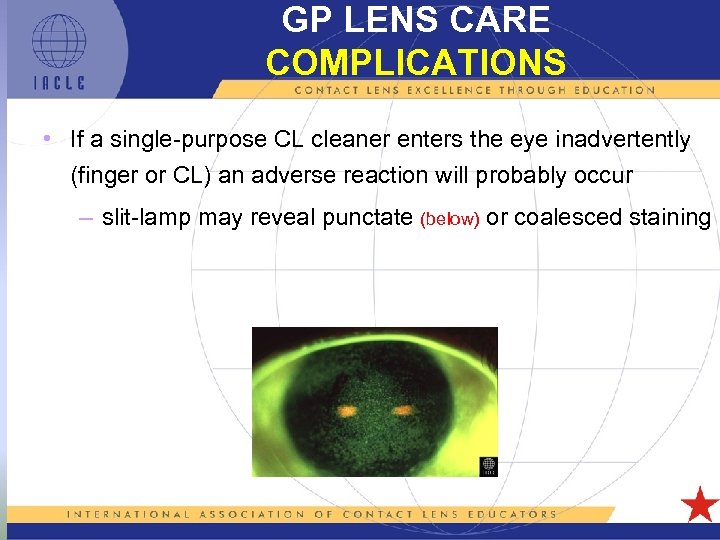 GP LENS CARE COMPLICATIONS • If a single-purpose CL cleaner enters the eye inadvertently (finger or CL) an adverse reaction will probably occur – slit-lamp may reveal punctate (below) or coalesced staining
GP LENS CARE COMPLICATIONS • If a single-purpose CL cleaner enters the eye inadvertently (finger or CL) an adverse reaction will probably occur – slit-lamp may reveal punctate (below) or coalesced staining
 GP LENS CARE STAINING MANAGEMENT • Remove CL immediately • Irrigate eye with saline (dilutes, rinses, & lubricates) • Examine for possible staining with a slit-lamp (previous slide) • Ocular lubricant may be useful (preservative-free best) • Epithelial ‘repair’ should be rapid – monitor eye for 24 -48 hours if staining significant • CLs should not be worn ( recovery rate) until epithelium appears normal
GP LENS CARE STAINING MANAGEMENT • Remove CL immediately • Irrigate eye with saline (dilutes, rinses, & lubricates) • Examine for possible staining with a slit-lamp (previous slide) • Ocular lubricant may be useful (preservative-free best) • Epithelial ‘repair’ should be rapid – monitor eye for 24 -48 hours if staining significant • CLs should not be worn ( recovery rate) until epithelium appears normal
 GP LENS CARE LENS REPLACEMENT • Purpose: regular CL replacement = preventative eye care – problems CL deposits – deposits can: • irritate lids, especially upper lids • deposit build-up deposit cycle • act as footholds for micro-organisms • Schedule: – GP CLs should be replaced at least annually. – more often (eg. six-monthly [Woods & Efron, 1996]), if the patient is wearing EW CLs &/or is a heavy depositor • CL deposit susceptibility should be considered when prescribing a replacement regimen – GP CL programmed replacement schemes do exist
GP LENS CARE LENS REPLACEMENT • Purpose: regular CL replacement = preventative eye care – problems CL deposits – deposits can: • irritate lids, especially upper lids • deposit build-up deposit cycle • act as footholds for micro-organisms • Schedule: – GP CLs should be replaced at least annually. – more often (eg. six-monthly [Woods & Efron, 1996]), if the patient is wearing EW CLs &/or is a heavy depositor • CL deposit susceptibility should be considered when prescribing a replacement regimen – GP CL programmed replacement schemes do exist
 GP LENS CARE AT DISPENSING • Upon receipt & before dispensing, GP CLs should be: – cleaned & soaked in conditioning solution overnight – after soaking, parameters should be verified • cleaning & soaking restores lens parameters (many custom-lens are shipped dry) • also removes any manufacturing process residues • lens wettability should be optimal at dispensing time – confirm lens wettability by observing CL surfaces in vitro (slit-lamp, stereo microscope, or a magnifier [specular reflection probably best])
GP LENS CARE AT DISPENSING • Upon receipt & before dispensing, GP CLs should be: – cleaned & soaked in conditioning solution overnight – after soaking, parameters should be verified • cleaning & soaking restores lens parameters (many custom-lens are shipped dry) • also removes any manufacturing process residues • lens wettability should be optimal at dispensing time – confirm lens wettability by observing CL surfaces in vitro (slit-lamp, stereo microscope, or a magnifier [specular reflection probably best])
 GP LENS CARE TRIAL SET DISINFECTION/STORAGE • Clean CLs with alcohol-based cleaner immediately after use (Ghajar et al. , 1989) – with a brief cleaning & a thorough rinsing, GP lens parameter stability is unaffected by such cleaners (Lowther, 1987) 5 L 11306 -91
GP LENS CARE TRIAL SET DISINFECTION/STORAGE • Clean CLs with alcohol-based cleaner immediately after use (Ghajar et al. , 1989) – with a brief cleaning & a thorough rinsing, GP lens parameter stability is unaffected by such cleaners (Lowther, 1987) 5 L 11306 -91
 GP LENS CARE TRIAL SET DISINFECTION/STORAGE • For storage, either: − − container that suspends the CL (opposite) without stress (recommended) or, flat-pack with CL placed concave-up to make removal easy while wet storage in soaking solution may suit a busy GP CL practice. . . dry storage might be easier to administer in a practice that sees few GP CL patients − trial fits with just-wetted, normally dry-stored CLs may be bad GP CL ‘marketing • Wet storage necessitates frequent & scheduled solution changes (14 -28 days) • GP CLs should be cleaned again immediately before a trial fit 5 L 40 LW 1 -98
GP LENS CARE TRIAL SET DISINFECTION/STORAGE • For storage, either: − − container that suspends the CL (opposite) without stress (recommended) or, flat-pack with CL placed concave-up to make removal easy while wet storage in soaking solution may suit a busy GP CL practice. . . dry storage might be easier to administer in a practice that sees few GP CL patients − trial fits with just-wetted, normally dry-stored CLs may be bad GP CL ‘marketing • Wet storage necessitates frequent & scheduled solution changes (14 -28 days) • GP CLs should be cleaned again immediately before a trial fit 5 L 40 LW 1 -98
 GP LENS CARE SUMMARY • Due of their non-absorbent nature, surface properties, rigidity, & durability, GP CLs are easier to care for than SCLs • However, as GP CLs are kept in regular use longer than SCLs, efficacious lens care is essential to successful long-term lens wear • Wearers should Clean, Rinse, And Disinfectant Lenses Every time (CRADLE) • Programmed replacement of GP CLs is an important factor in maintaining ocular health – a thorough repolish is probably the next best thing
GP LENS CARE SUMMARY • Due of their non-absorbent nature, surface properties, rigidity, & durability, GP CLs are easier to care for than SCLs • However, as GP CLs are kept in regular use longer than SCLs, efficacious lens care is essential to successful long-term lens wear • Wearers should Clean, Rinse, And Disinfectant Lenses Every time (CRADLE) • Programmed replacement of GP CLs is an important factor in maintaining ocular health – a thorough repolish is probably the next best thing
 GP LENS CARE PRODUCT UPDATES Due to research advances and changing market conditions, new LCP releases and older product retirements are regular events IACLE is keen to keep its resources up-to-date. Therefore, we invite members and other interested parties to inform IACLE of changes in the LCP marketplace Please forward information to: iacle@iacle. org As IACLE’s presentations are electronic resources, they can be updated easily & promptly IACLE will inform members of resource updates
GP LENS CARE PRODUCT UPDATES Due to research advances and changing market conditions, new LCP releases and older product retirements are regular events IACLE is keen to keep its resources up-to-date. Therefore, we invite members and other interested parties to inform IACLE of changes in the LCP marketplace Please forward information to: iacle@iacle. org As IACLE’s presentations are electronic resources, they can be updated easily & promptly IACLE will inform members of resource updates
 IACLE RESOURCES ERRORS, OMISSIONS, IMPROVEMENTS IACLE also invites users of its resources to help it evolve and improve them If you become aware of errors or omissions, or you have suggestions for improvements, you are invited to submit them to : iacle@iacle. org for consideration Contributions incorporated will be acknowledged in the relevant presentation’s Notes pane(s)
IACLE RESOURCES ERRORS, OMISSIONS, IMPROVEMENTS IACLE also invites users of its resources to help it evolve and improve them If you become aware of errors or omissions, or you have suggestions for improvements, you are invited to submit them to : iacle@iacle. org for consideration Contributions incorporated will be acknowledged in the relevant presentation’s Notes pane(s)
 IACLE INDUSTRY SPONSORS
IACLE INDUSTRY SPONSORS

 ALL REFERENCES USED (In alphabetical order) 5 L 1 -58
ALL REFERENCES USED (In alphabetical order) 5 L 1 -58
 Bontempo AR, Rapp J (1994). Lipid deposits on hydrophilic and rigid gas permeable contact lenses. CLAO J. 20(4): 242 – 245. Bontempo AR, Rapp J (1997). Protein-lipid interaction on the surface of a rigid gas permeable in vitro. Curr Eye Res. 16: 1258 - 1262. Brennan NA (1988). New technology in contact lens materials. J BCLA. (Trans BCLA Int Conf. ) 11(5): 23 – 28. Craig J (2002). Structure and Function of the Precursor Tear Film. In: Korb D et al. Eds. The Tear Film: Structure, Function, and Clinical Examination. Butterworth Heinemann, London. Fatt I (1978). Water flow conductivity and pore diameter in extended-wear gel lens materials. . Am J Optom Physiol Opt. 55(1): 43 – 47. Franklin V et al. (2001). Contact lens care: Part 4 – Contact lens deposition, discoloration and spoilation mechanisms. Optician 222(5808): 16 – 20. Franklin VJ (1997). Cleaning efficacy of single-purpose surfactant cleaners and multi-purpose solutions. Cont Lens & Ant Eye. 20(2): 63 – 68. Gachon AM et al. (1986). Protein migration through hydrogels: a tool for measuring porosity-application to hydrogels used as contact lenses. Anal Biochem. 157: 249 - 255. Garrett Q et al. (2000). Hydrogel Lens Monomer Constituents Modulate Protein Sorption. Invest Ophth Vis Sci. 41(7): 1687 - 1695. Gatin E et al. (2000). Correlations between permeability properties and the pore - size distribution of the porous media hydron useful as contact lenses. Phys Med. 16: 13 – 19. Ghajar M et al. (1989). Microbiological evaluation of Miraflow. J Am Optom Assoc. 60(8): 592 - 595. Guillon J-P, Guillon M (1994). The Role Of Tears In Contact Lens Performance And Its Measurement. In: Ruben M, Guillon M Eds. Contact Lens Practice. Chapman & Hall, London. Hart DE (1984). Lipid deposits which form on extended wear lenses. ICLC. 11(6): 348 – 360. Ide K et al. (1991). Deposits on gas-permeable hard contact lenses in extended wear by children. J Jpn CL Soc. 33: 172 – 178. Kanai A, Watanabe Y (1990). Deposits on the contact lens and their prevention. J Jpn CL Soc. 32: 71 – 78. Lamberts DW et al. (1995). Chapter 6: Wetting. In: CONTACT LENSES The CLAO Guide to Basic Science and Clinical Practice. Volume 1. Ed. Kastl PR. Kendall/Hunt Publishing Company, Dubuque. 101 – 102. 5 L 1 -59
Bontempo AR, Rapp J (1994). Lipid deposits on hydrophilic and rigid gas permeable contact lenses. CLAO J. 20(4): 242 – 245. Bontempo AR, Rapp J (1997). Protein-lipid interaction on the surface of a rigid gas permeable in vitro. Curr Eye Res. 16: 1258 - 1262. Brennan NA (1988). New technology in contact lens materials. J BCLA. (Trans BCLA Int Conf. ) 11(5): 23 – 28. Craig J (2002). Structure and Function of the Precursor Tear Film. In: Korb D et al. Eds. The Tear Film: Structure, Function, and Clinical Examination. Butterworth Heinemann, London. Fatt I (1978). Water flow conductivity and pore diameter in extended-wear gel lens materials. . Am J Optom Physiol Opt. 55(1): 43 – 47. Franklin V et al. (2001). Contact lens care: Part 4 – Contact lens deposition, discoloration and spoilation mechanisms. Optician 222(5808): 16 – 20. Franklin VJ (1997). Cleaning efficacy of single-purpose surfactant cleaners and multi-purpose solutions. Cont Lens & Ant Eye. 20(2): 63 – 68. Gachon AM et al. (1986). Protein migration through hydrogels: a tool for measuring porosity-application to hydrogels used as contact lenses. Anal Biochem. 157: 249 - 255. Garrett Q et al. (2000). Hydrogel Lens Monomer Constituents Modulate Protein Sorption. Invest Ophth Vis Sci. 41(7): 1687 - 1695. Gatin E et al. (2000). Correlations between permeability properties and the pore - size distribution of the porous media hydron useful as contact lenses. Phys Med. 16: 13 – 19. Ghajar M et al. (1989). Microbiological evaluation of Miraflow. J Am Optom Assoc. 60(8): 592 - 595. Guillon J-P, Guillon M (1994). The Role Of Tears In Contact Lens Performance And Its Measurement. In: Ruben M, Guillon M Eds. Contact Lens Practice. Chapman & Hall, London. Hart DE (1984). Lipid deposits which form on extended wear lenses. ICLC. 11(6): 348 – 360. Ide K et al. (1991). Deposits on gas-permeable hard contact lenses in extended wear by children. J Jpn CL Soc. 33: 172 – 178. Kanai A, Watanabe Y (1990). Deposits on the contact lens and their prevention. J Jpn CL Soc. 32: 71 – 78. Lamberts DW et al. (1995). Chapter 6: Wetting. In: CONTACT LENSES The CLAO Guide to Basic Science and Clinical Practice. Volume 1. Ed. Kastl PR. Kendall/Hunt Publishing Company, Dubuque. 101 – 102. 5 L 1 -59
 Lopez-Alemany et al. (2002). Porous structure of Purevision versus Focus Night & Day and conventional hydrogel contact lenses. J Biomed Mat Res 63(3): 319 – 325. Lowther GE (1987). Effect of some solutions on HGP contact lens parameters. J Am Optom Assoc. 58(3): 188 - 192. Luensmann D (2009). Protein Sorption to Contact Lenses and Intraocular Lenses. Ph. D Thesis, UWaterloo, CANADA. Morris J (2004). RGP lenses: Part 1 – materials, manufacturing and design. Optician 228(5971): 28 – 35. Nicolson PC 2003. Continuous wear contact lens surface chemistry and wearability. Eye & CL. : Science & Clinical Practice 29(1): S 30 – S 32. Phillips AJ, Czigler B (1985). Polyclens (Opti-clean) – A further study. Aust J Optom. 68(1): 36 – 39. Picolo MG et al. (1990). Rigid lens base curve stability upon hydrogen peroxide disinfection. Optom Vision Sci. 67(1): 19 21. Refojo M (1983 B). In: Lens Deposits – Adapted from the October 1982 meeting of CLAO J. 9(2): 172. Refojo M (1983 C). In: Lens Deposits – Adapted from the October 1982 meeting of CLAO J. 9(2): 173. Sano K (2000). Contact lens materials and lens contamination. J Jpn CL Soc. 42: 68 – 73. Schultz CL et al. (2000). Bacterial colonization of rigid gas permeable and hydrogel contact lenses by Staphylococcus aureus. J Indust Microbiol Biotech 24: 113 – 115. Sibley M (1983). In: Lens Deposits – Adapted from the October 1982 meeting of CLAO J. 9(2): 172. Tighe B J (2007). Contact lens materials Chap. 3 in: Contact Lenses 5 th ed. Phillips A J, Speedwell L. Butterworth Heinemann, Edinburgh. Vajdic CM et al. (1996). Do contact lens wearers have more ocular discomfort than spectacle wearers? Invest Ophth Vis Sci. 37(3): Suppl. 5178. van der Worp E (2010). RGPs – The low fat option? Global. CONTACT 2 -2010(55): 6 – 11. Wilson LA (1983). In: Lens Deposits – Adapted from the October 1982 meeting of CLAO J. 9(2): 183. Wood JM et al. (1983). Characterization of poly(2 -hydroxyethyl methacrylate) gels. Drug Dev Ind Pharm. 9(1 -2): 93 – 101. Woods CA, Efron N (1996). Regular replacement of daily-wear rigid gas-permeable contact lenses. J Brit Cont Lens Assoc. 19(3): 83 - 89. 5 L 1 -60
Lopez-Alemany et al. (2002). Porous structure of Purevision versus Focus Night & Day and conventional hydrogel contact lenses. J Biomed Mat Res 63(3): 319 – 325. Lowther GE (1987). Effect of some solutions on HGP contact lens parameters. J Am Optom Assoc. 58(3): 188 - 192. Luensmann D (2009). Protein Sorption to Contact Lenses and Intraocular Lenses. Ph. D Thesis, UWaterloo, CANADA. Morris J (2004). RGP lenses: Part 1 – materials, manufacturing and design. Optician 228(5971): 28 – 35. Nicolson PC 2003. Continuous wear contact lens surface chemistry and wearability. Eye & CL. : Science & Clinical Practice 29(1): S 30 – S 32. Phillips AJ, Czigler B (1985). Polyclens (Opti-clean) – A further study. Aust J Optom. 68(1): 36 – 39. Picolo MG et al. (1990). Rigid lens base curve stability upon hydrogen peroxide disinfection. Optom Vision Sci. 67(1): 19 21. Refojo M (1983 B). In: Lens Deposits – Adapted from the October 1982 meeting of CLAO J. 9(2): 172. Refojo M (1983 C). In: Lens Deposits – Adapted from the October 1982 meeting of CLAO J. 9(2): 173. Sano K (2000). Contact lens materials and lens contamination. J Jpn CL Soc. 42: 68 – 73. Schultz CL et al. (2000). Bacterial colonization of rigid gas permeable and hydrogel contact lenses by Staphylococcus aureus. J Indust Microbiol Biotech 24: 113 – 115. Sibley M (1983). In: Lens Deposits – Adapted from the October 1982 meeting of CLAO J. 9(2): 172. Tighe B J (2007). Contact lens materials Chap. 3 in: Contact Lenses 5 th ed. Phillips A J, Speedwell L. Butterworth Heinemann, Edinburgh. Vajdic CM et al. (1996). Do contact lens wearers have more ocular discomfort than spectacle wearers? Invest Ophth Vis Sci. 37(3): Suppl. 5178. van der Worp E (2010). RGPs – The low fat option? Global. CONTACT 2 -2010(55): 6 – 11. Wilson LA (1983). In: Lens Deposits – Adapted from the October 1982 meeting of CLAO J. 9(2): 183. Wood JM et al. (1983). Characterization of poly(2 -hydroxyethyl methacrylate) gels. Drug Dev Ind Pharm. 9(1 -2): 93 – 101. Woods CA, Efron N (1996). Regular replacement of daily-wear rigid gas-permeable contact lenses. J Brit Cont Lens Assoc. 19(3): 83 - 89. 5 L 1 -60
 GP CLs CLEANERS • • • • • Alcon OPTI-FREE Supra. Clens • Optikem Intn’l Sereine® Contact Lens II Daily Cleaner Alcon Opti-Cleaner Alcon OPTI-FREE Daily Cleaner • Sauflon Delta GP Cleaner ® II Daily Cleaner Alcon POLYCLENS AMO Total Care Cleaner Avizor GP Cleaner Boston Advance Lens Cleaner Boston Original Formula Cleaner Contopharma i-clean! (Miraflow clone? ) CIBA Vision Miraflow Cleaner (avail. ? ) CIBA Vision Miraflow Extra Strength Cleaner EYEYE Crystal Cleaner Lobob® Optimum Extra Strength Cleaner Laboratory/Professional Use ONLY ® Sterile Cleaning Solution Lobob • Boston® Laboratory Lens Cleaner Meni. Care Plus MPS • Meni. Care Progent Menicon Spray & Clean • Meni. Lab Disinfectant/Cleaner Menicon Unique p. H
GP CLs CLEANERS • • • • • Alcon OPTI-FREE Supra. Clens • Optikem Intn’l Sereine® Contact Lens II Daily Cleaner Alcon Opti-Cleaner Alcon OPTI-FREE Daily Cleaner • Sauflon Delta GP Cleaner ® II Daily Cleaner Alcon POLYCLENS AMO Total Care Cleaner Avizor GP Cleaner Boston Advance Lens Cleaner Boston Original Formula Cleaner Contopharma i-clean! (Miraflow clone? ) CIBA Vision Miraflow Cleaner (avail. ? ) CIBA Vision Miraflow Extra Strength Cleaner EYEYE Crystal Cleaner Lobob® Optimum Extra Strength Cleaner Laboratory/Professional Use ONLY ® Sterile Cleaning Solution Lobob • Boston® Laboratory Lens Cleaner Meni. Care Plus MPS • Meni. Care Progent Menicon Spray & Clean • Meni. Lab Disinfectant/Cleaner Menicon Unique p. H
 GP LENS CARE SOAKING SOLUTIONS Lobob Hard Lens Soaking Solution
GP LENS CARE SOAKING SOLUTIONS Lobob Hard Lens Soaking Solution
 GP LENS CARE CONDITIONING SOLUTIONS • Boston® Conditioning Solution • Boston Advance Comfort Formula Conditioning Solution • Optikem International Sereine Wetting and Soaking Solution
GP LENS CARE CONDITIONING SOLUTIONS • Boston® Conditioning Solution • Boston Advance Comfort Formula Conditioning Solution • Optikem International Sereine Wetting and Soaking Solution
 GP LENS CARE WETTING SOLUTIONS • Lobob Hard Lens Wetting Solutions • Lobob OPTIMUM Wetting and Rewetting Drop • Meni. Care GP WRW (wetting & rewetting)
GP LENS CARE WETTING SOLUTIONS • Lobob Hard Lens Wetting Solutions • Lobob OPTIMUM Wetting and Rewetting Drop • Meni. Care GP WRW (wetting & rewetting)
 GP LENS CARE MULTI-PURPOSE SOLUTIONS • Alcon OPTI-FREE® GP Multi-Purpose Solution • AMO Total Care 1 • Boston Simplus Multi-Action Solution • Lobob Optimum Cleaning/Disinfecting/Storage Solution • Menicon Menicare GP CDS (cleaning, disinfecting, soaking) USA only • Menicon Meni. Care Plus • Menicon Unique-p. H Multi-Purpose Solution (formerly Alcon) • Sauflon delta DSW (disinfecting, soaking, wetting)
GP LENS CARE MULTI-PURPOSE SOLUTIONS • Alcon OPTI-FREE® GP Multi-Purpose Solution • AMO Total Care 1 • Boston Simplus Multi-Action Solution • Lobob Optimum Cleaning/Disinfecting/Storage Solution • Menicon Menicare GP CDS (cleaning, disinfecting, soaking) USA only • Menicon Meni. Care Plus • Menicon Unique-p. H Multi-Purpose Solution (formerly Alcon) • Sauflon delta DSW (disinfecting, soaking, wetting)
 GP LENS CARE LUBRICATING DROPS • Alcon Clerz® Plus Lens Drops • Alcon OPTI-FREE® Repleni. SH® Rewetting Drops • AMO blink Contacts Lubricating Eye Drops • AMO Complete Blink-N-Clean Lens Drops • Boston® Rewetting Drops • Menicon Rewetting Drops
GP LENS CARE LUBRICATING DROPS • Alcon Clerz® Plus Lens Drops • Alcon OPTI-FREE® Repleni. SH® Rewetting Drops • AMO blink Contacts Lubricating Eye Drops • AMO Complete Blink-N-Clean Lens Drops • Boston® Rewetting Drops • Menicon Rewetting Drops
 GP LENS CARE PROTEIN REMOVERS • Alcon OPTI-FREE® Supra. Clens® Daily Protein Remover • Boston® One Step Liquid Enzymatic Cleaner • Menicon Progent (protein remover, disinfectant, intensive cleaner (For professional use only)
GP LENS CARE PROTEIN REMOVERS • Alcon OPTI-FREE® Supra. Clens® Daily Protein Remover • Boston® One Step Liquid Enzymatic Cleaner • Menicon Progent (protein remover, disinfectant, intensive cleaner (For professional use only)
 GP LENS CARE MISCELLANEOUS CARE PRODUCTS • Boston® Laboratory Cleaner (availability? ) (lab-only product) • Paragon Fluoro-Solve (availability? ) (lab-only product) • Menicon Progent (protein remover, disinfectant, intensive cleaner (For professional use only)
GP LENS CARE MISCELLANEOUS CARE PRODUCTS • Boston® Laboratory Cleaner (availability? ) (lab-only product) • Paragon Fluoro-Solve (availability? ) (lab-only product) • Menicon Progent (protein remover, disinfectant, intensive cleaner (For professional use only)
 5 L 1 -69
5 L 1 -69


