Carbon Nanostructures Lecture-presentation on “Basics of Nanochemistry and


Carbon Nanostructures Lecture-presentation on “Basics of Nanochemistry and Nanotechnology” by L.K.Tastanova

Objectives of lecture –presentation: To study compositions, production methods and properties of different carbon nanostructures.

Plan of lecture –presentation: 1. Fullerenes 2. Nanotubes 3. Other Carbon Nanostructures

1. Fullerenes Ts, to achieve acceptable stability, molecules in self-assembled aggregates should be joined by a high number of weak noncovalent interactions. Moreover, these interactions must be more favorable energetically than the competing interactions with the solvent and must be able to overwhelm the entropic advantages of disintegration of the ordered aggregate into disordered or dissociated states.

1. Fullerenes Fullerenes are allotropic form of carbon. They consist of a spherical, ellipsoid, or cylindrical arrangement of dozens of carbon atoms. Fullerenes were named after Richard Buckminster Fuller, an architect known for the design of geodesic domes which resemble spherical fullerenes in appearance.
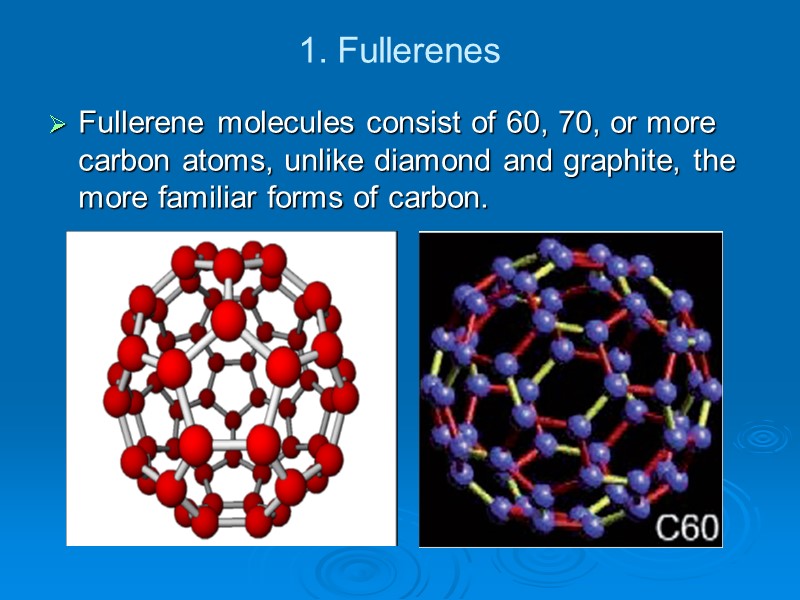
1. Fullerenes Fullerene molecules consist of 60, 70, or more carbon atoms, unlike diamond and graphite, the more familiar forms of carbon.

1. Fullerenes The 1996 Nobel Prize in Chemistry was awarded to Professors Robert F. Curl, Jr., Richard E. Smalley, and Sir Harold W. Kroto for their discovery. Robert F. Curl, Jr. Richard E. Smalley Sir Harold W. Kroto

1. Fullerenes Fullerenes occur only in small amounts naturally. They were found in the natural environment, but only in the ppm-range. Some places are Shunga/Russia, New Zealand and Sudbury/Canada. It takes from 600 million to 2 billion years to form shungite (very rarely found mineral).

1. Fullerenes Methods of production of fullerenes: Production of fullerenes in a benzene flame. Vaporization of graphite rods. Catalytic chemical vapor deposition from ethanol vapor. Fullerene is expensive product – about 30 times more expensive than gold.

1. Fullerenes Properties of fullerenes: Good solvents for fullerenes are CS2, toluene, xylene, and odichlorobenzene while they are insoluble in water and stable at air. Thin layers of fullerenes and solutions are coloured, because of ππ*-electron transitions: C60 purple/violetred, C70 brick-red, C76 light yellow-green, C2v -C78 maroon, D3- C78 golden, C84 brown and C86 olive-green.

1. Fullerenes A wide variety of synthetic protocols, successfully applied to C60, have established this novel form of carbon as a useful building block in organic synthesis. The main advantage gained upon modification of fullerenes is a substantial increase in their solubility, in addition to the presence of new functionalities

1. Fullerenes Combination of the unique structural, physicochemical, and electronic properties of the fullerenes with the special characteristics of the added groups eventually leads to the development of new materials with enormous potential in fascinating and widespread technological and biological applications.
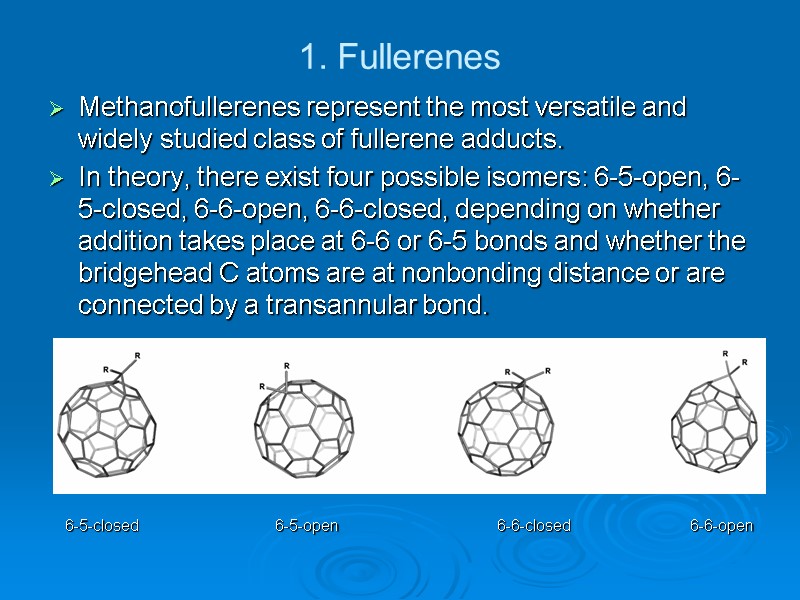
1. Fullerenes Methanofullerenes represent the most versatile and widely studied class of fullerene adducts. In theory, there exist four possible isomers: 6-5-open, 6-5-closed, 6-6-open, 6-6-closed, depending on whether addition takes place at 6-6 or 6-5 bonds and whether the bridgehead C atoms are at nonbonding distance or are connected by a transannular bond. 6-5-closed 6-5-open 6-6-closed 6-6-open
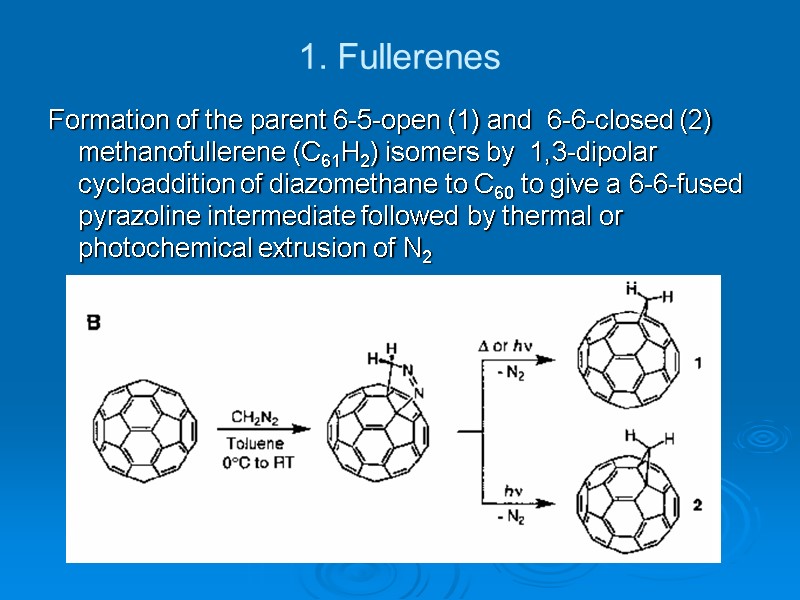
1. Fullerenes Formation of the parent 6-5-open (1) and 6-6-closed (2) methanofullerene (C61H2) isomers by 1,3-dipolar cycloaddition of diazomethane to C60 to give a 6-6-fused pyrazoline intermediate followed by thermal or photochemical extrusion of N2
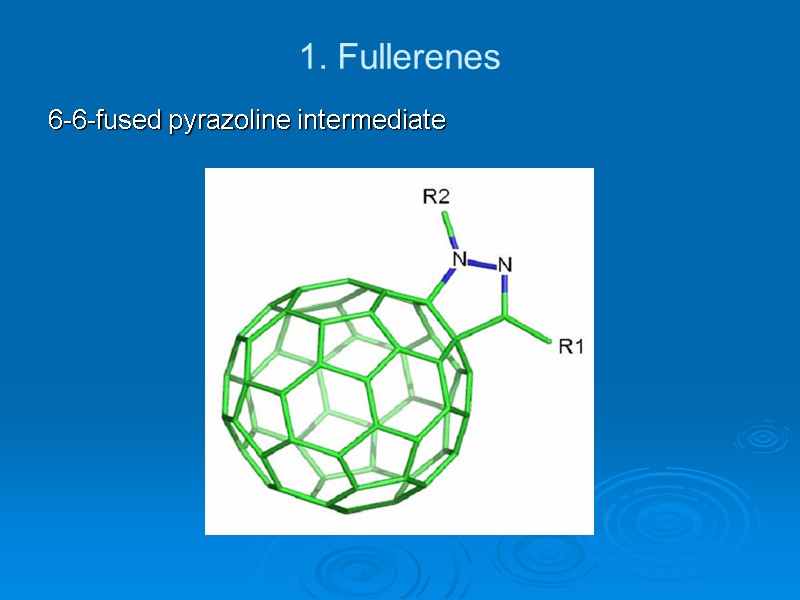
1. Fullerenes 6-6-fused pyrazoline intermediate
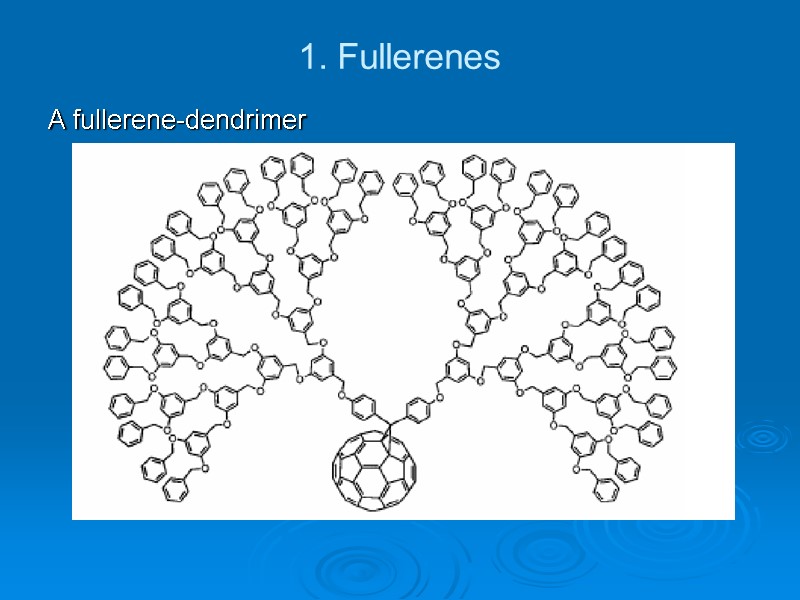
1. Fullerenes A fullerene-dendrimer

1. Fullerenes In 1991 superconductivity in alkali doped fullerides at moderately high temperatures was discovered. The chemistry of fullerenes also includes the synthesis of endohedral fullerenes having a metal atom inside the fullerene cage. Moreover, an unexpected ferromagnetic behavior has been recently described in fullerenic materials.

1. Fullerenes Utilizations of Fullerenes; Soft ferromagnetics, Organic conductors, Lubricant materials, Superconductivity, discovered in fullerene films dopped with alkaline metals. The temperature of transition into this state, at 45 K is exceeded only by ceramic superconductors, but fullerene films have higher critical-current values. Applications in catalysis is also of interest.
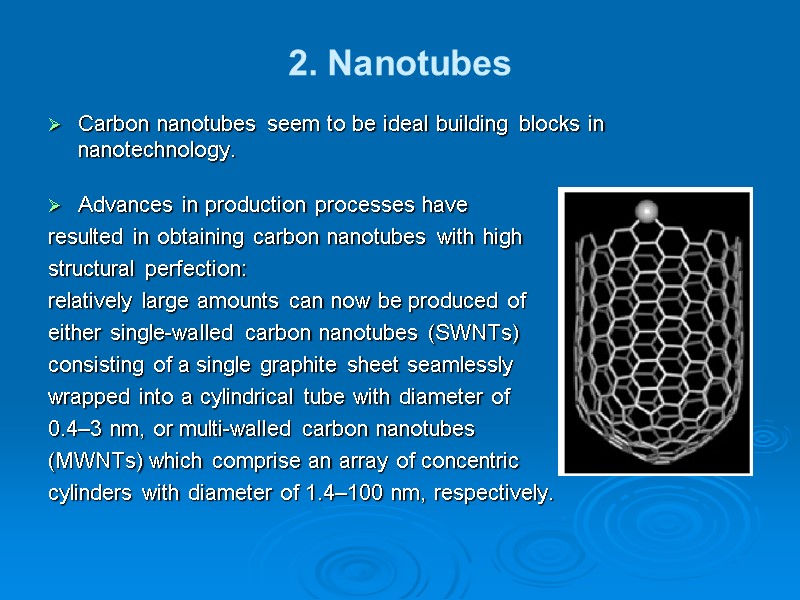
2. Nanotubes Carbon nanotubes seem to be ideal building blocks in nanotechnology. Advances in production processes have resulted in obtaining carbon nanotubes with high structural perfection: relatively large amounts can now be produced of either single-walled carbon nanotubes (SWNTs) consisting of a single graphite sheet seamlessly wrapped into a cylindrical tube with diameter of 0.4–3 nm, or multi-walled carbon nanotubes (MWNTs) which comprise an array of concentric cylinders with diameter of 1.4–100 nm, respectively.
2. Nanotubes Nanotubes are mostly classified according to the type of rolling of graphite plane which is characterized by two numbers n and m that determine the division of rolling direction to the vectors of graphite grid translation. According to the values of parameters (n, m) there are: 1) «armchair» n=m 2) zigzag m=0 or n=0 3) spiral (hiral) nanotubes m ≠ n
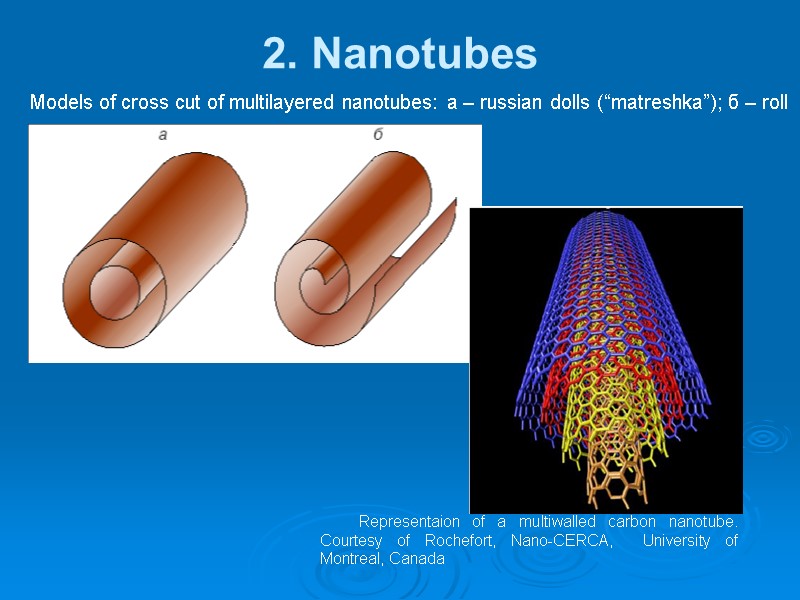
2. Nanotubes Models of cross cut of multilayered nanotubes: a – russian dolls (“matreshka”); б – roll Representaion of a multiwalled carbon nanotube. Courtesy of Rochefort, Nano-CERCA, University of Montreal, Canada

2. Nanotubes Carbon nanotubes are the strongest and stiffest materials yet discovered in terms of tensile strength and electric modulus respectively. A carbon nanotube has tensile strength about 20 times that of high-strength steel alloys, and a density half that of aluminum. Carbon nanotubes demonstrate superconductive properties, and single nanotubes up to 4 centimeters in length have been synthesized.

2. Nanotubes Under excessive tensile strain, the tubes will undergo plastic deformation, which means the deformation is permanent. This deformation begins at strains of approximately 5% and can increase the maximum strain the tubes undergo before fracture by releasing strain energy.
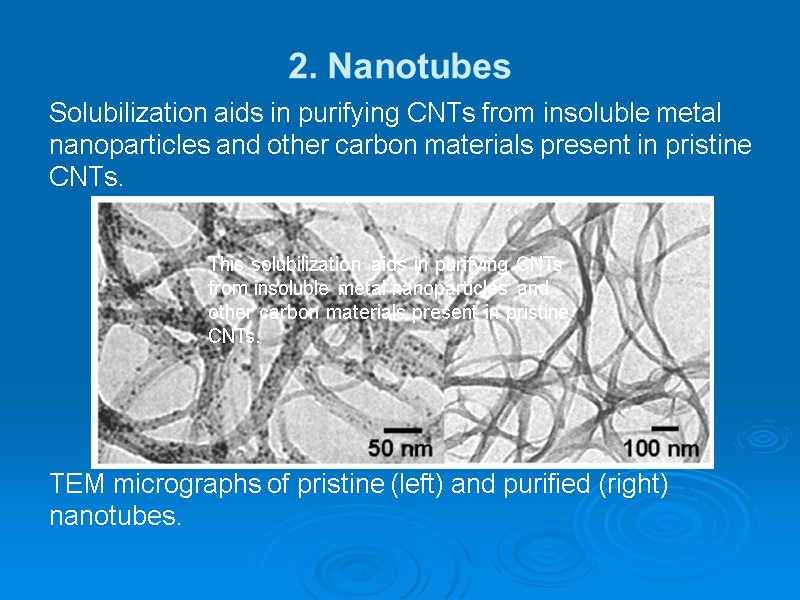
2. Nanotubes Solubilization aids in purifying CNTs from insoluble metal nanoparticles and other carbon materials present in pristine CNTs. TEM micrographs of pristine (left) and purified (right) nanotubes. This solubilization aids in purifying CNTs from insoluble metal nanoparticles and other carbon materials present in pristine CNTs.

2. Nanotubes Another central aspect of nanotube chemistry, which yet awaits exploration, is its function and performance in donor–acceptor ensembles. Toward this direction, the covalent linking of electron donors, such as ferrocene, onto the network of CNTs was pursued with the aim of potential use in photovoltaics. Indeed, utilizing ferrocene-modified glycine and paraformaldehyde, soluble CNTs decorated with many ferrocene (Fc) units all around their skeleton were obtained.
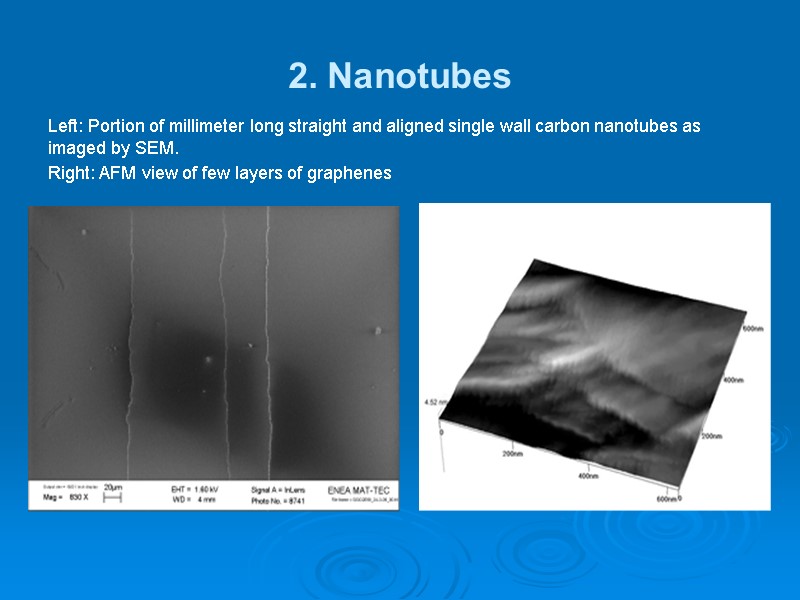
2. Nanotubes Left: Portion of millimeter long straight and aligned single wall carbon nanotubes as imaged by SEM. Right: AFM view of few layers of graphenes
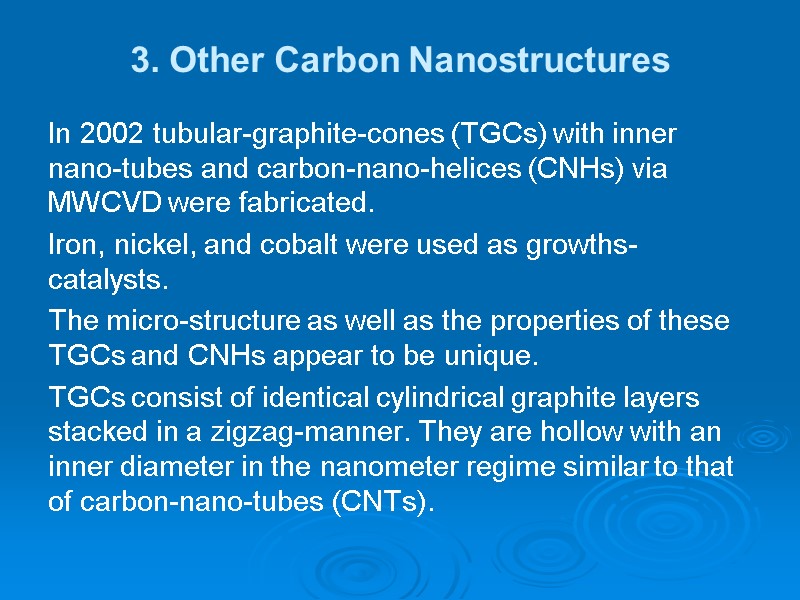
3. Other Carbon Nanostructures In 2002 tubular-graphite-cones (TGCs) with inner nano-tubes and carbon-nano-helices (CNHs) via MWCVD were fabricated. Iron, nickel, and cobalt were used as growths-catalysts. The micro-structure as well as the properties of these TGCs and CNHs appear to be unique. TGCs consist of identical cylindrical graphite layers stacked in a zigzag-manner. They are hollow with an inner diameter in the nanometer regime similar to that of carbon-nano-tubes (CNTs).
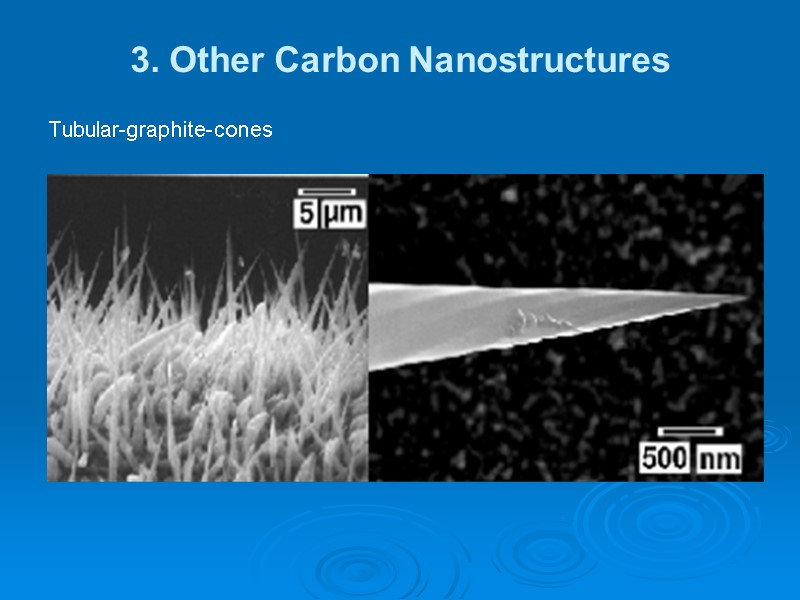
3. Other Carbon Nanostructures Tubular-graphite-cones

3. Other Carbon Nanostructures CNHs on the other hand, that look like an italian-noodles (fussili) in the SEM, consist of carbon-ribbons twisted in a spiral-manner. These ribbons have a width of about 70-80nm and show lengths of some 10 micrometers. Their huge area to volume ratio makes them a potential candidate as storage-device in biotechnological applications.

3. Other Carbon Nanostructures Carbon-nano-helices

Check Yourself What is one of the great challenges facing chemistry and materials science? What conditions are to be meat in order to obtain stable and well-shaped final assembly? How do they achieve acceptable stability of aggregates? What advantages were gained upon modification of fullerenes? How structurally different derivatives of C60 can be obtained? What properties of porphyrin moiety are useful for self-assembling? What properties of C60 are useful for the reaction? What do the rates of forward and back electron transfer depend on? What interactions are the driving force for self-assembling?

Literature: 1. Roco M. C. J. Nanoparticle Res., 2001, v. 3, №5–6, 2001, p. 353–360. 2. NSTC, National Nanotechnology Initiative and Its Implementation Plan, Washington, D.C., 2000. 3. Societal Implications of Nanoscience and Nanotechnology. Eds.M. C. Roco, W. S.Bainbridgeю Dordrecht: Kluver Acad. Publ., 2001. 4. NSTC, National Nanotechnology Initiative and Its Implementation Plan, Washington, D.C., 2002. 5. Gleiter H. Nanostructured materials – State-oftheart and perspectives. // Z/ Metallkunde., 1995. V.86. P.78-83. 6. Charitidis C., Logothetidis S. Nanomechanical and nanotribological properties of carbon based films // Thin Solid Films, 2005. V.482. P.120–125.
38378-8._carbon_nanostructures.ppt
- Количество слайдов: 32

