8ed71e8d20e1197bd1ec8d91d2323af1.ppt
- Количество слайдов: 57

Cancer Registry and Burden of cancer Hai-Rim Shin Data Analysis and Interpretation Group International Agency for Research on Cancer Lyon, France
Outlines • Cancer Registry • Definition of registry: registration • Types of cancer registries • Essential variables- required • Quality indices: Quality control • Software: Can. Reg Use of Cancer Registry and cancer control • Cancer Burden • GLOBOCAN project January 2010 NCD Seminar, Lausanne
Cancer Registry : Registration Cancer Registry The office or institution which is responsible for the collection, storage, analysis and interpretation of data on persons with cancer. Cancer registration The process of continuing systematic collection of data on the occurrence, characteristics, and outcome of reportable neoplasms with the purpose of helping to assess (prevent) and control the impact of malignant disease in the community. January 2010 NCD Seminar, Lausanne
Cancer Registry Types 1. Population based cancer registry (PBCR) 2. Hospital cancer registry 3. Pathology registry January 2010 NCD Seminar, Lausanne
Population based Cancer Registry v All cases in a DEFINED population are registered v True (unbiased) profile of cancer in the community incidence, stage distribution, survival, etc. v Calculation of incidence rates (because population at risk is quantified) v The main interest is for epidemiology and public health January 2010 NCD Seminar, Lausanne

Hospital based Cancer Registry v Records all cases of cancer treated in a given hospital v The population from which the cases come is not defined v The main interest is clinical care hospital administration January 2010 NCD Seminar, Lausanne

Pathology Tumor Registry Collects information from one or more laboratories on histologically diagnosed cancers • The population from which the tumour tissue has come is not defined • The information - has high diagnostic quality - but is difficult to generalize • January 2010 NCD Seminar, Lausanne
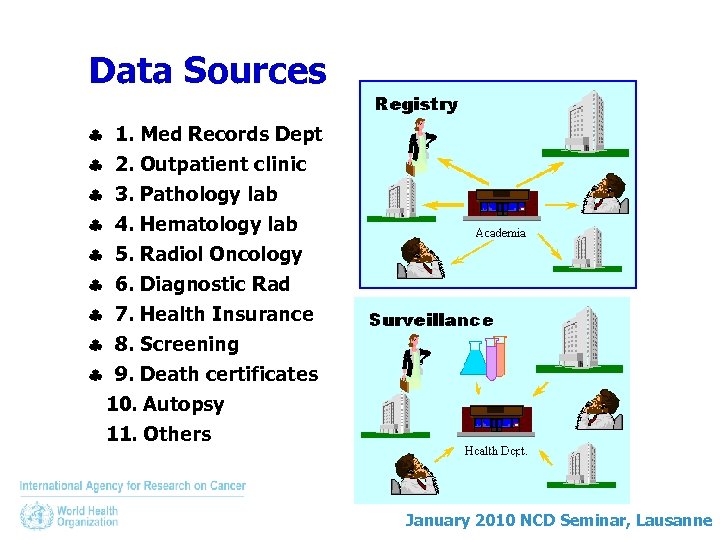
Data Sources 1. Med Records Dept 2. Outpatient clinic 3. Pathology lab 4. Hematology lab 5. Radiol Oncology 6. Diagnostic Rad 7. Health Insurance 8. Screening 9. Death certificates 10. Autopsy 11. Others January 2010 NCD Seminar, Lausanne
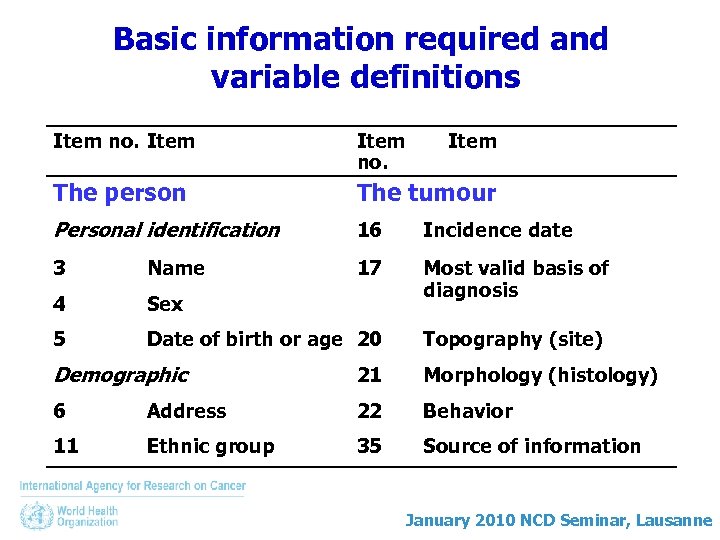
Basic information required and variable definitions Item no. Item The person The tumour Personal identification 16 Incidence date 3 Name 17 4 Sex Most valid basis of diagnosis 5 Date of birth or age 20 Topography (site) Demographic 21 Morphology (histology) 6 Address 22 Behavior 11 Ethnic group 35 Source of information January 2010 NCD Seminar, Lausanne
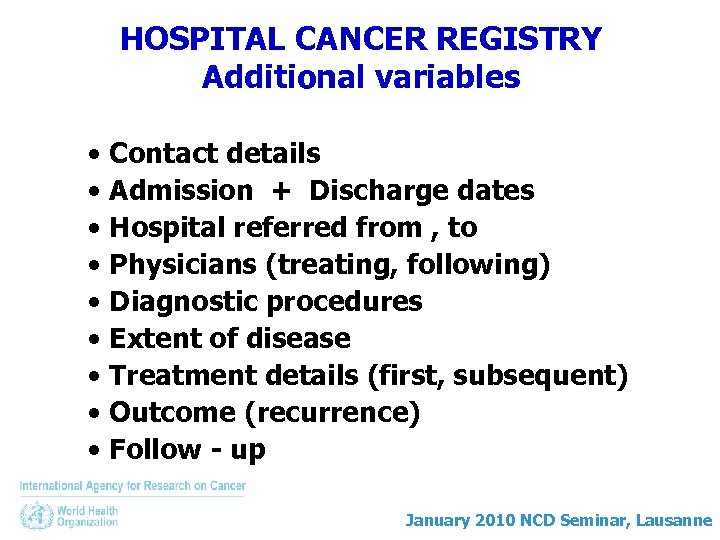
HOSPITAL CANCER REGISTRY Additional variables • Contact details • Admission + Discharge dates • Hospital referred from , to • Physicians (treating, following) • Diagnostic procedures • Extent of disease • Treatment details (first, subsequent) • Outcome (recurrence) • Follow - up January 2010 NCD Seminar, Lausanne
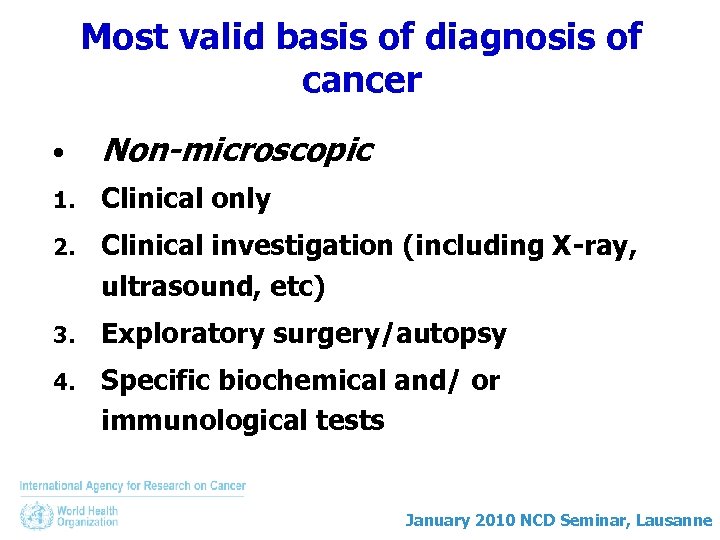
Most valid basis of diagnosis of cancer • Non-microscopic 1. Clinical only 2. Clinical investigation (including X-ray, ultrasound, etc) 3. Exploratory surgery/autopsy 4. Specific biochemical and/ or immunological tests January 2010 NCD Seminar, Lausanne
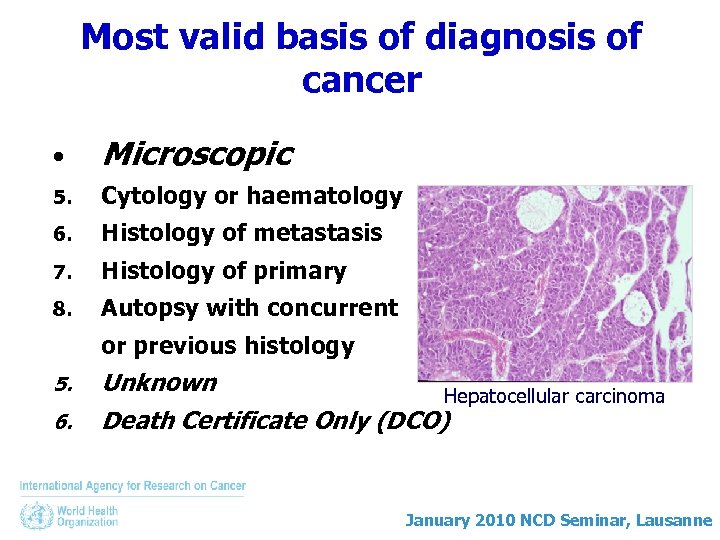
Most valid basis of diagnosis of cancer • Microscopic 5. Cytology or haematology 6. Histology of metastasis 7. Histology of primary 8. Autopsy with concurrent or previous histology 5. 6. Unknown Hepatocellular carcinoma Death Certificate Only (DCO) January 2010 NCD Seminar, Lausanne

Quality : Quality Control Quality of Data The registry data – reliable and of good quality Should be complete, consistent and accurate Quality Control The mechanism by which the quality of data can be assessed * a formal ongoing programme * ad hoc survey to assess completeness and consistency of case finding, abstracting, and coding as well as the accuracy of reporting January 2010 NCD Seminar, Lausanne
Evaluation of data quality in the cancer registry v Completeness v Comparability v Validity or accuracy v Timeliness January 2010 NCD Seminar, Lausanne

Completeness • The extent to which all of the incident cancers occurring in the population are included in the registry database • Coverage January 2010 NCD Seminar, Lausanne
Comparability The system used for classification and coding of neoplasms; • The definition of incidence, i. e. what is defined as a case, and what is the definition of the incidence date; • The distinction between a primary cancer (new case) and an extension, recurrence or metastasis of an existing one (multiple primary); • The recoding of cancers detected in asymptomatic individuals • January 2010 NCD Seminar, Lausanne
International standards for classification and coding of neoplasm 1. ICD-O-3 (2000, WHO) Topography: location of the tumour in the body (T code: C 16) Morphology: microscopic appearance and cellular origin of the tumor (M code: 8000) Behavior: whether the tumour is malignant, benign, in situ or uncertain (/3) Grade: the extent of defferentiation of tumour A standard coding scheme is also provided for recording the basis of diagnosis of cancers Comparability January 2010 NCD Seminar, Lausanne

Date of diagnosis: Incidence date SEER Program Coding and Staging Manual 2007 (pp 61 -64) ENCR, 1999 Standards recommended for the definitions of incidence 1. Date of first histological or cytological confirmation 2. Date of admission to the hospital 3. Date of first evaluation (outpatient clinic) 4. Date of diagnosis other than 1, 2, 3 5. Date of death, if no information is available 6. Date of death – at autopsy Comparability January 2010 NCD Seminar, Lausanne
Multiple primaries IARC Multiple Primary Rule (2000 and 2004) International rules for multiple primary cancers ICD-O-3 rd ed 2004. IARC Internal Report No. 2004 /02 January 2010 NCD Seminar, Lausanne
Incidental diagnosis refers to the detection of cancer incidentally 1. Screen detected cancers Aim of screening : to detect cancers that are asymptomatic at an earlier stage - increasing with prevalent cancers - tend reduce incidence rates of colon and cervix cancer via the removal of premalignant lesions 2. Autopsy diagnosis ? In Asia Comparability January 2010 NCD Seminar, Lausanne
Validity (accuracy) 1. Re-abstracting and recoding 2. Histological verification the index of validity: the percentage of cases morphologically verified 3. Death Certificate Only (DCO) 4. Missing information 5. Internal consistency: IARC, IACR CHECK program January 2010 NCD Seminar, Lausanne
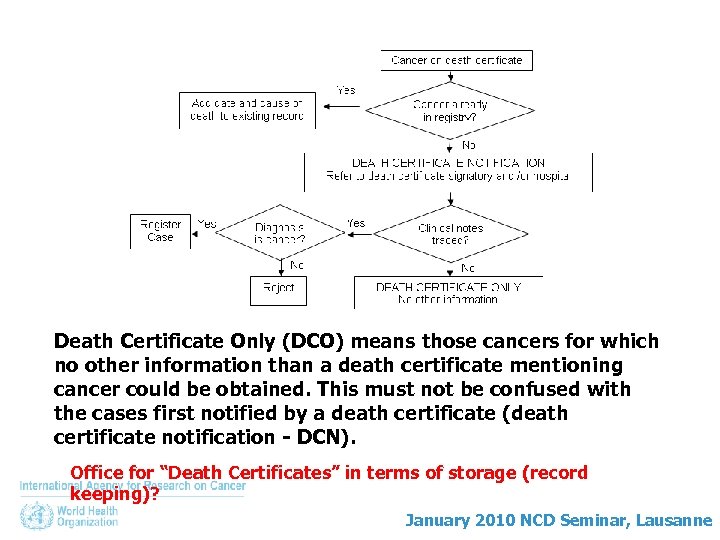
Death Certificate Only (DCO) means those cancers for which no other information than a death certificate mentioning cancer could be obtained. This must not be confused with the cases first notified by a death certificate (death certificate notification - DCN). Office for “Death Certificates” in terms of storage (record keeping)? January 2010 NCD Seminar, Lausanne
Timeliness • Rapid reporting of information on cancer cases is another priority • There are no international guidelines for timeliness at present, but -North American agencies have set out specific standards for the relevant registries - SEER: with 22 month of the end of the diagnosis year January 2010 NCD Seminar, Lausanne
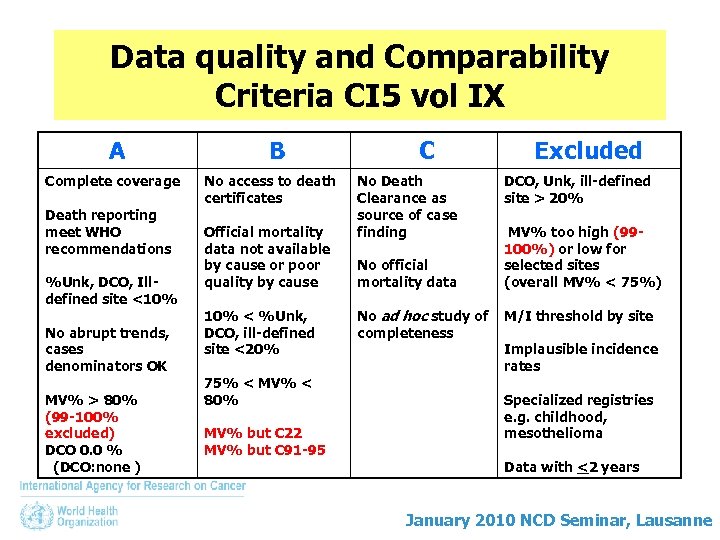
Data quality and Comparability Criteria CI 5 vol IX A Complete coverage Death reporting meet WHO recommendations %Unk, DCO, Illdefined site <10% No abrupt trends, cases denominators OK MV% > 80% (99 -100% excluded) DCO 0. 0 % (DCO: none ) B No access to death certificates Official mortality data not available by cause or poor quality by cause C No Death Clearance as source of case finding v No official mortality data Excluded DCO, Unk, ill-defined site > 20% MV% too high (99100%) or low for selected sites (overall MV% < 75%) v 10% < %Unk, DCO, ill-defined site <20% 75% < MV% < 80% MV% but C 22 MV% but C 91 -95 No ad hoc study of completeness M/I threshold by site Implausible incidence rates v Specialized registries e. g. childhood, mesothelioma Data with <2 years January 2010 NCD Seminar, Lausanne

Software for registration • • Cancer registries need a tool to input, store, check and analyze their data. Cancer registration data that are collected and coded in a standard way make possible the production of comparable cancer incidence among various countries. January 2010 NCD Seminar, Lausanne
Can. Reg 5 • • The goal of the Can. Reg 5 project is to make available an easy to use and flexible software package to support cancer registries in accomplishing these tasks. Can. Reg 5 contains modules for: • data entry • quality control • basic analysis of the data Provides online help Currently beta version Responsible Officer: Ervic Morten, DEP January 2010 NCD Seminar, Lausanne
Multiple Document Interface January 2010 NCD Seminar, Lausanne

January 2010 NCD Seminar, Lausanne

Purposes and Uses of Cancer Registration 1 Epidemiological Research Descriptive Epidemiology Analytic Epidemiology 2 Health Care Planning and Monitoring Patient Care Survival Screening Prevention January 2010 NCD Seminar, Lausanne
Use of Cancer Registry data • Analyses of cancer registry data • Record linkage studies • Sources of cases for case-control studies • Source of reference rates January 2010 NCD Seminar, Lausanne
Analysis of cancer registry data Geographical variations • Time trends • Analyses by sex and ethnic group • Analysis of other risk factors occupation place of birth civil status religion • January 2010 NCD Seminar, Lausanne
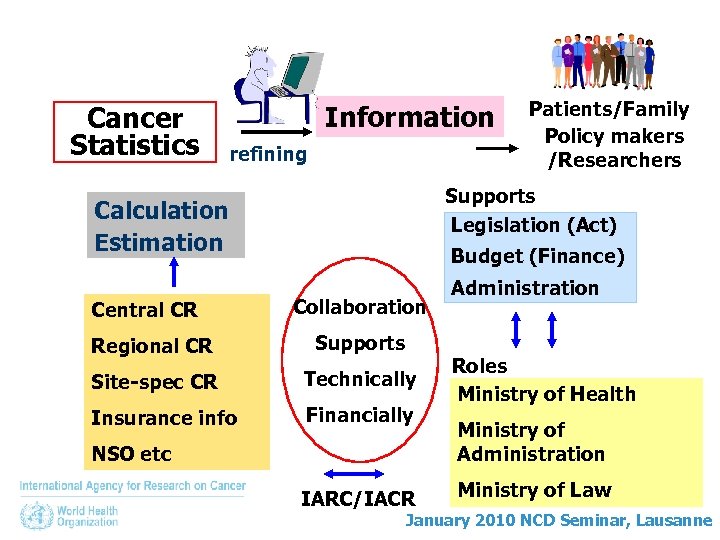
Cancer Statistics Information refining Supports Legislation (Act) Calculation Estimation Central CR Patients/Family Policy makers /Researchers Budget (Finance) Collaboration Regional CR Supports Site-spec CR Technically Insurance info Financially NSO etc IARC/IACR Administration Roles Ministry of Health Ministry of Administration Ministry of Law January 2010 NCD Seminar, Lausanne
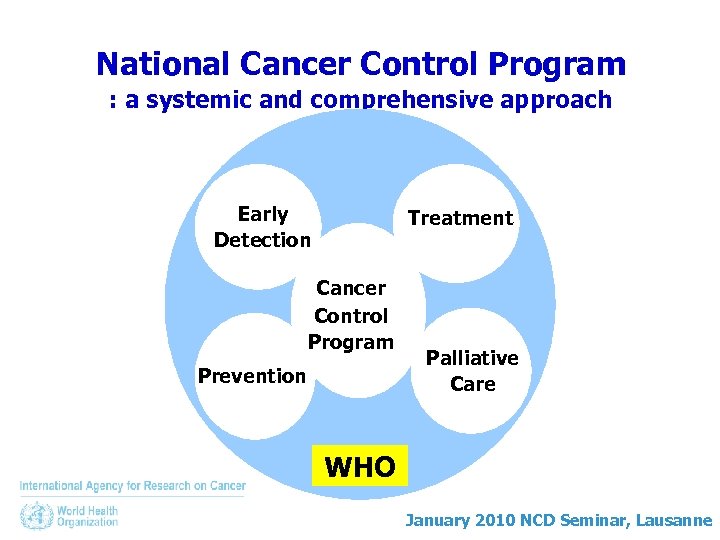
National Cancer Control Program : a systemic and comprehensive approach Early Detection Treatment Cancer Control Program Prevention Palliative Care WHO January 2010 NCD Seminar, Lausanne
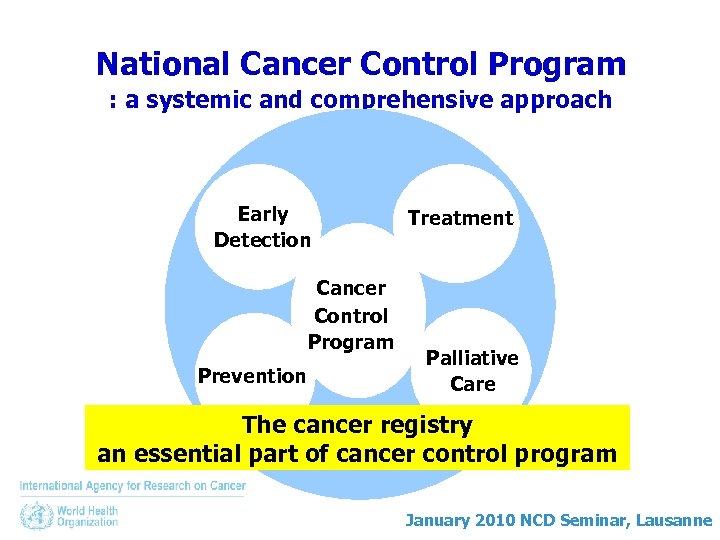
National Cancer Control Program : a systemic and comprehensive approach Early Detection Cancer Control Program Prevention Treatment Palliative Care The cancer registry an essential part of cancer control program January 2010 NCD Seminar, Lausanne
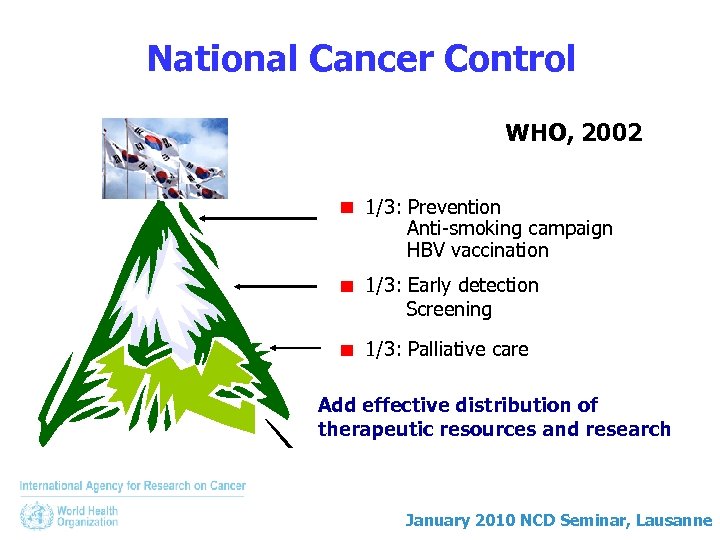
National Cancer Control WHO, 2002 1/3: Prevention Anti-smoking campaign HBV vaccination 1/3: Early detection Screening 1/3: Palliative care Add effective distribution of therapeutic resources and research January 2010 NCD Seminar, Lausanne
January 2010 NCD Seminar, Lausanne
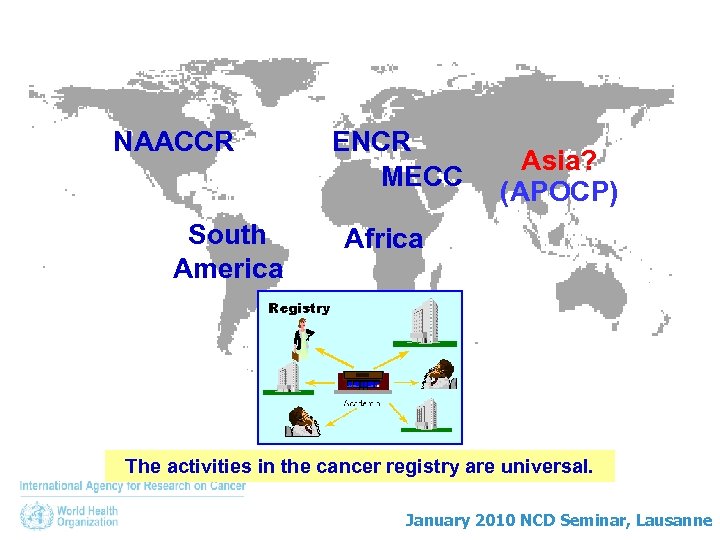
NAACCR South America ENCR MECC Asia? (APOCP) Africa The activities in the cancer registry are universal. January 2010 NCD Seminar, Lausanne
Outlines • Cancer Registry • • Types of cancer registries • Essential variables- required • • Definition of registry: registration Quality indices: Quality control Cancer Burden • GLOBOCAN project January 2010 NCD Seminar, Lausanne
Burden of Cancer Incidence, Mortality, Morbidity (Prevalence): by site, age group, sex • Summary measurements: DALY (disability adjusted life year), YLLs (years life lost due to premature death), YLD (years lived with disability), Hea. LY (healthy life years lost), etc • Economic burden: Medical cost, non. Medical cost • January 2010 NCD Seminar, Lausanne

Estimate of the Global Burden of Cancer: The GLOBOCAN project The aim of the GLOBOCAN project is to provide contemporary estimates of the incidence of, and mortality from the major types of cancer, at national level, for every country in the world. GLOBOCAN 2008 in Feb 2010 Members of Editorial board: Jacques Ferlay, Hai-Rim Shin (DEA, IARC) Max Parkin, Fraddie Bray (Cancer team for GBD) Coli Mathers (WHO HQ) January 2010 NCD Seminar, Lausanne
Estimate fo the Global Burden of Cancer: The GLOBOCAN project Data sources (1) Incidence and survival data: • Provided by cancer registries thru the International Association of Cancer Registries (IACR) • Mostly regional. • Not always recent (generally 5 year late): request time to be compiled and published. Detailed information (site, histology, laterality, grade, stage). • January 2010 NCD Seminar, Lausanne
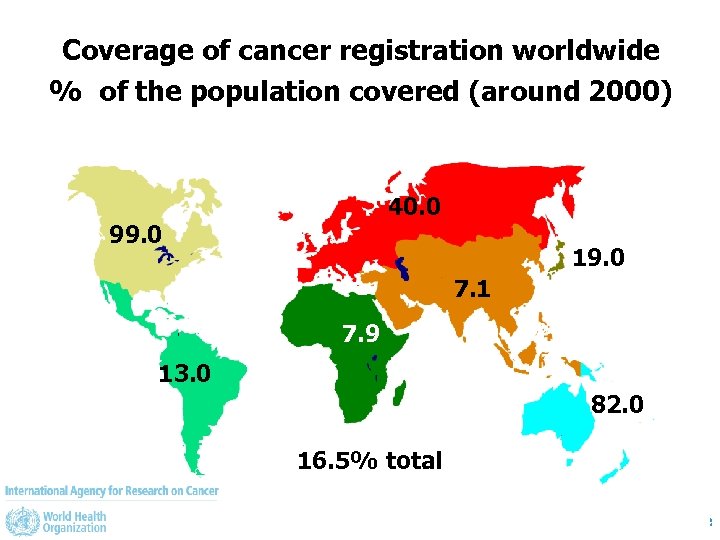
Coverage of cancer registration worldwide % of the population covered (around 2000) 40. 0 99. 0 19. 0 7. 1 7. 9 13. 0 82. 0 16. 5% total January 2010 NCD Seminar, Lausanne
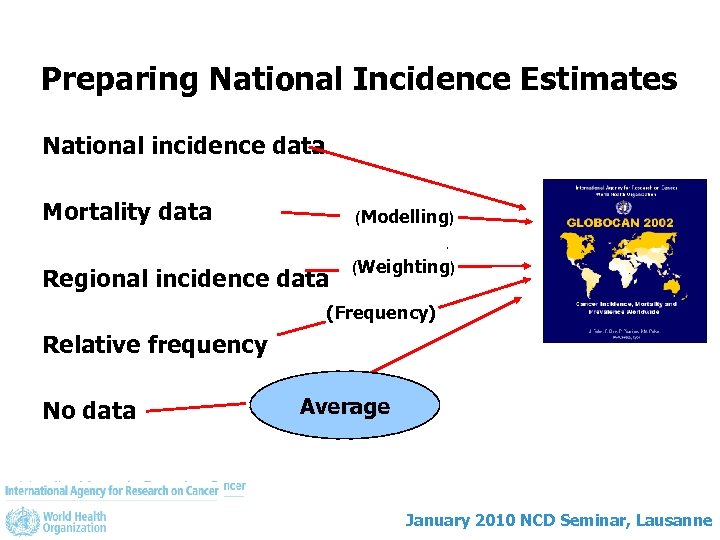
Preparing National Incidence Estimates National incidence data Mortality data (Modelling) Regional incidence data (Weighting) (Frequency) Relative frequency No data Average January 2010 NCD Seminar, Lausanne
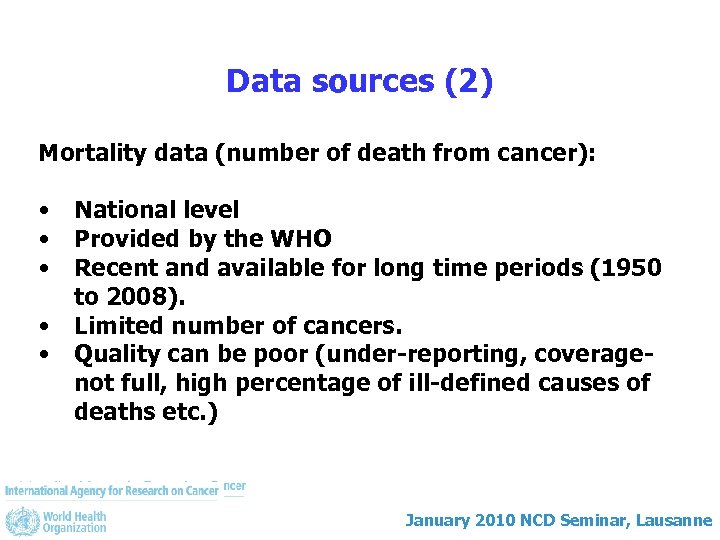
Data sources (2) Mortality data (number of death from cancer): • • • National level Provided by the WHO Recent and available for long time periods (1950 to 2008). Limited number of cancers. Quality can be poor (under-reporting, coveragenot full, high percentage of ill-defined causes of deaths etc. ) January 2010 NCD Seminar, Lausanne
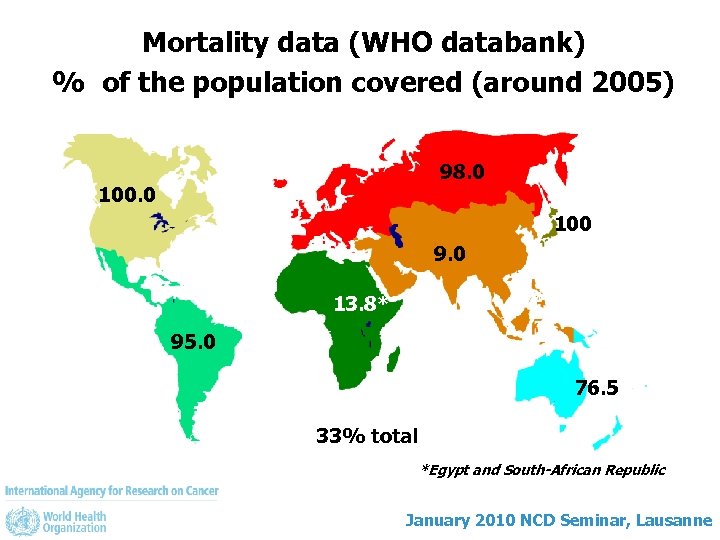
Mortality data (WHO databank) % of the population covered (around 2005) 98. 0 100 9. 0 13. 8* 95. 0 76. 5 33% total *Egypt and South-African Republic January 2010 NCD Seminar, Lausanne
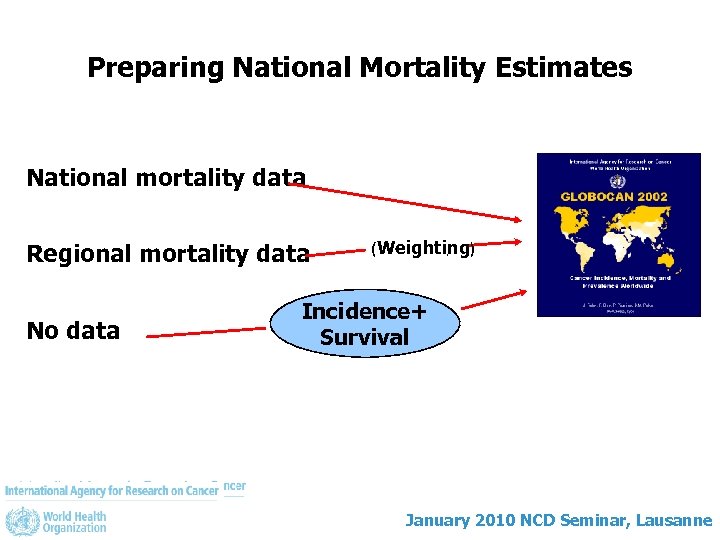
Preparing National Mortality Estimates National mortality data Regional mortality data No data (Weighting) Incidence+ Survival January 2010 NCD Seminar, Lausanne

The results are presented for 170 countries of the world, plus build-in areas (six WHO regions, more and less developed countries and the world) Data available for 27 major cancers, for men and women, and for 5 age groups: 0 -14, 15 -44, 4554, 55 -64, 65+ Accessible thru the Internet or using a Windowsbased PC software January 2010 NCD Seminar, Lausanne
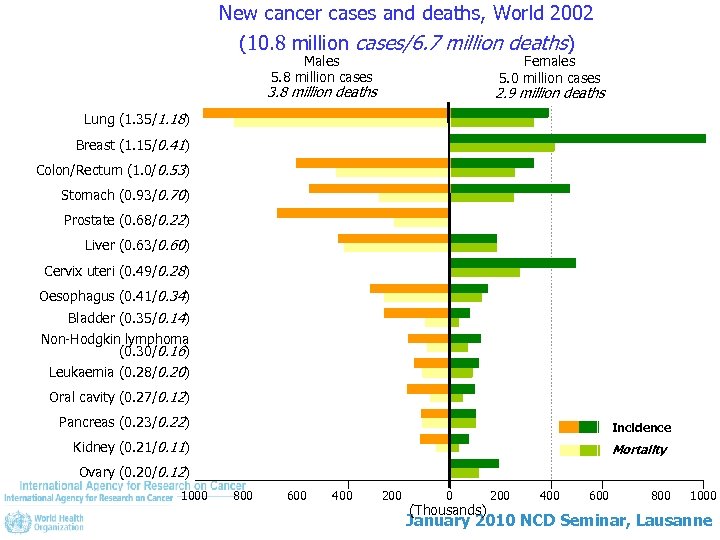
New cancer cases and deaths, World 2002 (10. 8 million cases/6. 7 million deaths) Males 5. 8 million cases Females 5. 0 million cases 3. 8 million deaths 2. 9 million deaths Lung (1. 35/1. 18) Breast (1. 15/0. 41) Colon/Rectum (1. 0/0. 53) Stomach (0. 93/0. 70) Prostate (0. 68/0. 22) Liver (0. 63/0. 60) Cervix uteri (0. 49/0. 28) Oesophagus (0. 41/0. 34) Bladder (0. 35/0. 14) Non-Hodgkin lymphoma (0. 30/0. 16) Leukaemia (0. 28/0. 20) Oral cavity (0. 27/0. 12) Pancreas (0. 23/0. 22) Incidence Kidney (0. 21/0. 11) Mortality Ovary (0. 20/0. 12) 1000 800 600 400 200 0 (Thousands) 200 400 600 800 1000 January 2010 NCD Seminar, Lausanne
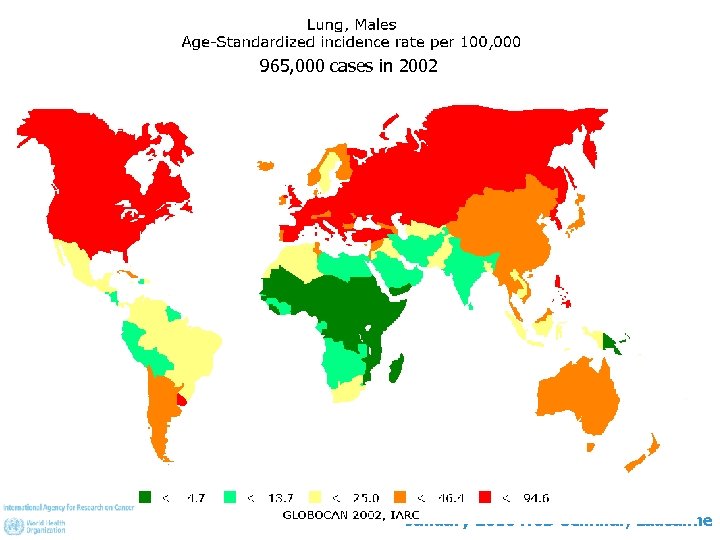
965, 000 cases in 2002 January 2010 NCD Seminar, Lausanne
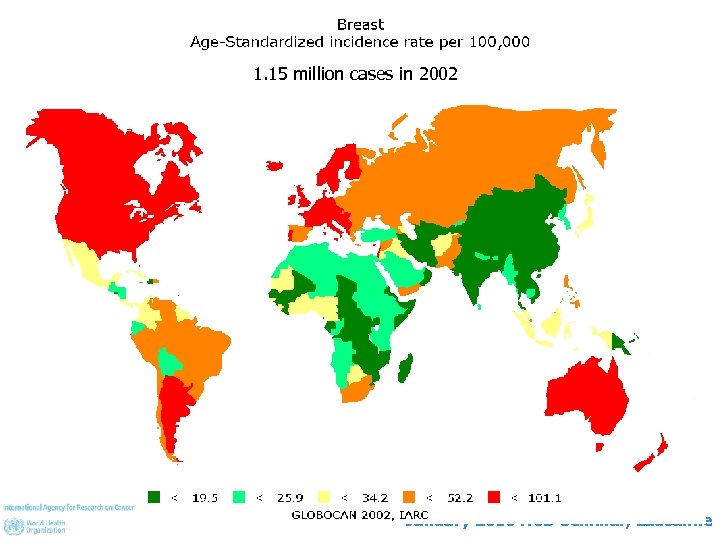
1. 15 million cases in 2002 January 2010 NCD Seminar, Lausanne
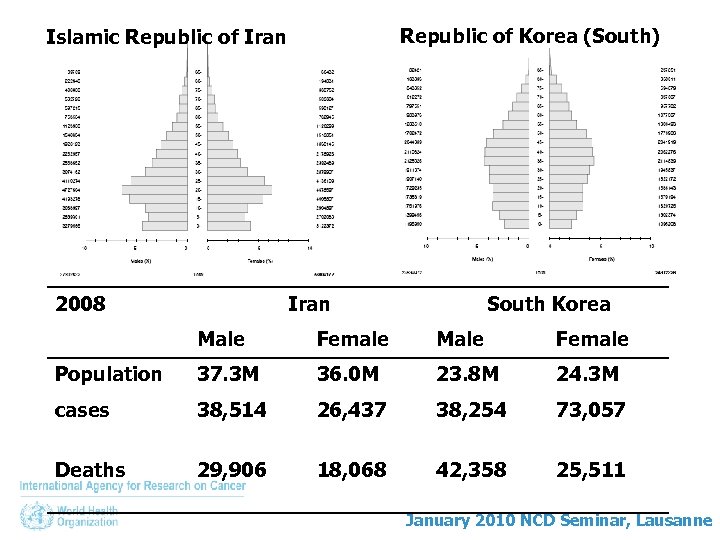
Republic of Korea (South) Islamic Republic of Iran 2008 Iran South Korea Male Female Population 37. 3 M 36. 0 M 23. 8 M 24. 3 M cases 38, 514 26, 437 38, 254 73, 057 Deaths 29, 906 18, 068 42, 358 25, 511 January 2010 NCD Seminar, Lausanne
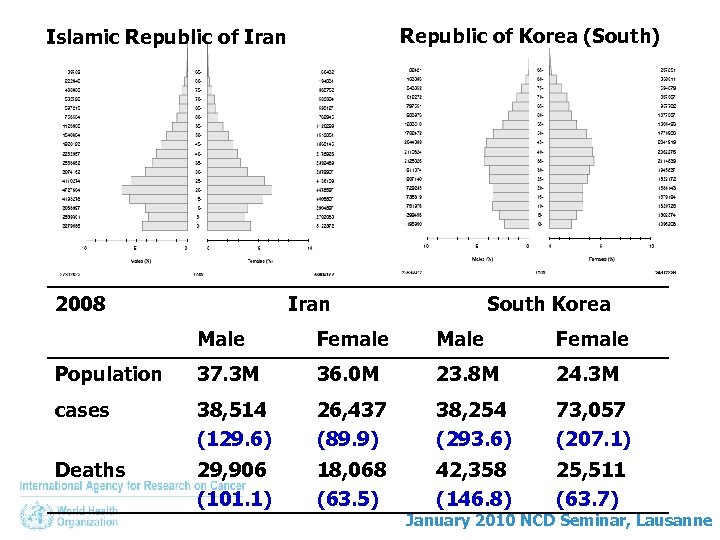
Republic of Korea (South) Islamic Republic of Iran 2008 Iran South Korea Male Female Population 37. 3 M 36. 0 M 23. 8 M 24. 3 M cases 38, 514 (129. 6) 26, 437 (89. 9) 38, 254 (293. 6) 73, 057 (207. 1) Deaths 29, 906 (101. 1) 18, 068 (63. 5) 42, 358 (146. 8) 25, 511 (63. 7) January 2010 NCD Seminar, Lausanne
Burden of cancer • • Incidence, Mortality, Prevalence, Survival Causes of cancer • Alcohol Drinking Infectious Agents • Occupation • Environment • Radiation • Diet • Reproductive factors • Obesity • Physical inactivity • • • Tobacco Smoking Genetic • HRT/Oral Contraceptive Population Attributable Fraction Cancer Control (planning/monitoring/Evaluation) January 2010 NCD Seminar, Lausanne

The burden of cancer is rising markedly worldwide with estimates indicating that there will be double the current number of new cases per year by 2030. The majority of the increase is expected in low- and middle-income countries where health services are least able to meet the impending challenge. January 2010 NCD Seminar, Lausanne

References (cancer registry and control) 1. Parkin, D. M. The evolution of the population-based cancer registry. Nat Rev Cancer, 6: 603 -12, 2006. 2. Curado, M. P. , Edwards, B. , Shin, H. R. , Storm, H. , Feray, J. , Heanue, M. , and Boyle, P. Cancer Incidence in Five Continents. IARC Scientific Publications No. 160. Lyon: IARC, 2007. 3. Bray, F. , and Parkin, D. M. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer, 45: 747 -55, 2009. 4. Parkin, D. M. , and Bray, F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. Eur J Cancer, 45: 756 -64, 2009. 5. Curado, M. P. , Voti, L. , and Sortino-Rachou, A. M. Cancer registration data and quality indicators in low and middle income countries: their interpretation and potential use for the improvement of cancer care. Cancer Causes Control, 20: 751 -6, 2009. January 2010 NCD Seminar, Lausanne

References (cancer registry and control) 6. Das, B. , Clegg, L. X. , Feuer, E. J. , and Pickle, L. W. A new method to evaluate the completeness of case ascertainment by a cancer registry. Cancer Causes Control, 19: 515 -25, 2008. 7. Fallah, M. , and Kharazmi, E. Global cancer incidences are substantially under-estimated due to under-ascertainment in elderly cancer cases. Asian Pac J Cancer Prev, 10: 223 -6, 2009. 8. Wingo, P. A. , Howe, H. L. , Thun, M. J. , Ballard-Barbash, R. , Ward, E. , Brown, M. L. , Sylvester, J. , Friedell, G. H. , Alley, L. , Rowland, J. H. , and Edwards, B. K. A national framework for cancer surveillance in the United States. Cancer Causes Control, 16: 151 -70, 2005. 9. Parkin, D. M. The role of cancer registries in cancer control. Int J Clin Oncol, 13: 102 -11, 2008. 10. Moore, M. A. , Shin, H. R. , Curado, M. P. , and Sobue, T. Establishment of an Asian Cancer Registry Network - problems and perspectives. Asian Pac J Cancer Prev, 9: 815 -32, 2008. January 2010 NCD Seminar, Lausanne

Thank you for your time and attention! Questions and Comments January 2010 NCD Seminar, Lausanne
8ed71e8d20e1197bd1ec8d91d2323af1.ppt