6337633d0083d73b7480fc3e6de960ca.ppt
- Количество слайдов: 29
 Can we eradicate HIV… What do we need to answer the question? Daria Hazuda Merck and Co July 2010
Can we eradicate HIV… What do we need to answer the question? Daria Hazuda Merck and Co July 2010
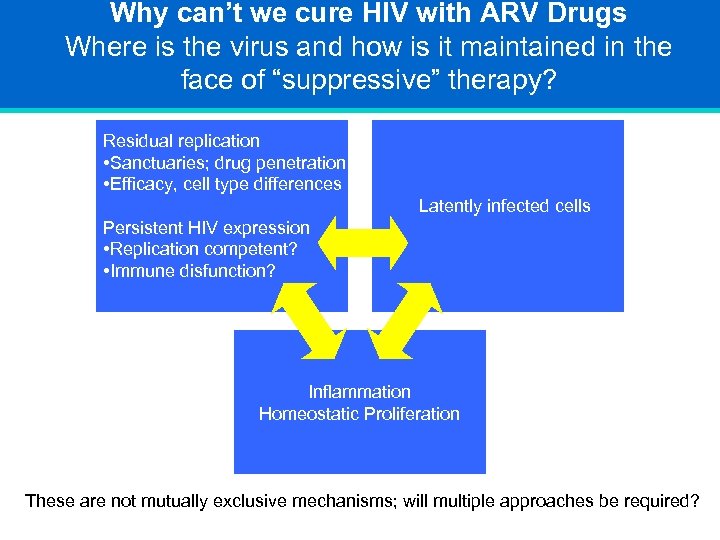 Why can’t we cure HIV with ARV Drugs Where is the virus and how is it maintained in the face of “suppressive” therapy? Residual replication • Sanctuaries; drug penetration • Efficacy, cell type differences Latently infected cells Persistent HIV expression • Replication competent? • Immune disfunction? Inflammation Homeostatic Proliferation These are not mutually exclusive mechanisms; will multiple approaches be required?
Why can’t we cure HIV with ARV Drugs Where is the virus and how is it maintained in the face of “suppressive” therapy? Residual replication • Sanctuaries; drug penetration • Efficacy, cell type differences Latently infected cells Persistent HIV expression • Replication competent? • Immune disfunction? Inflammation Homeostatic Proliferation These are not mutually exclusive mechanisms; will multiple approaches be required?
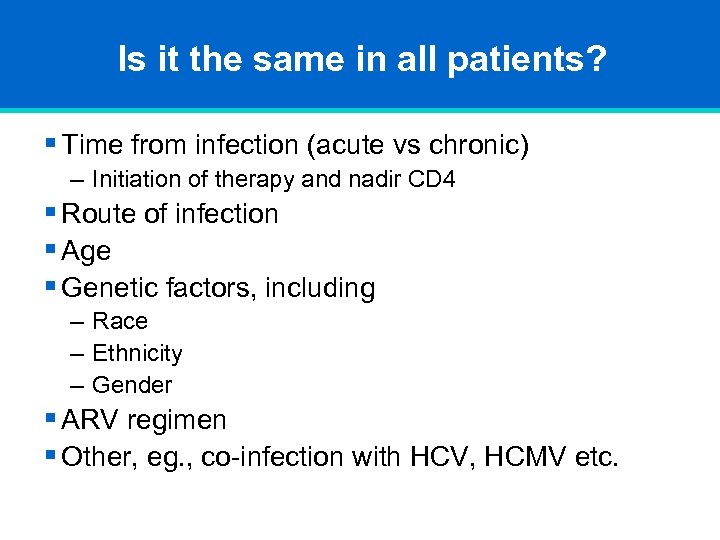 Is it the same in all patients? § Time from infection (acute vs chronic) – Initiation of therapy and nadir CD 4 § Route of infection § Age § Genetic factors, including – Race – Ethnicity – Gender § ARV regimen § Other, eg. , co-infection with HCV, HCMV etc.
Is it the same in all patients? § Time from infection (acute vs chronic) – Initiation of therapy and nadir CD 4 § Route of infection § Age § Genetic factors, including – Race – Ethnicity – Gender § ARV regimen § Other, eg. , co-infection with HCV, HCMV etc.
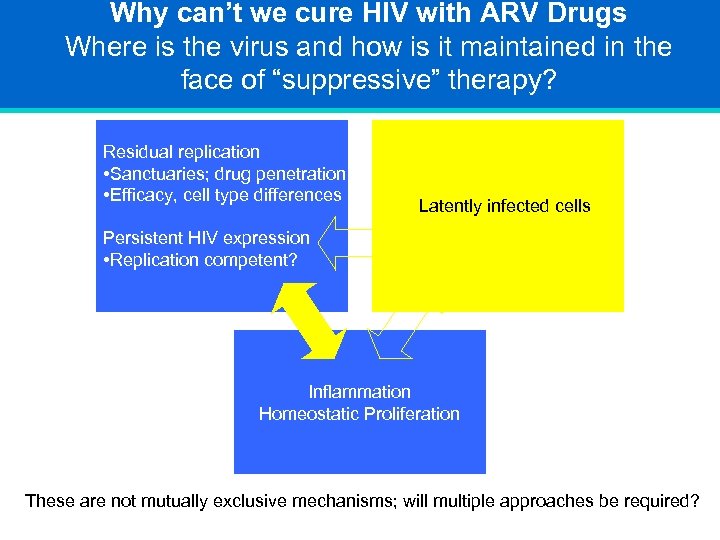 Why can’t we cure HIV with ARV Drugs Where is the virus and how is it maintained in the face of “suppressive” therapy? Residual replication • Sanctuaries; drug penetration • Efficacy, cell type differences Latently infected cells Persistent HIV expression • Replication competent? Inflammation Homeostatic Proliferation These are not mutually exclusive mechanisms; will multiple approaches be required?
Why can’t we cure HIV with ARV Drugs Where is the virus and how is it maintained in the face of “suppressive” therapy? Residual replication • Sanctuaries; drug penetration • Efficacy, cell type differences Latently infected cells Persistent HIV expression • Replication competent? Inflammation Homeostatic Proliferation These are not mutually exclusive mechanisms; will multiple approaches be required?
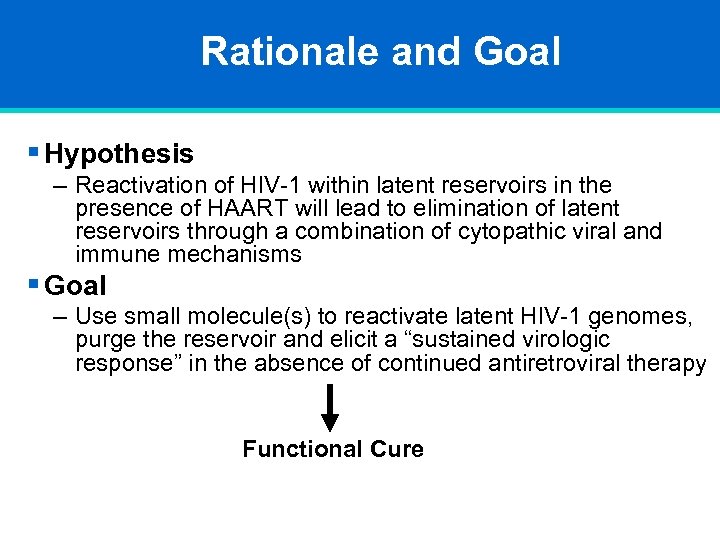 Rationale and Goal § Hypothesis – Reactivation of HIV-1 within latent reservoirs in the presence of HAART will lead to elimination of latent reservoirs through a combination of cytopathic viral and immune mechanisms § Goal – Use small molecule(s) to reactivate latent HIV-1 genomes, purge the reservoir and elicit a “sustained virologic response” in the absence of continued antiretroviral therapy Functional Cure
Rationale and Goal § Hypothesis – Reactivation of HIV-1 within latent reservoirs in the presence of HAART will lead to elimination of latent reservoirs through a combination of cytopathic viral and immune mechanisms § Goal – Use small molecule(s) to reactivate latent HIV-1 genomes, purge the reservoir and elicit a “sustained virologic response” in the absence of continued antiretroviral therapy Functional Cure
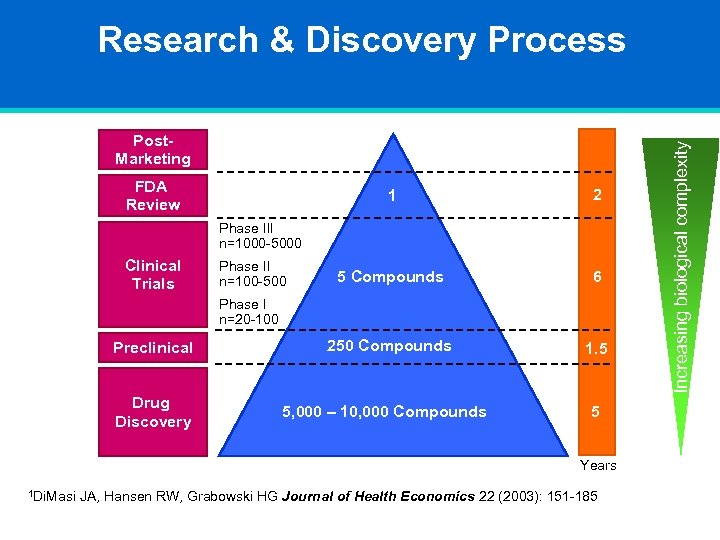 Post. Marketing FDA Review 1 2 5 Compounds 6 250 Compounds 1. 5 Phase III n=1000 -5000 Clinical Trials Phase II n=100 -500 Phase I n=20 -100 Preclinical Drug Discovery 5, 000 – 10, 000 Compounds 5 Years 1 Di. Masi JA, Hansen RW, Grabowski HG Journal of Health Economics 22 (2003): 151 -185 Increasing biological complexity Research & Discovery Process
Post. Marketing FDA Review 1 2 5 Compounds 6 250 Compounds 1. 5 Phase III n=1000 -5000 Clinical Trials Phase II n=100 -500 Phase I n=20 -100 Preclinical Drug Discovery 5, 000 – 10, 000 Compounds 5 Years 1 Di. Masi JA, Hansen RW, Grabowski HG Journal of Health Economics 22 (2003): 151 -185 Increasing biological complexity Research & Discovery Process
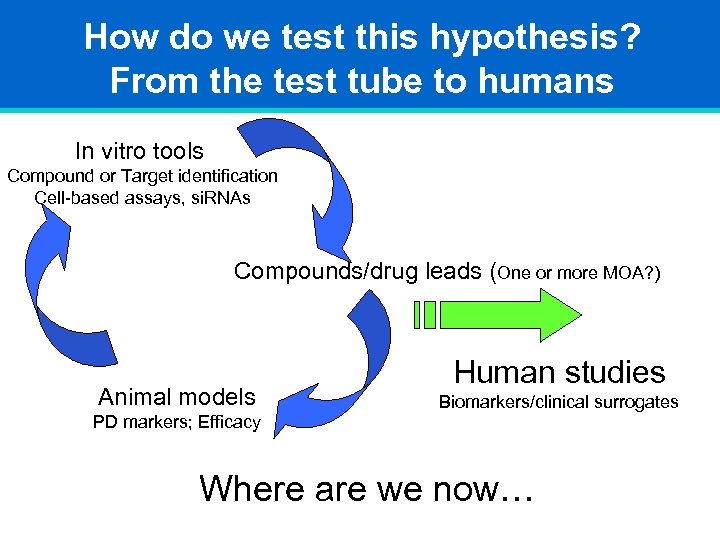 How do we test this hypothesis? From the test tube to humans In vitro tools Compound or Target identification Cell-based assays, si. RNAs Compounds/drug leads (One or more MOA? ) Animal models Human studies Biomarkers/clinical surrogates PD markers; Efficacy Where are we now…
How do we test this hypothesis? From the test tube to humans In vitro tools Compound or Target identification Cell-based assays, si. RNAs Compounds/drug leads (One or more MOA? ) Animal models Human studies Biomarkers/clinical surrogates PD markers; Efficacy Where are we now…
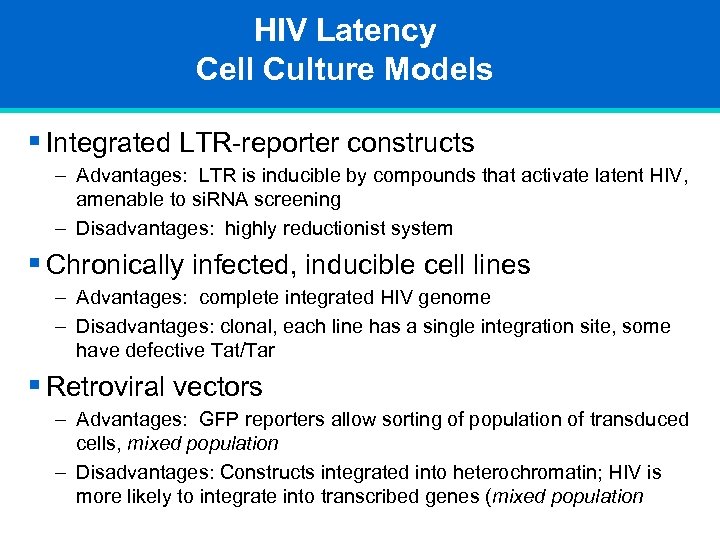 HIV Latency Cell Culture Models § Integrated LTR-reporter constructs – Advantages: LTR is inducible by compounds that activate latent HIV, amenable to si. RNA screening – Disadvantages: highly reductionist system § Chronically infected, inducible cell lines – Advantages: complete integrated HIV genome – Disadvantages: clonal, each line has a single integration site, some have defective Tat/Tar § Retroviral vectors – Advantages: GFP reporters allow sorting of population of transduced cells, mixed population – Disadvantages: Constructs integrated into heterochromatin; HIV is more likely to integrate into transcribed genes (mixed population
HIV Latency Cell Culture Models § Integrated LTR-reporter constructs – Advantages: LTR is inducible by compounds that activate latent HIV, amenable to si. RNA screening – Disadvantages: highly reductionist system § Chronically infected, inducible cell lines – Advantages: complete integrated HIV genome – Disadvantages: clonal, each line has a single integration site, some have defective Tat/Tar § Retroviral vectors – Advantages: GFP reporters allow sorting of population of transduced cells, mixed population – Disadvantages: Constructs integrated into heterochromatin; HIV is more likely to integrate into transcribed genes (mixed population
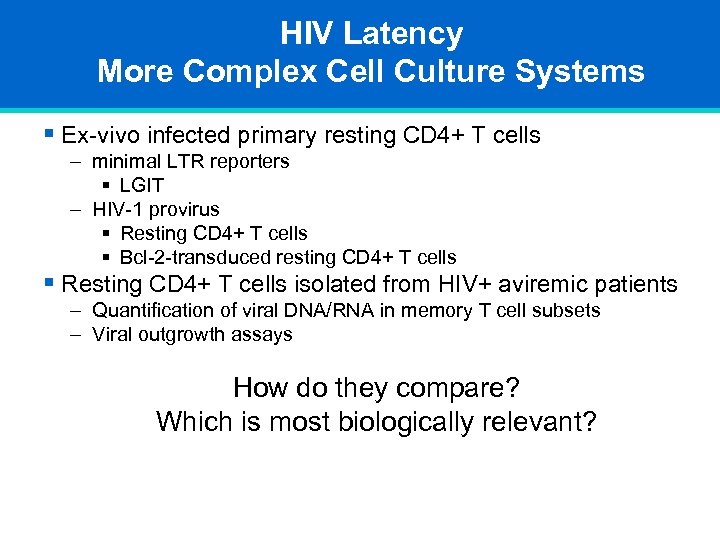 HIV Latency More Complex Cell Culture Systems § Ex-vivo infected primary resting CD 4+ T cells – minimal LTR reporters § LGIT – HIV-1 provirus § Resting CD 4+ T cells § Bcl-2 -transduced resting CD 4+ T cells § Resting CD 4+ T cells isolated from HIV+ aviremic patients – Quantification of viral DNA/RNA in memory T cell subsets – Viral outgrowth assays How do they compare? Which is most biologically relevant?
HIV Latency More Complex Cell Culture Systems § Ex-vivo infected primary resting CD 4+ T cells – minimal LTR reporters § LGIT – HIV-1 provirus § Resting CD 4+ T cells § Bcl-2 -transduced resting CD 4+ T cells § Resting CD 4+ T cells isolated from HIV+ aviremic patients – Quantification of viral DNA/RNA in memory T cell subsets – Viral outgrowth assays How do they compare? Which is most biologically relevant?
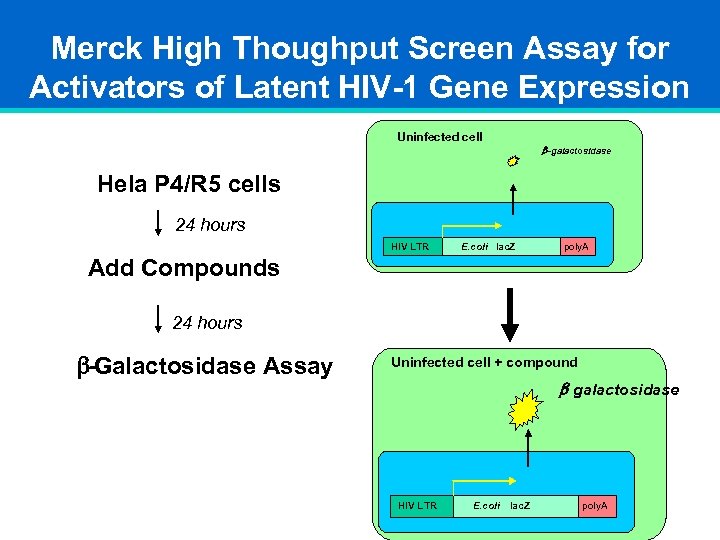 Merck High Thoughput Screen Assay for Activators of Latent HIV-1 Gene Expression Uninfected cell b -galactosidase Hela P 4/R 5 cells 24 hours HIV LTR E. coli lac. Z poly. A Add Compounds 24 hours b-Galactosidase Assay Uninfected cell + compound b- galactosidase HIV LTR E. coli lac. Z poly. A
Merck High Thoughput Screen Assay for Activators of Latent HIV-1 Gene Expression Uninfected cell b -galactosidase Hela P 4/R 5 cells 24 hours HIV LTR E. coli lac. Z poly. A Add Compounds 24 hours b-Galactosidase Assay Uninfected cell + compound b- galactosidase HIV LTR E. coli lac. Z poly. A
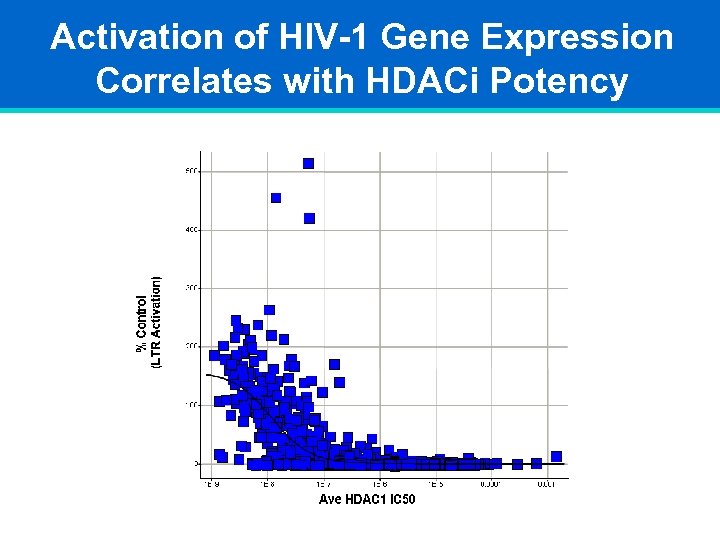 Activation of HIV-1 Gene Expression Correlates with HDACi Potency
Activation of HIV-1 Gene Expression Correlates with HDACi Potency
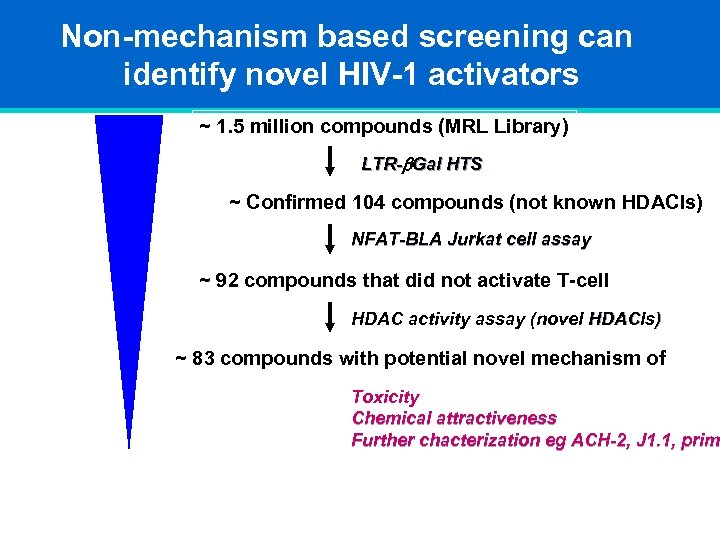 Non-mechanism based screening can identify novel HIV-1 activators ~ 1. 5 million compounds (MRL Library) LTR-b. Gal HTS ~ Confirmed 104 compounds (not known HDACIs) NFAT-BLA Jurkat cell assay ~ 92 compounds that did not activate T-cell HDAC activity assay (novel HDACIs) ~ 83 compounds with potential novel mechanism of Toxicity Chemical attractiveness Further chacterization eg ACH-2, J 1. 1, prima prim
Non-mechanism based screening can identify novel HIV-1 activators ~ 1. 5 million compounds (MRL Library) LTR-b. Gal HTS ~ Confirmed 104 compounds (not known HDACIs) NFAT-BLA Jurkat cell assay ~ 92 compounds that did not activate T-cell HDAC activity assay (novel HDACIs) ~ 83 compounds with potential novel mechanism of Toxicity Chemical attractiveness Further chacterization eg ACH-2, J 1. 1, prima prim
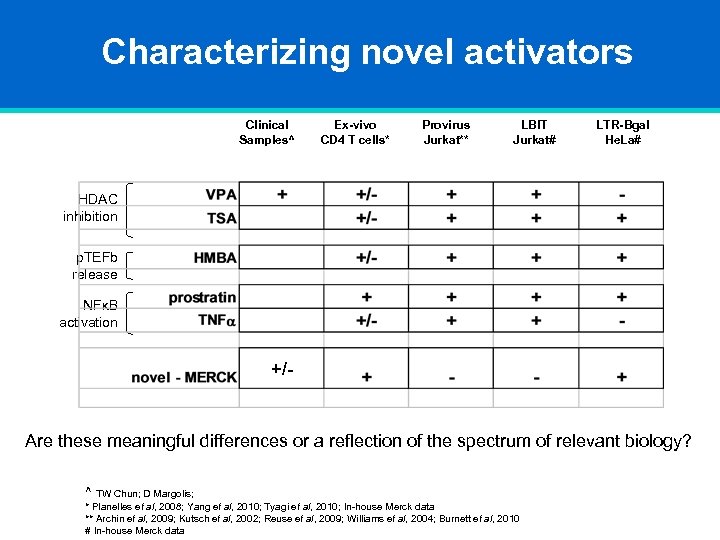 Characterizing novel activators Clinical Samples^ Ex-vivo CD 4 T cells* Provirus Jurkat** LBIT Jurkat# LTR-Bgal He. La# HDAC inhibition p. TEFb release NFκB activation +/- Are these meaningful differences or a reflection of the spectrum of relevant biology? ^ TW Chun; D Margolis; * Planelles et al, 2008; Yang et al, 2010; Tyagi et al, 2010; In-house Merck data ** Archin et al, 2009; Kutsch et al, 2002; Reuse et al, 2009; Williams et al, 2004; Burnett et al, 2010 # In-house Merck data
Characterizing novel activators Clinical Samples^ Ex-vivo CD 4 T cells* Provirus Jurkat** LBIT Jurkat# LTR-Bgal He. La# HDAC inhibition p. TEFb release NFκB activation +/- Are these meaningful differences or a reflection of the spectrum of relevant biology? ^ TW Chun; D Margolis; * Planelles et al, 2008; Yang et al, 2010; Tyagi et al, 2010; In-house Merck data ** Archin et al, 2009; Kutsch et al, 2002; Reuse et al, 2009; Williams et al, 2004; Burnett et al, 2010 # In-house Merck data
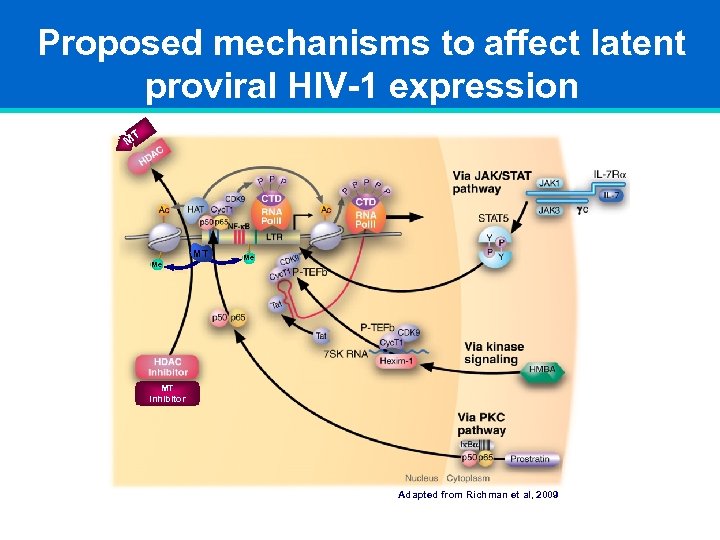 Proposed mechanisms to affect latent proviral HIV-1 expression MT MT Me Me MT Inhibitor Adapted from Richman et al, 2009
Proposed mechanisms to affect latent proviral HIV-1 expression MT MT Me Me MT Inhibitor Adapted from Richman et al, 2009
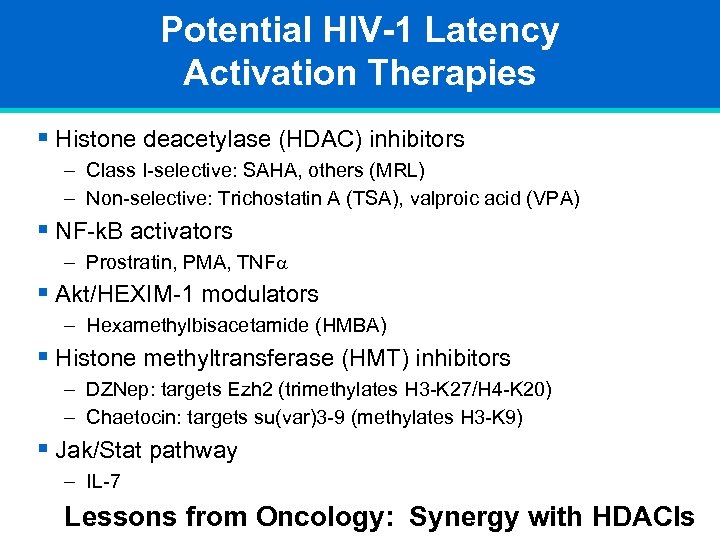 Potential HIV-1 Latency Activation Therapies § Histone deacetylase (HDAC) inhibitors – Class I-selective: SAHA, others (MRL) – Non-selective: Trichostatin A (TSA), valproic acid (VPA) § NF-k. B activators – Prostratin, PMA, TNF § Akt/HEXIM-1 modulators – Hexamethylbisacetamide (HMBA) § Histone methyltransferase (HMT) inhibitors – DZNep: targets Ezh 2 (trimethylates H 3 -K 27/H 4 -K 20) – Chaetocin: targets su(var)3 -9 (methylates H 3 -K 9) § Jak/Stat pathway – IL-7 Lessons from Oncology: Synergy with HDACIs
Potential HIV-1 Latency Activation Therapies § Histone deacetylase (HDAC) inhibitors – Class I-selective: SAHA, others (MRL) – Non-selective: Trichostatin A (TSA), valproic acid (VPA) § NF-k. B activators – Prostratin, PMA, TNF § Akt/HEXIM-1 modulators – Hexamethylbisacetamide (HMBA) § Histone methyltransferase (HMT) inhibitors – DZNep: targets Ezh 2 (trimethylates H 3 -K 27/H 4 -K 20) – Chaetocin: targets su(var)3 -9 (methylates H 3 -K 9) § Jak/Stat pathway – IL-7 Lessons from Oncology: Synergy with HDACIs
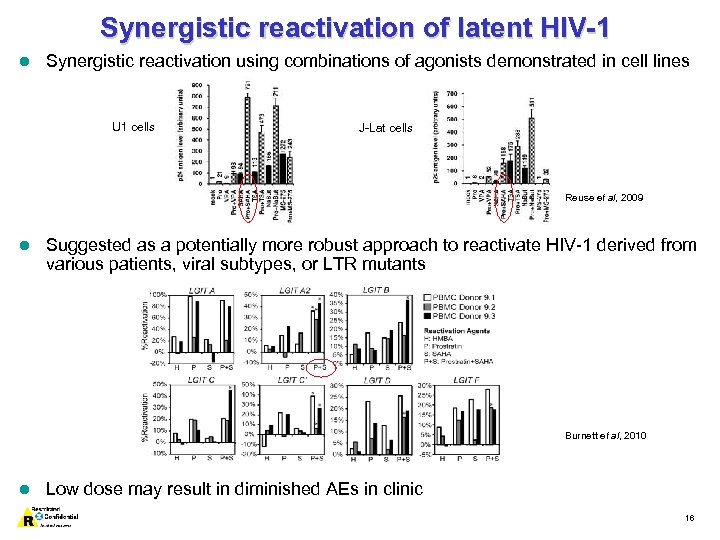 Synergistic reactivation of latent HIV-1 l Synergistic reactivation using combinations of agonists demonstrated in cell lines U 1 cells J-Lat cells Reuse et al, 2009 l Suggested as a potentially more robust approach to reactivate HIV-1 derived from various patients, viral subtypes, or LTR mutants Burnett et al, 2010 l Low dose may result in diminished AEs in clinic 16
Synergistic reactivation of latent HIV-1 l Synergistic reactivation using combinations of agonists demonstrated in cell lines U 1 cells J-Lat cells Reuse et al, 2009 l Suggested as a potentially more robust approach to reactivate HIV-1 derived from various patients, viral subtypes, or LTR mutants Burnett et al, 2010 l Low dose may result in diminished AEs in clinic 16
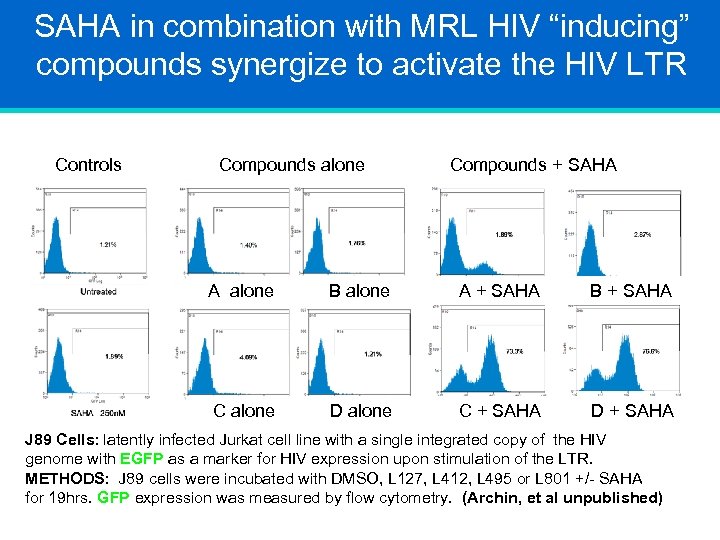 SAHA in combination with MRL HIV “inducing” compounds synergize to activate the HIV LTR Controls Compounds alone Compounds + SAHA A alone B alone A + SAHA B + SAHA C alone D alone C + SAHA D + SAHA J 89 Cells: latently infected Jurkat cell line with a single integrated copy of the HIV genome with EGFP as a marker for HIV expression upon stimulation of the LTR. METHODS: J 89 cells were incubated with DMSO, L 127, L 412, L 495 or L 801 +/- SAHA for 19 hrs. GFP expression was measured by flow cytometry. (Archin, et al unpublished)
SAHA in combination with MRL HIV “inducing” compounds synergize to activate the HIV LTR Controls Compounds alone Compounds + SAHA A alone B alone A + SAHA B + SAHA C alone D alone C + SAHA D + SAHA J 89 Cells: latently infected Jurkat cell line with a single integrated copy of the HIV genome with EGFP as a marker for HIV expression upon stimulation of the LTR. METHODS: J 89 cells were incubated with DMSO, L 127, L 412, L 495 or L 801 +/- SAHA for 19 hrs. GFP expression was measured by flow cytometry. (Archin, et al unpublished)
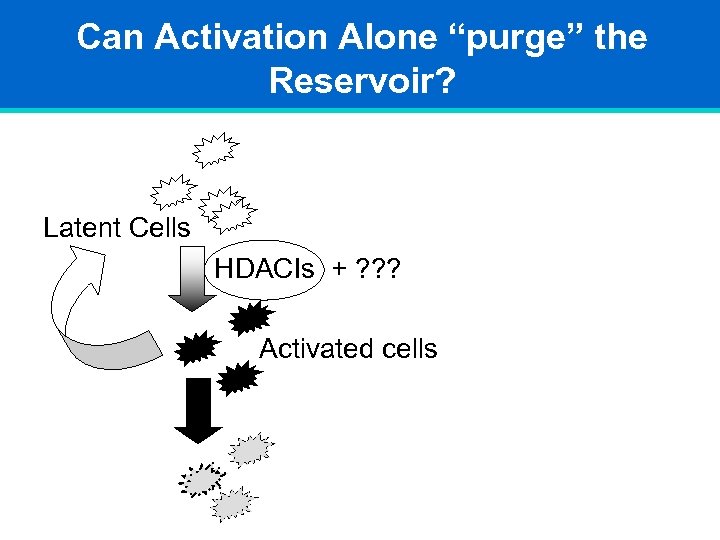 Can Activation Alone “purge” the Reservoir? Latent Cells HDACIs + ? ? ? OR Activated cells Immune Modulator?
Can Activation Alone “purge” the Reservoir? Latent Cells HDACIs + ? ? ? OR Activated cells Immune Modulator?
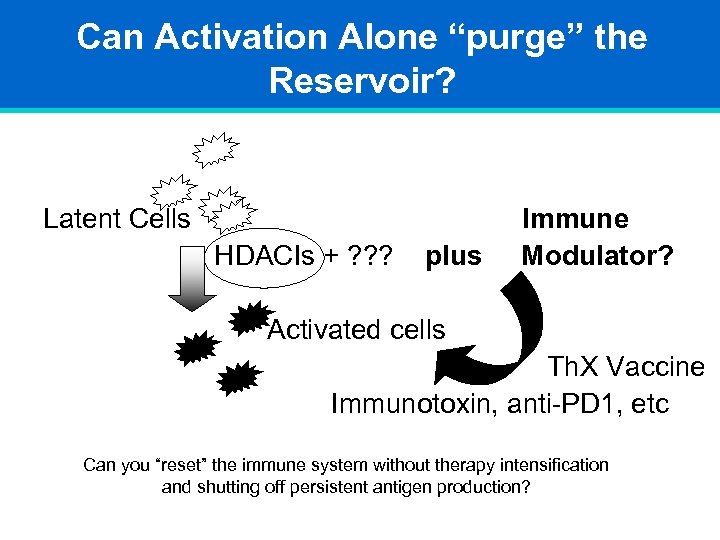 Can Activation Alone “purge” the Reservoir? Latent Cells HDACIs + ? ? ? plus Immune Modulator? Activated cells Th. X Vaccine Immunotoxin, anti-PD 1, etc Can you “reset” the immune system without therapy intensification and shutting off persistent antigen production?
Can Activation Alone “purge” the Reservoir? Latent Cells HDACIs + ? ? ? plus Immune Modulator? Activated cells Th. X Vaccine Immunotoxin, anti-PD 1, etc Can you “reset” the immune system without therapy intensification and shutting off persistent antigen production?
 HIV-1 Latency Pre-clinical In Vivo Models § Animal models are critical for understanding viral persistence and testing novel concepts – Can model HAART in HIV-infected humans (eg, RTIs and In. STIs) – Parameters such as time of infection and HAART initiation can be standardized. – It is possible to extensively evaluate reservoirs in tissues eg, GALT & CNS – Viral rebound as an critical endpoint can be monitored. § Rodent Models – SCID-hu mouse (human transplants of thymus, fetal liver or PBMCs
HIV-1 Latency Pre-clinical In Vivo Models § Animal models are critical for understanding viral persistence and testing novel concepts – Can model HAART in HIV-infected humans (eg, RTIs and In. STIs) – Parameters such as time of infection and HAART initiation can be standardized. – It is possible to extensively evaluate reservoirs in tissues eg, GALT & CNS – Viral rebound as an critical endpoint can be monitored. § Rodent Models – SCID-hu mouse (human transplants of thymus, fetal liver or PBMCs
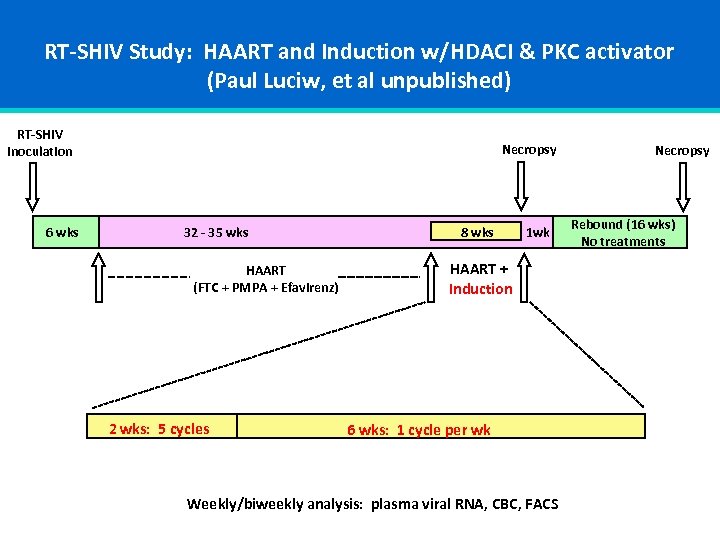 RT-SHIV Study: HAART and Induction w/HDACI & PKC activator (Paul Luciw, et al unpublished) RT-SHIV Inoculation 6 wks Necropsy 32 - 35 wks HAART (FTC + PMPA + Efavirenz) 2 wks: 5 cycles 8 wks 1 wk HAART + Induction 6 wks: 1 cycle per wk Weekly/biweekly analysis: plasma viral RNA, CBC, FACS Necropsy Rebound (16 wks) No treatments
RT-SHIV Study: HAART and Induction w/HDACI & PKC activator (Paul Luciw, et al unpublished) RT-SHIV Inoculation 6 wks Necropsy 32 - 35 wks HAART (FTC + PMPA + Efavirenz) 2 wks: 5 cycles 8 wks 1 wk HAART + Induction 6 wks: 1 cycle per wk Weekly/biweekly analysis: plasma viral RNA, CBC, FACS Necropsy Rebound (16 wks) No treatments
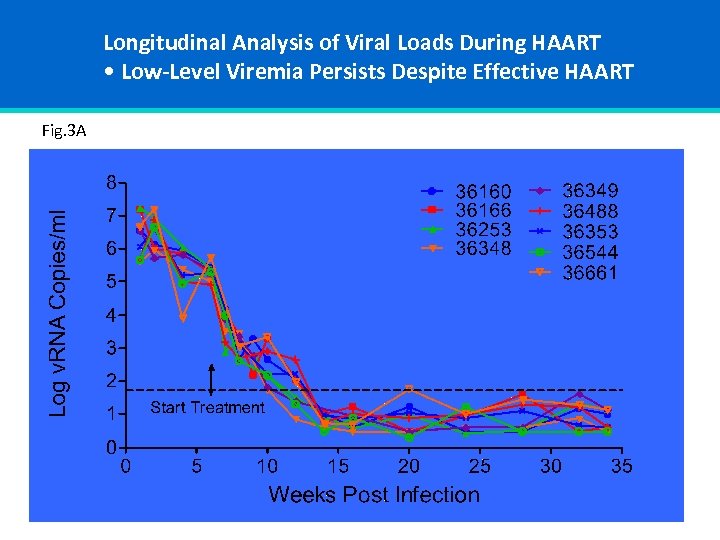 Longitudinal Analysis of Viral Loads During HAART • Low-Level Viremia Persists Despite Effective HAART Fig. 3 A
Longitudinal Analysis of Viral Loads During HAART • Low-Level Viremia Persists Despite Effective HAART Fig. 3 A
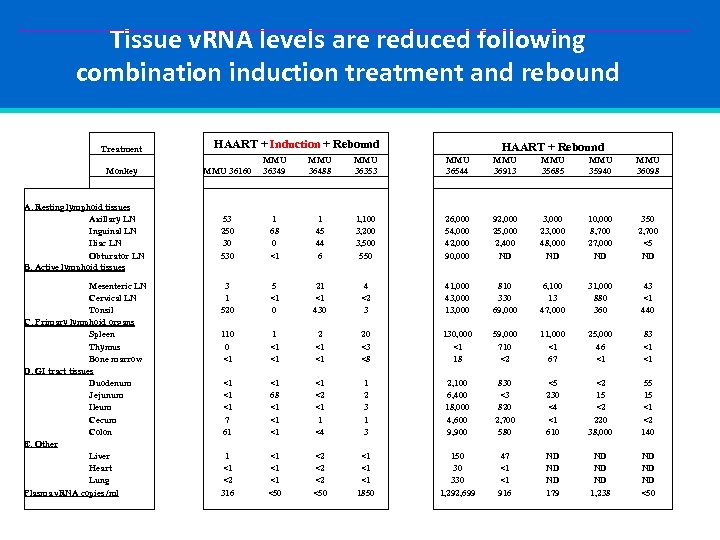 Tissue v. RNA levels are reduced following combination induction treatment and rebound Treatment Monkey A. Resting lymphoid tissues Axillary LN Inguinal LN Iliac LN Obturator LN B. Active lymphoid tissues Mesenteric LN Cervical LN Tonsil C. Primary lymphoid organs Spleen Thymus Bone marrow D. GI tract tissues Duodenum Jejunum Ileum Cecum Colon E. Other Liver Heart Lung Plasma v. RNA copies /ml HAART + Induction + Rebound HAART + Rebound MMU 36160 MMU 36349 MMU 36488 MMU 36353 MMU 36544 MMU 36913 MMU 35685 MMU 35940 MMU 36098 53 250 30 530 1 68 0 <1 1 45 44 6 1, 100 3, 200 3, 500 550 26, 000 54, 000 42, 000 90, 000 92, 000 25, 000 2, 400 ND 3, 000 23, 000 48, 000 ND 10, 000 8, 700 27, 000 ND 350 2, 700 <5 ND 3 1 520 5 <1 0 21 <1 430 4 <2 3 41, 000 43, 000 13, 000 810 330 69, 000 6, 100 13 47, 000 31, 000 880 360 43 <1 440 110 0 <1 1 <1 <1 20 <3 <8 130, 000 <1 18 59, 000 710 <2 11, 000 <1 67 25, 000 46 <1 83 <1 <1 <1 7 61 <1 68 <1 <1 <2 <1 1 <4 1 2 3 1 3 2, 100 6, 400 18, 000 4, 600 9, 900 830 <3 820 2, 700 580 <5 230 <4 <1 610 <2 15 <2 220 38, 000 55 15 <1 <2 140 1 <1 <2 316 <1 <1 <1 <50 <2 <2 <2 <50 <1 <1 <1 1850 150 30 330 1, 292, 699 47 <1 <1 916 ND ND ND 179 ND ND ND 1, 238 ND ND ND <50
Tissue v. RNA levels are reduced following combination induction treatment and rebound Treatment Monkey A. Resting lymphoid tissues Axillary LN Inguinal LN Iliac LN Obturator LN B. Active lymphoid tissues Mesenteric LN Cervical LN Tonsil C. Primary lymphoid organs Spleen Thymus Bone marrow D. GI tract tissues Duodenum Jejunum Ileum Cecum Colon E. Other Liver Heart Lung Plasma v. RNA copies /ml HAART + Induction + Rebound HAART + Rebound MMU 36160 MMU 36349 MMU 36488 MMU 36353 MMU 36544 MMU 36913 MMU 35685 MMU 35940 MMU 36098 53 250 30 530 1 68 0 <1 1 45 44 6 1, 100 3, 200 3, 500 550 26, 000 54, 000 42, 000 90, 000 92, 000 25, 000 2, 400 ND 3, 000 23, 000 48, 000 ND 10, 000 8, 700 27, 000 ND 350 2, 700 <5 ND 3 1 520 5 <1 0 21 <1 430 4 <2 3 41, 000 43, 000 13, 000 810 330 69, 000 6, 100 13 47, 000 31, 000 880 360 43 <1 440 110 0 <1 1 <1 <1 20 <3 <8 130, 000 <1 18 59, 000 710 <2 11, 000 <1 67 25, 000 46 <1 83 <1 <1 <1 7 61 <1 68 <1 <1 <2 <1 1 <4 1 2 3 1 3 2, 100 6, 400 18, 000 4, 600 9, 900 830 <3 820 2, 700 580 <5 230 <4 <1 610 <2 15 <2 220 38, 000 55 15 <1 <2 140 1 <1 <2 316 <1 <1 <1 <50 <2 <2 <2 <50 <1 <1 <1 1850 150 30 330 1, 292, 699 47 <1 <1 916 ND ND ND 179 ND ND ND 1, 238 ND ND ND <50
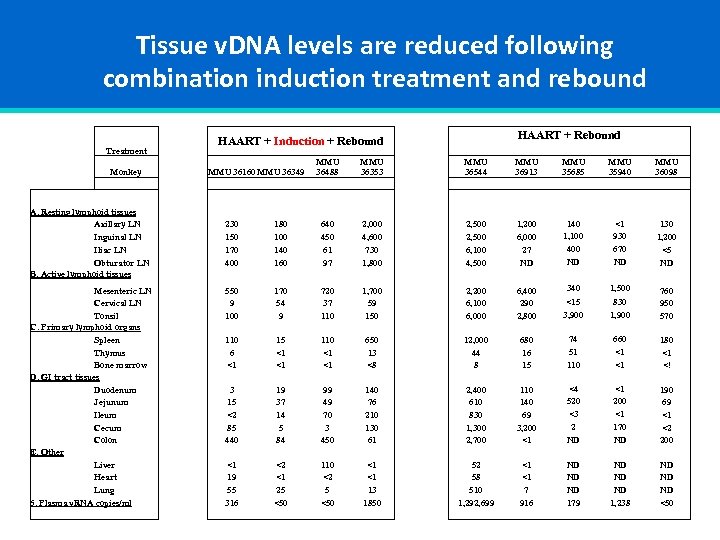 Tissue v. DNA levels are reduced following combination induction treatment and rebound Treatment Monkey A. Resting lymphoid tissues Axillary LN Inguinal LN Iliac LN Obturator LN B. Active lymphoid tissues Mesenteric LN Cervical LN Tonsil C. Primary lymphoid organs Spleen Thymus Bone marrow D. GI tract tissues Duodenum Jejunum Ileum Cecum Colon E. Other Liver Heart Lung 6. Plasma v. RNA copies/ml HAART + Rebound HAART + Induction + Rebound MMU 36488 MMU 36160 MMU 36349 MMU 36353 MMU 36544 MMU 36913 MMU 35685 MMU 35940 MMU 36098 230 150 170 400 180 100 140 160 640 450 61 97 2, 000 4, 600 730 1, 800 2, 500 6, 100 4, 500 1, 200 6, 000 27 ND 140 1, 100 400 ND <1 930 670 ND 130 1, 200 <5 ND 550 9 100 170 54 9 720 37 110 1, 700 59 150 2, 200 6, 100 6, 000 6, 400 290 2, 800 340 1, 500 <15 3, 900 830 1, 900 760 950 570 110 6 <1 15 <1 <1 110 <1 <1 650 13 <8 12, 000 44 8 680 16 15 74 51 110 660 <1 <1 180 <1
Tissue v. DNA levels are reduced following combination induction treatment and rebound Treatment Monkey A. Resting lymphoid tissues Axillary LN Inguinal LN Iliac LN Obturator LN B. Active lymphoid tissues Mesenteric LN Cervical LN Tonsil C. Primary lymphoid organs Spleen Thymus Bone marrow D. GI tract tissues Duodenum Jejunum Ileum Cecum Colon E. Other Liver Heart Lung 6. Plasma v. RNA copies/ml HAART + Rebound HAART + Induction + Rebound MMU 36488 MMU 36160 MMU 36349 MMU 36353 MMU 36544 MMU 36913 MMU 35685 MMU 35940 MMU 36098 230 150 170 400 180 100 140 160 640 450 61 97 2, 000 4, 600 730 1, 800 2, 500 6, 100 4, 500 1, 200 6, 000 27 ND 140 1, 100 400 ND <1 930 670 ND 130 1, 200 <5 ND 550 9 100 170 54 9 720 37 110 1, 700 59 150 2, 200 6, 100 6, 000 6, 400 290 2, 800 340 1, 500 <15 3, 900 830 1, 900 760 950 570 110 6 <1 15 <1 <1 110 <1 <1 650 13 <8 12, 000 44 8 680 16 15 74 51 110 660 <1 <1 180 <1
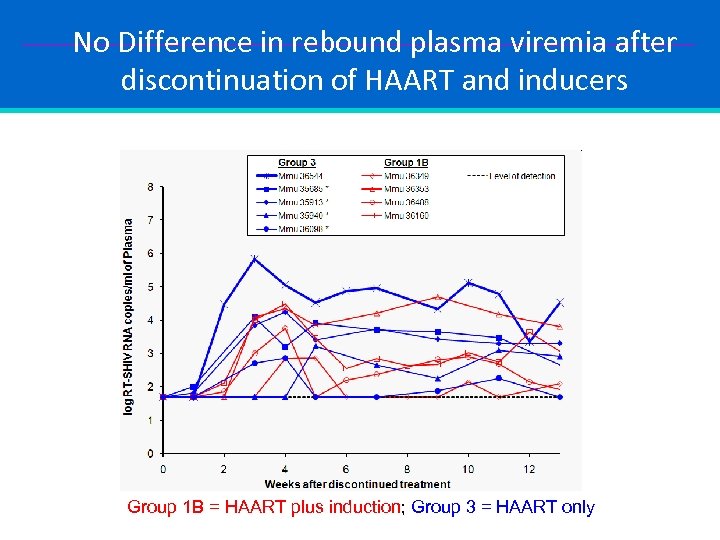 No Difference in rebound plasma viremia after discontinuation of HAART and inducers Group 1 B = HAART plus induction; Group 3 = HAART only
No Difference in rebound plasma viremia after discontinuation of HAART and inducers Group 1 B = HAART plus induction; Group 3 = HAART only
 Summary and Outstanding Issues § Multiple and perhaps “inter-dependent” processes contribute to the inability to eradicate HIV with ARV therapy – Will any one approach be sufficient for eradication? – Will the same combination of interventions work for all patients? § Various interventions which can address at least some of these issues are being explored including small molecules which may activate latent viral gene expression – Activators can manifest differential activity in different cell based assays; Are these differences biologically meaningful or reflect a specturm of biological mechanisms relevant in “non-uniform” systems? – HDACIs appear to be the most robust, provide an anchor for combinations? – Will activation therapy be sufficient without modulation of the immune response; can the immune response be modulated wiithout blocking pesistent viremia? § Evaluating these approaches individually and in combination in well validated animal models will be critical to understand many of these issues – What data will provide sufficient evidence to justify clinical evaluation? – What clinical surrogates can be used to provide an early signal of efficacy and would be sufficiently robust to trigger therapy interruption?
Summary and Outstanding Issues § Multiple and perhaps “inter-dependent” processes contribute to the inability to eradicate HIV with ARV therapy – Will any one approach be sufficient for eradication? – Will the same combination of interventions work for all patients? § Various interventions which can address at least some of these issues are being explored including small molecules which may activate latent viral gene expression – Activators can manifest differential activity in different cell based assays; Are these differences biologically meaningful or reflect a specturm of biological mechanisms relevant in “non-uniform” systems? – HDACIs appear to be the most robust, provide an anchor for combinations? – Will activation therapy be sufficient without modulation of the immune response; can the immune response be modulated wiithout blocking pesistent viremia? § Evaluating these approaches individually and in combination in well validated animal models will be critical to understand many of these issues – What data will provide sufficient evidence to justify clinical evaluation? – What clinical surrogates can be used to provide an early signal of efficacy and would be sufficiently robust to trigger therapy interruption?
 Acknowledgements § Amy Espeseth § Marta Majdan § Camil Sayegh § Chris Tan Collaborators: § David Margolis § Una O’Doherty § Doug Richman § Jeff Lifson § Paul Luciw § Tom North § Warner Greene § Eric Verdin Many others
Acknowledgements § Amy Espeseth § Marta Majdan § Camil Sayegh § Chris Tan Collaborators: § David Margolis § Una O’Doherty § Doug Richman § Jeff Lifson § Paul Luciw § Tom North § Warner Greene § Eric Verdin Many others

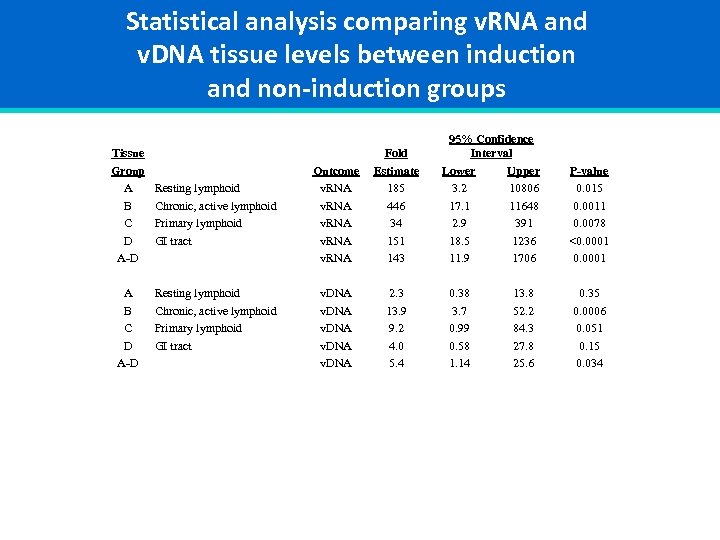 Statistical analysis comparing v. RNA and v. DNA tissue levels between induction and non-induction groups Tissue Group A B C D A-D Resting lymphoid Chronic, active lymphoid Primary lymphoid GI tract Outcome v. RNA Fold Estimate 185 446 34 151 143 v. DNA 2. 3 13. 9 9. 2 4. 0 5. 4 95% Confidence Interval Lower Upper 3. 2 10806 17. 1 11648 2. 9 391 18. 5 1236 11. 9 1706 0. 38 3. 7 0. 99 0. 58 1. 14 13. 8 52. 2 84. 3 27. 8 25. 6 P-value 0. 015 0. 0011 0. 0078 <0. 0001 0. 35 0. 0006 0. 051 0. 15 0. 034
Statistical analysis comparing v. RNA and v. DNA tissue levels between induction and non-induction groups Tissue Group A B C D A-D Resting lymphoid Chronic, active lymphoid Primary lymphoid GI tract Outcome v. RNA Fold Estimate 185 446 34 151 143 v. DNA 2. 3 13. 9 9. 2 4. 0 5. 4 95% Confidence Interval Lower Upper 3. 2 10806 17. 1 11648 2. 9 391 18. 5 1236 11. 9 1706 0. 38 3. 7 0. 99 0. 58 1. 14 13. 8 52. 2 84. 3 27. 8 25. 6 P-value 0. 015 0. 0011 0. 0078 <0. 0001 0. 35 0. 0006 0. 051 0. 15 0. 034


