8dcaf359ca1b440869229febec1dc926.ppt
- Количество слайдов: 1
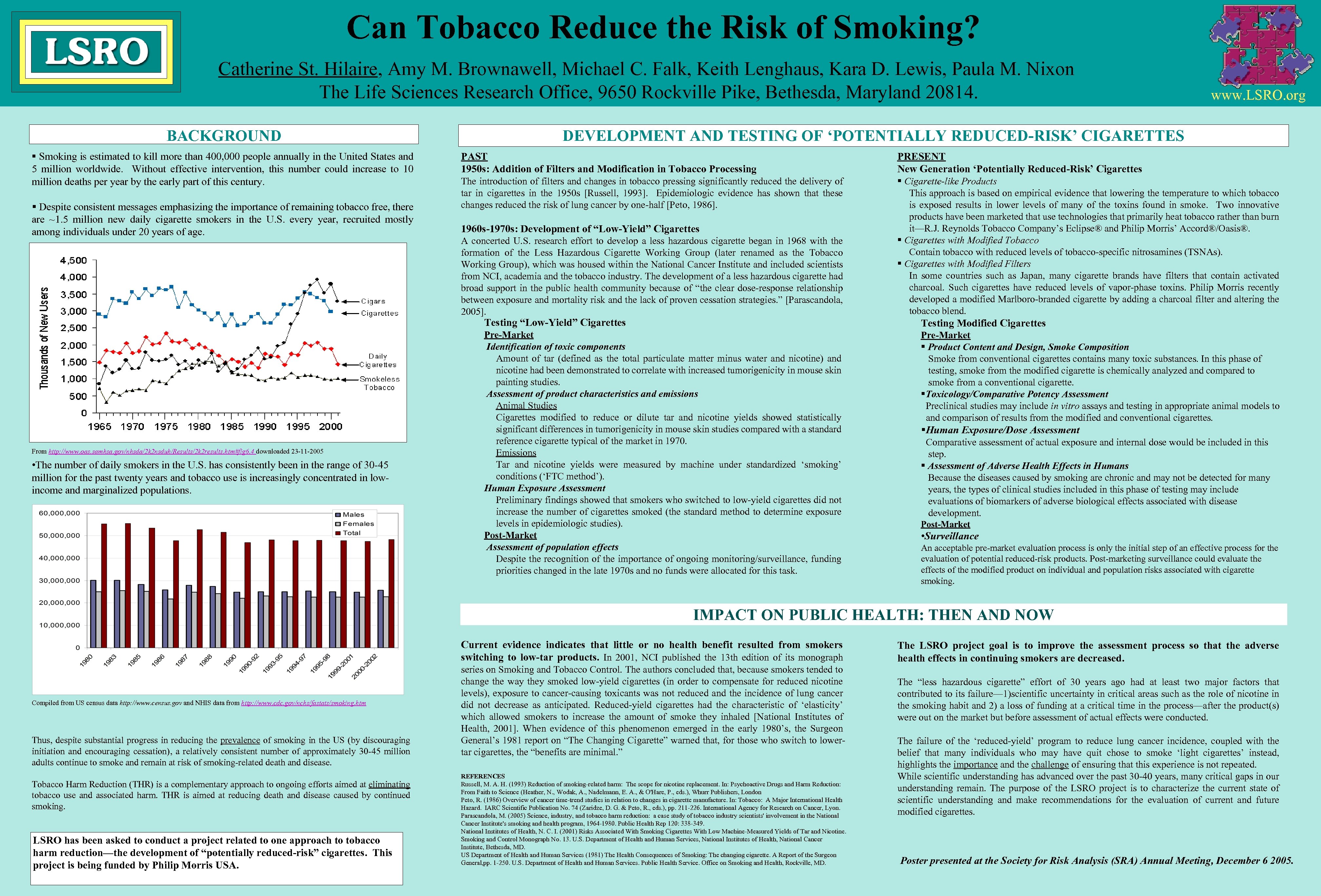
Can Tobacco Reduce the Risk of Smoking? Catherine St. Hilaire, Amy M. Brownawell, Michael C. Falk, Keith Lenghaus, Kara D. Lewis, Paula M. Nixon The Life Sciences Research Office, 9650 Rockville Pike, Bethesda, Maryland 20814. BACKGROUND § Smoking is estimated to kill more than 400, 000 people annually in the United States and 5 million worldwide. Without effective intervention, this number could increase to 10 million deaths per year by the early part of this century. § Despite consistent messages emphasizing the importance of remaining tobacco free, there are ~1. 5 million new daily cigarette smokers in the U. S. every year, recruited mostly among individuals under 20 years of age. www. LSRO. org DEVELOPMENT AND TESTING OF ‘POTENTIALLY REDUCED-RISK’ CIGARETTES PAST 1950 s: Addition of Filters and Modification in Tobacco Processing The introduction of filters and changes in tobacco pressing significantly reduced the delivery of tar in cigarettes in the 1950 s [Russell, 1993]. Epidemiologic evidence has shown that these changes reduced the risk of lung cancer by one-half [Peto, 1986]. 1960 s-1970 s: Development of “Low-Yield” Cigarettes A concerted U. S. research effort to develop a less hazardous cigarette began in 1968 with the formation of the Less Hazardous Cigarette Working Group (later renamed as the Tobacco Working Group), which was housed within the National Cancer Institute and included scientists from NCI, academia and the tobacco industry. The development of a less hazardous cigarette had broad support in the public health community because of “the clear dose-response relationship between exposure and mortality risk and the lack of proven cessation strategies. ” [Parascandola, 2005]. PRESENT New Generation ‘Potentially Reduced-Risk’ Cigarettes § Cigarette-like Products This approach is based on empirical evidence that lowering the temperature to which tobacco is exposed results in lower levels of many of the toxins found in smoke. Two innovative products have been marketed that use technologies that primarily heat tobacco rather than burn it—R. J. Reynolds Tobacco Company’s Eclipse® and Philip Morris’ Accord®/Oasis®. § Cigarettes with Modified Tobacco Contain tobacco with reduced levels of tobacco-specific nitrosamines (TSNAs). § Cigarettes with Modified Filters In some countries such as Japan, many cigarette brands have filters that contain activated charcoal. Such cigarettes have reduced levels of vapor-phase toxins. Philip Morris recently developed a modified Marlboro-branded cigarette by adding a charcoal filter and altering the tobacco blend. Testing “Low-Yield” Cigarettes § From http: //www. oas. samhsa. gov/nhsda/2 k 2 nsduh/Results/2 k 2 results. htm#fig 6. 4 downloaded 23 -11 -2005 • The number of daily smokers in the U. S. has consistently been in the range of 30 -45 million for the past twenty years and tobacco use is increasingly concentrated in lowincome and marginalized populations. Testing Modified Cigarettes Pre-Market Identification of toxic components Amount of tar (defined as the total particulate matter minus water and nicotine) and nicotine had been demonstrated to correlate with increased tumorigenicity in mouse skin painting studies. Assessment of product characteristics and emissions Animal Studies Cigarettes modified to reduce or dilute tar and nicotine yields showed statistically significant differences in tumorigenicity in mouse skin studies compared with a standard reference cigarette typical of the market in 1970. Emissions Tar and nicotine yields were measured by machine under standardized ‘smoking’ conditions (‘FTC method’). Human Exposure Assessment Preliminary findings showed that smokers who switched to low-yield cigarettes did not increase the number of cigarettes smoked (the standard method to determine exposure levels in epidemiologic studies). Post-Market Assessment of population effects Despite the recognition of the importance of ongoing monitoring/surveillance, funding priorities changed in the late 1970 s and no funds were allocated for this task. Pre-Market § Product Content and Design, Smoke Composition Smoke from conventional cigarettes contains many toxic substances. In this phase of testing, smoke from the modified cigarette is chemically analyzed and compared to smoke from a conventional cigarette. §Toxicology/Comparative Potency Assessment Preclinical studies may include in vitro assays and testing in appropriate animal models to and comparison of results from the modified and conventional cigarettes. §Human Exposure/Dose Assessment Comparative assessment of actual exposure and internal dose would be included in this step. § Assessment of Adverse Health Effects in Humans Because the diseases caused by smoking are chronic and may not be detected for many years, the types of clinical studies included in this phase of testing may include evaluations of biomarkers of adverse biological effects associated with disease development. Post-Market • Surveillance An acceptable pre-market evaluation process is only the initial step of an effective process for the evaluation of potential reduced-risk products. Post-marketing surveillance could evaluate the effects of the modified product on individual and population risks associated with cigarette smoking. IMPACT ON PUBLIC HEALTH: THEN AND NOW Current evidence indicates that little or no health benefit resulted from smokers switching to low-tar products. In 2001, NCI published the 13 th edition of its monograph Compiled from US census data http: //www. census. gov and NHIS data from http: //www. cdc. gov/nchs/fastats/smoking. htm Thus, despite substantial progress in reducing the prevalence of smoking in the US (by discouraging initiation and encouraging cessation), a relatively consistent number of approximately 30 -45 million adults continue to smoke and remain at risk of smoking-related death and disease. Tobacco Harm Reduction (THR) is a complementary approach to ongoing efforts aimed at eliminating tobacco use and associated harm. THR is aimed at reducing death and disease caused by continued smoking. LSRO has been asked to conduct a project related to one approach to tobacco harm reduction—the development of “potentially reduced-risk” cigarettes. This project is being funded by Philip Morris USA. series on Smoking and Tobacco Control. The authors concluded that, because smokers tended to change the way they smoked low-yield cigarettes (in order to compensate for reduced nicotine levels), exposure to cancer-causing toxicants was not reduced and the incidence of lung cancer did not decrease as anticipated. Reduced-yield cigarettes had the characteristic of ‘elasticity’ which allowed smokers to increase the amount of smoke they inhaled [National Institutes of Health, 2001]. When evidence of this phenomenon emerged in the early 1980’s, the Surgeon General’s 1981 report on “The Changing Cigarette” warned that, for those who switch to lowertar cigarettes, the “benefits are minimal. ” REFERENCES Russell, M. A. H. (1993) Reduction of smoking-related harm: The scope for nicotine replacement. In: Psychoactive Drugs and Harm Reduction: From Faith to Science (Heather, N. , Wodak, A. , Nadelmann, E. A. , & O'Hare, P. , eds. ), Whurr Publishers, London Peto, R. (1986) Overview of cancer time-trend studies in relation to changes in cigarette manufacture. In: Tobacco: A Major International Health Hazard. IARC Scientific Publication No. 74 (Zaridze, D. G. & Peto, R. , eds. ), pp. 211 -226. International Agency for Research on Cancer, Lyon. Parascandola, M. (2005) Science, industry, and tobacco harm reduction: a case study of tobacco industry scientists' involvement in the National Cancer Institute's smoking and health program, 1964 -1980. Public Health Rep 120: 338 -349. National Institutes of Health, N. C. I. (2001) Risks Associated With Smoking Cigarettes With Low Machine-Measured Yields of Tar and Nicotine. Smoking and Control Monograph No. 13. U. S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Bethesda, MD. US Department of Health and Human Services (1981) The Health Consequences of Smoking: The changing cigarette. A Report of the Surgeon General, pp. 1 -250. U. S. Department of Health and Human Services. Public Health Service. Office on Smoking and Health, Rockville, MD. The LSRO project goal is to improve the assessment process so that the adverse health effects in continuing smokers are decreased. The “less hazardous cigarette” effort of 30 years ago had at least two major factors that contributed to its failure— 1)scientific uncertainty in critical areas such as the role of nicotine in the smoking habit and 2) a loss of funding at a critical time in the process—after the product(s) were out on the market but before assessment of actual effects were conducted. The failure of the ‘reduced-yield’ program to reduce lung cancer incidence, coupled with the belief that many individuals who may have quit chose to smoke ‘light cigarettes’ instead, highlights the importance and the challenge of ensuring that this experience is not repeated. While scientific understanding has advanced over the past 30 -40 years, many critical gaps in our understanding remain. The purpose of the LSRO project is to characterize the current state of scientific understanding and make recommendations for the evaluation of current and future modified cigarettes. Poster presented at the Society for Risk Analysis (SRA) Annual Meeting, December 6 2005.
8dcaf359ca1b440869229febec1dc926.ppt