d749b2e7c6852b72dc8f3e37a4509149.ppt
- Количество слайдов: 37

CAMPBELL BIOLOGY IN FOCUS Urry • Cain • Wasserman • Minorsky • Jackson • Reece 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge © 2014 Pearson Education, Inc.

Do now: (think back to most recent lab) §Write down the reaction for the decomposition of hydrogen peroxide. Identify the reactants and the products. §What was the enzyme used to facilitate this reaction? Why was this enzyme necessary? §What was the purpose of the guaiacol? §What effect do you think p. H would have on the enzyme?
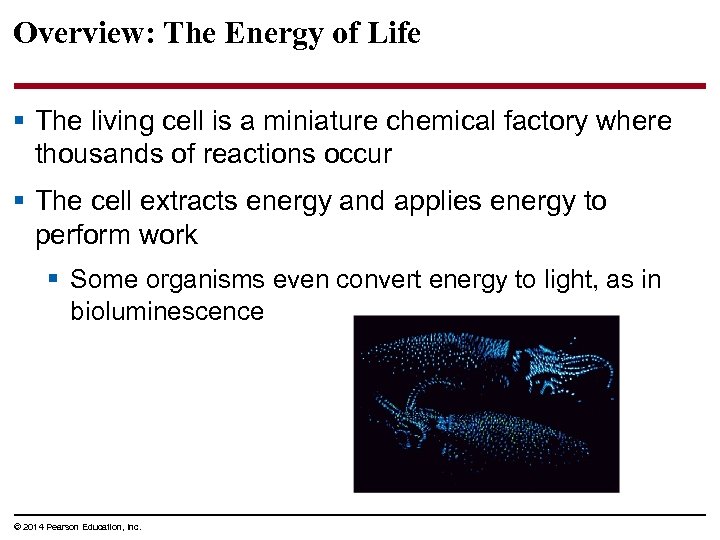
Overview: The Energy of Life § The living cell is a miniature chemical factory where thousands of reactions occur § The cell extracts energy and applies energy to perform work § Some organisms even convert energy to light, as in bioluminescence © 2014 Pearson Education, Inc.
Concept 6. 1: An organism’s metabolism transforms matter and energy § Metabolism is the totality of an organism’s chemical reactions § Energy is the capacity to cause change § Catabolic pathways release energy by breaking down complex molecules into simpler compounds § Ex: cellular respiration, hydrolysis § Anabolic pathways consume energy to build complex molecules from simpler ones § Dehydration synthesis of lipids, carbohydrates, proteins © 2014 Pearson Education, Inc.
Free-Energy Change ( G), Stability, and Equilibrium § A living system’s free energy is energy that can do work when temperature and pressure are uniform, as in a living cell § The change in free energy (∆G) during a chemical reaction is the difference between the free energy of the final state and the free energy of the initial state ∆G = Gfinal state – Ginitial state § Only processes with a negative ∆G are spontaneous © 2014 Pearson Education, Inc.
Free Energy and Metabolism § The concept of free energy can be applied to the chemistry of life’s processes © 2014 Pearson Education, Inc.
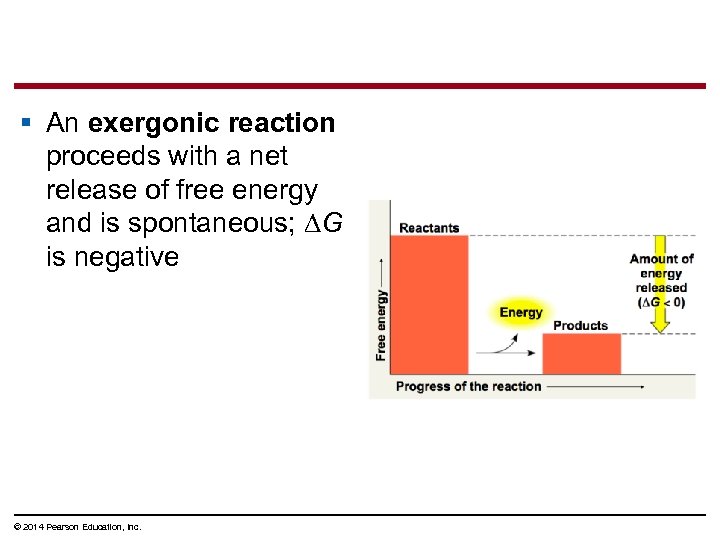
§ An exergonic reaction proceeds with a net release of free energy and is spontaneous; ∆G is negative © 2014 Pearson Education, Inc.
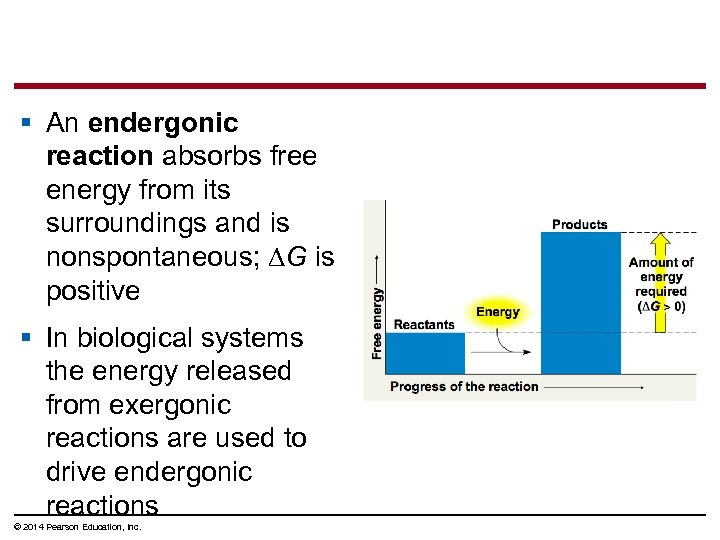
§ An endergonic reaction absorbs free energy from its surroundings and is nonspontaneous; ∆G is positive § In biological systems the energy released from exergonic reactions are used to drive endergonic reactions © 2014 Pearson Education, Inc.
The Activation Energy Barrier § Every chemical reaction between molecules involves bond breaking and bond forming § The initial energy needed to start a chemical reaction is called the free energy of activation, or activation energy (EA) § Activation energy is often supplied in the form of thermal energy that the reactant molecules absorb from their surroundings § Why is obtaining thermal energy not an effective strategy for biological reactions? © 2014 Pearson Education, Inc.
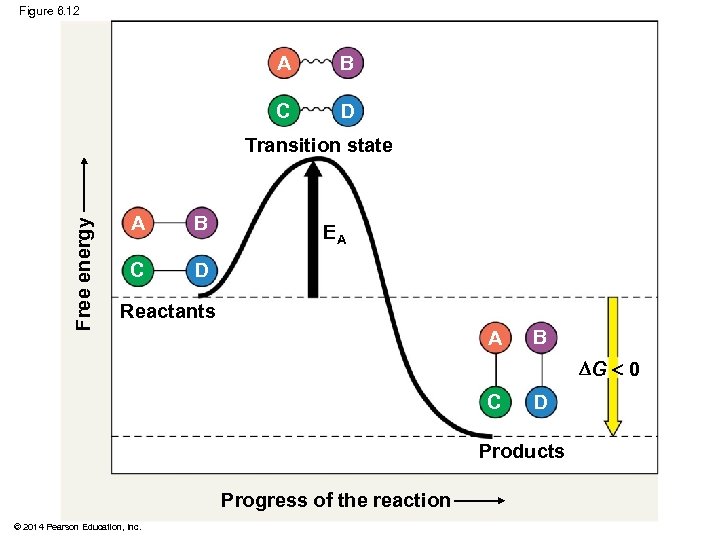
Figure 6. 12 A B C D Free energy Transition state A B C D EA Reactants A B G 0 C D Products Progress of the reaction © 2014 Pearson Education, Inc.
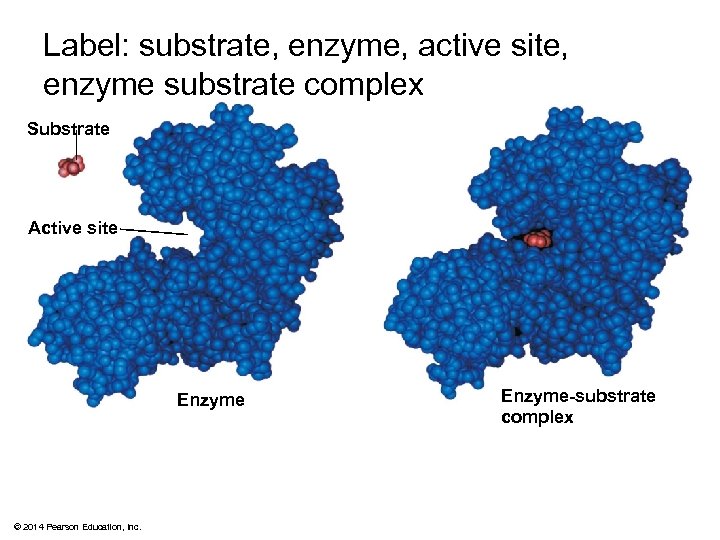
Label: substrate, enzyme, active site, enzyme substrate complex Substrate Active site Enzyme © 2014 Pearson Education, Inc. Enzyme-substrate complex
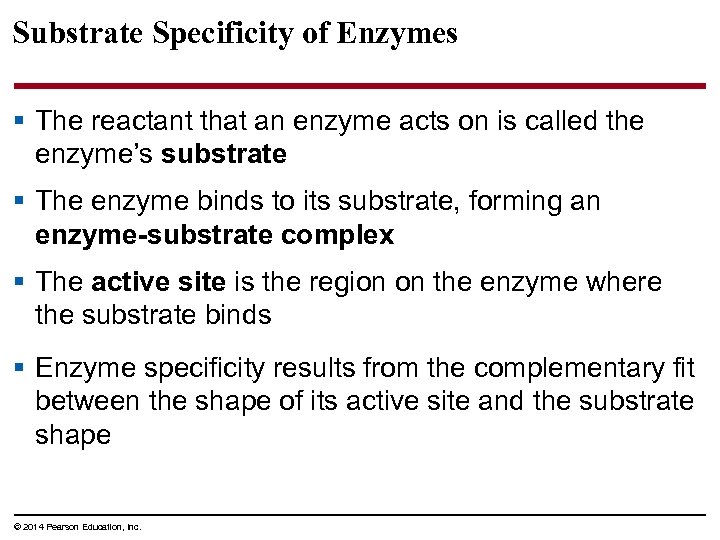
Substrate Specificity of Enzymes § The reactant that an enzyme acts on is called the enzyme’s substrate § The enzyme binds to its substrate, forming an enzyme-substrate complex § The active site is the region on the enzyme where the substrate binds § Enzyme specificity results from the complementary fit between the shape of its active site and the substrate shape © 2014 Pearson Education, Inc.
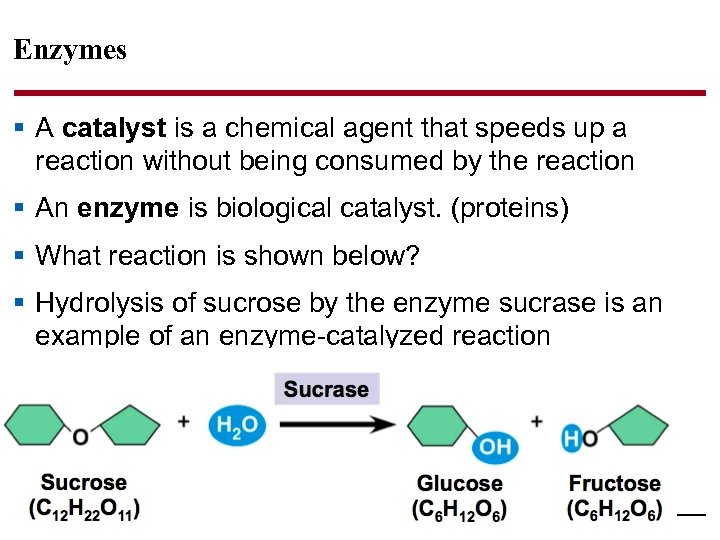
Enzymes § A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction § An enzyme is biological catalyst. (proteins) § What reaction is shown below? § Hydrolysis of sucrose by the enzyme sucrase is an example of an enzyme-catalyzed reaction © 2014 Pearson Education, Inc.
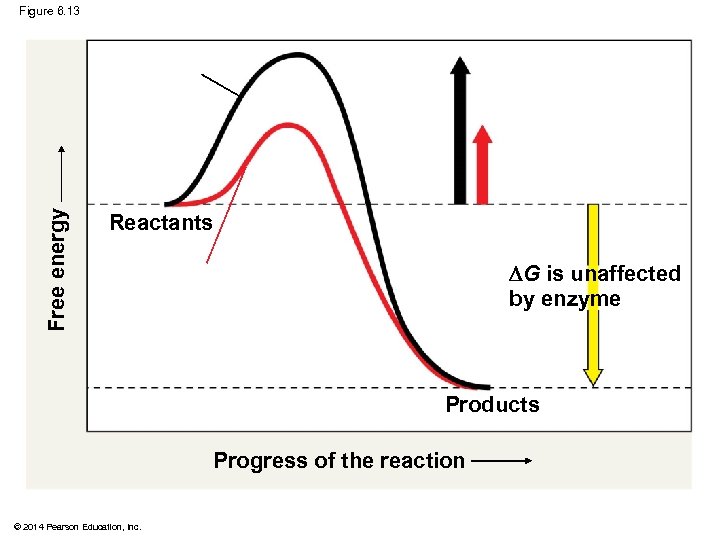
Free energy Figure 6. 13 Reactants G is unaffected by enzyme Products Progress of the reaction © 2014 Pearson Education, Inc.
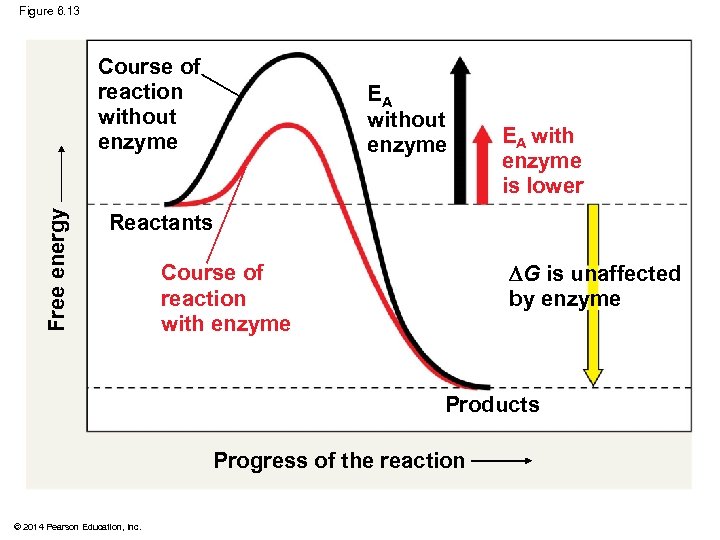
Figure 6. 13 Free energy Course of reaction without enzyme EA with enzyme is lower Reactants G is unaffected by enzyme Course of reaction with enzyme Products Progress of the reaction © 2014 Pearson Education, Inc.
How Enzymes Speed Up Reactions § Enzymes catalyze reactions by lowering the EA barrier § Enzymes do not affect the change in free energy (∆G); instead, they hasten reactions that would occur eventually Animation: How Enzymes Work © 2014 Pearson Education, Inc.
§ Enzymes change shape due to chemical interactions with the substrate § What is the difference between the lock and key model vs the induced fit model? § This induced fit of the enzyme to the substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the reaction Video: Enzyme Induced Fit © 2014 Pearson Education, Inc.
In case you were curious…. § In an enzymatic reaction, the substrate binds to the active site of the enzyme § The active site can lower an EA barrier by § Orienting substrates correctly § Straining substrate bonds § Providing a favorable microenvironment § Covalently bonding to the substrate © 2014 Pearson Education, Inc.
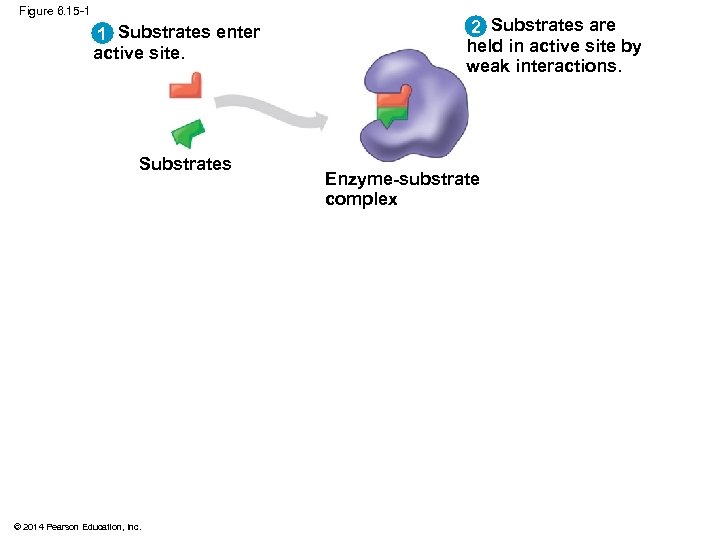
Figure 6. 15 -1 1 Substrates enter active site. Substrates © 2014 Pearson Education, Inc. 2 Substrates are held in active site by weak interactions. Enzyme-substrate complex
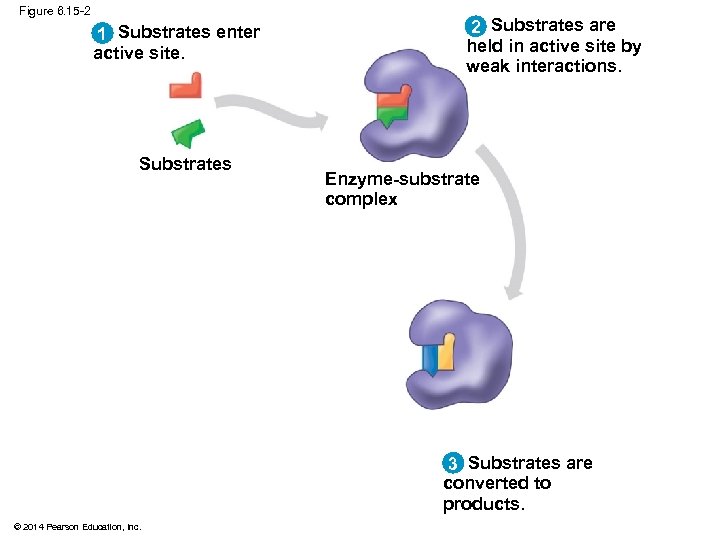
Figure 6. 15 -2 1 Substrates enter active site. Substrates 2 Substrates are held in active site by weak interactions. Enzyme-substrate complex 3 Substrates are converted to products. © 2014 Pearson Education, Inc.
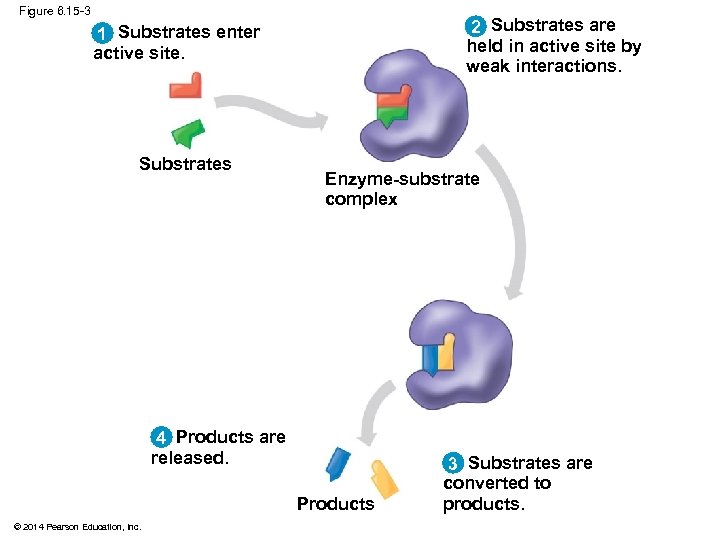
Figure 6. 15 -3 2 Substrates are held in active site by weak interactions. 1 Substrates enter active site. Substrates Enzyme-substrate complex 4 Products are released. Products © 2014 Pearson Education, Inc. 3 Substrates are converted to products.
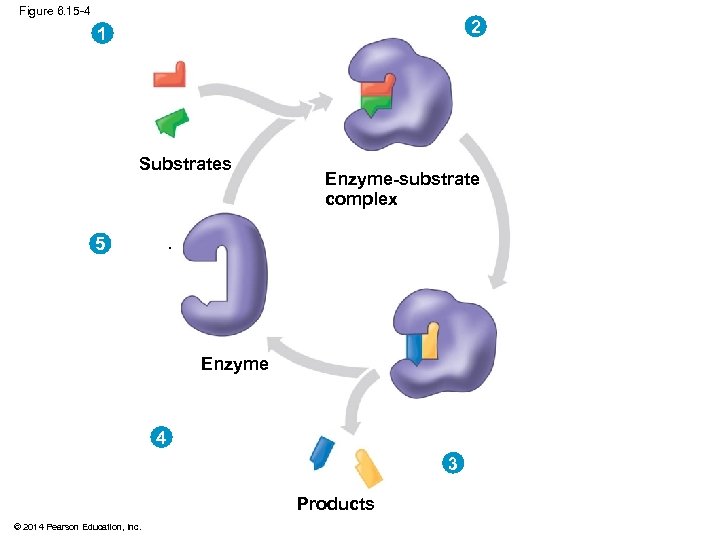
Figure 6. 15 -4 2 1 Substrates Enzyme-substrate complex . 5 Enzyme . 4 3 Products © 2014 Pearson Education, Inc.
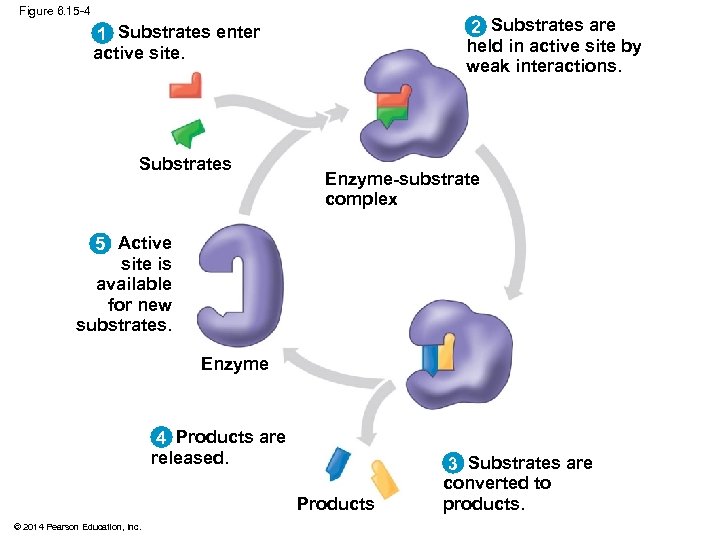
Figure 6. 15 -4 2 Substrates are held in active site by weak interactions. 1 Substrates enter active site. Substrates Enzyme-substrate complex 5 Active site is available for new substrates. Enzyme 4 Products are released. Products © 2014 Pearson Education, Inc. 3 Substrates are converted to products.
Effects of Local Conditions on Enzyme Activity § An enzyme’s activity can be affected by § General environmental factors, such as temperature and p. H § Chemicals that specifically influence the enzyme © 2014 Pearson Education, Inc.
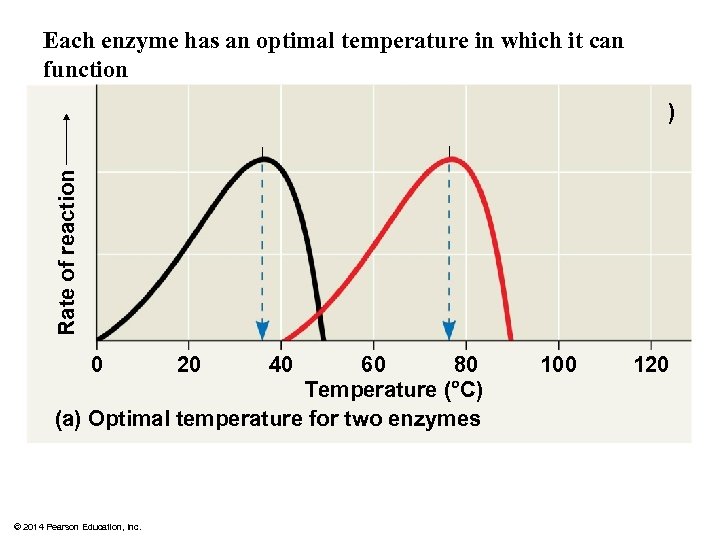
Each enzyme has an optimal temperature in which it can function Rate of reaction ) 80 60 Temperature ( C) (a) Optimal temperature for two enzymes 0 © 2014 Pearson Education, Inc. 20 40 100 120
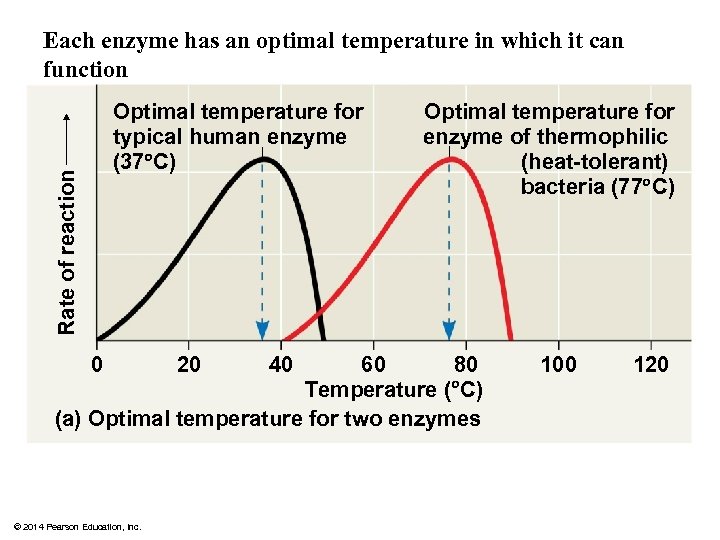
Each enzyme has an optimal temperature in which it can function Rate of reaction Optimal temperature for typical human enzyme (37 C) 80 60 Temperature ( C) (a) Optimal temperature for two enzymes 0 © 2014 Pearson Education, Inc. 20 40 Optimal temperature for enzyme of thermophilic (heat-tolerant) bacteria (77 C) 100 120
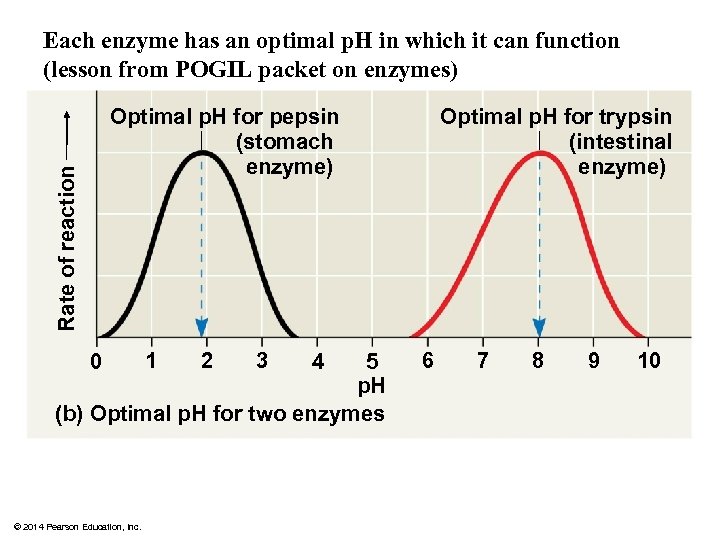
Each enzyme has an optimal p. H in which it can function (lesson from POGIL packet on enzymes) Rate of reaction Optimal p. H for pepsin (stomach enzyme) 0 1 2 3 5 p. H (b) Optimal p. H for two enzymes © 2014 Pearson Education, Inc. 4 Optimal p. H for trypsin (intestinal enzyme) 6 7 8 9 10
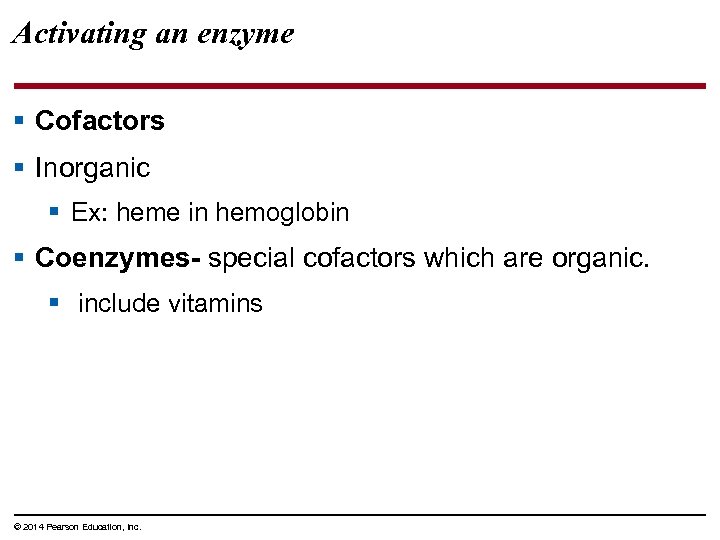
Activating an enzyme § Cofactors § Inorganic § Ex: heme in hemoglobin § Coenzymes- special cofactors which are organic. § include vitamins © 2014 Pearson Education, Inc.
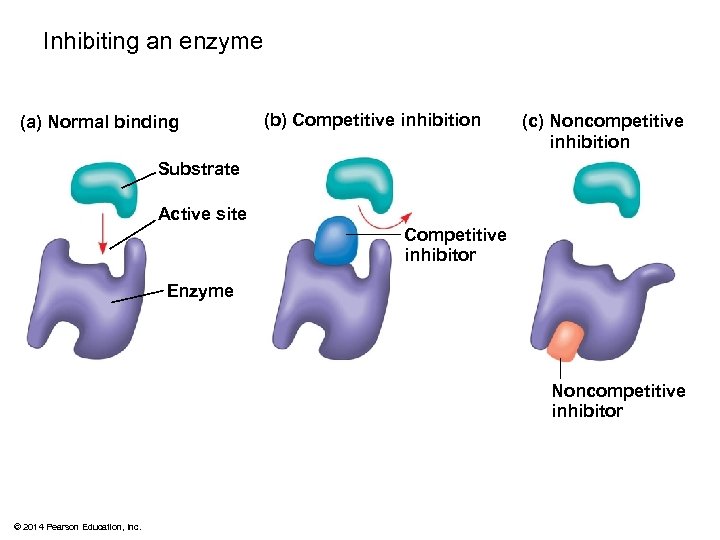
Inhibiting an enzyme (a) Normal binding (b) Competitive inhibition (c) Noncompetitive inhibition Substrate Active site Competitive inhibitor Enzyme Noncompetitive inhibitor © 2014 Pearson Education, Inc.

Enzyme Inhibitors § Competitive inhibitors bind to the active site of an enzyme, competing with the substrate § Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective § Examples of inhibitors include toxins, poisons, pesticides, and antibiotics © 2014 Pearson Education, Inc.
Regulation of Enzymes § Sometimes a cell needs a reaction to occur, sometimes it needs it to stop. § Analogous to when a car needs to break, and when it needs to accelerate. How does it regulate? § Allosteric regulation may either inhibit or stimulate an enzyme’s activity § Allosteric regulation occurs when a regulatory molecule binds to a protein at one site and affects the protein’s function at another site © 2014 Pearson Education, Inc.
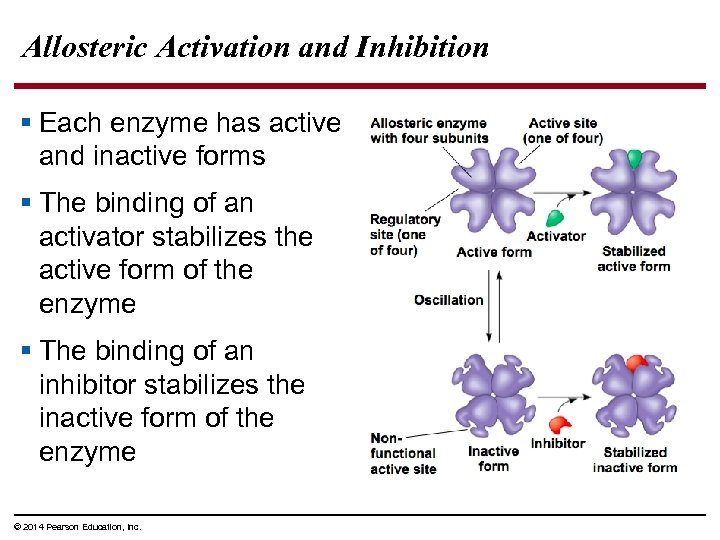
Allosteric Activation and Inhibition § Each enzyme has active and inactive forms § The binding of an activator stabilizes the active form of the enzyme § The binding of an inhibitor stabilizes the inactive form of the enzyme © 2014 Pearson Education, Inc.
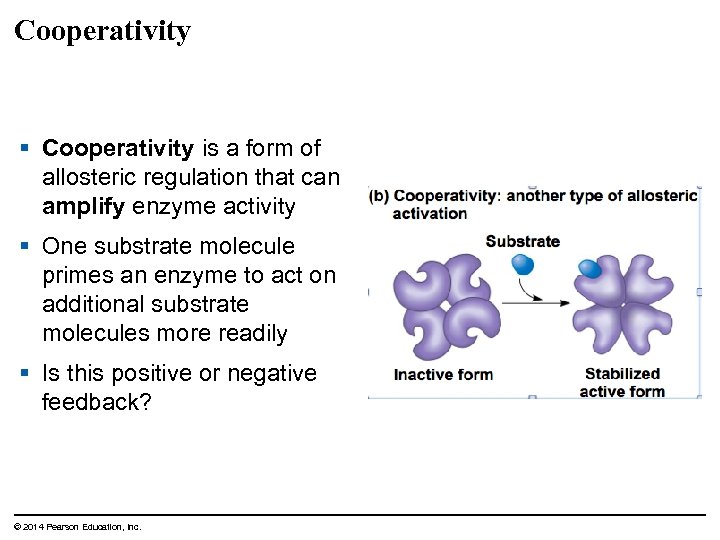
Cooperativity § Cooperativity is a form of allosteric regulation that can amplify enzyme activity § One substrate molecule primes an enzyme to act on additional substrate molecules more readily § Is this positive or negative feedback? © 2014 Pearson Education, Inc.
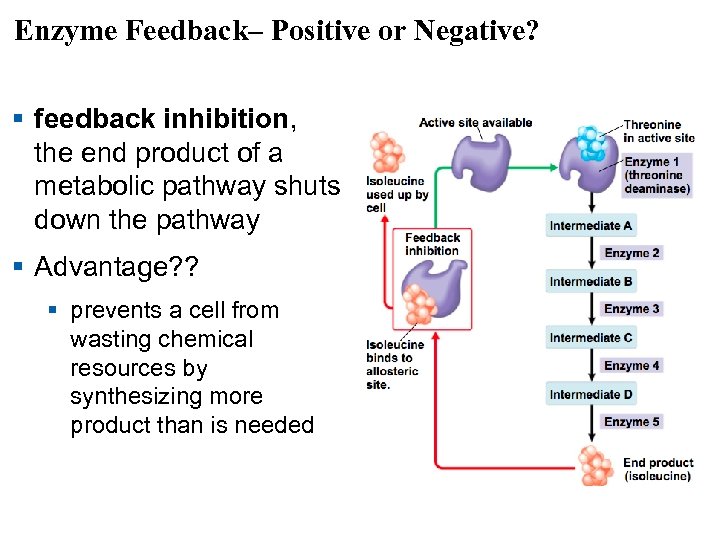
Enzyme Feedback– Positive or Negative? § feedback inhibition, the end product of a metabolic pathway shuts down the pathway § Advantage? ? § prevents a cell from wasting chemical resources by synthesizing more product than is needed
Specific Localization of Enzymes Within the Cell § Structures within the cell help bring order to metabolic pathways § Some enzymes act as structural components of membranes § In eukaryotic cells, enzymes for cellular respiration are located in mitochondria © 2014 Pearson Education, Inc.
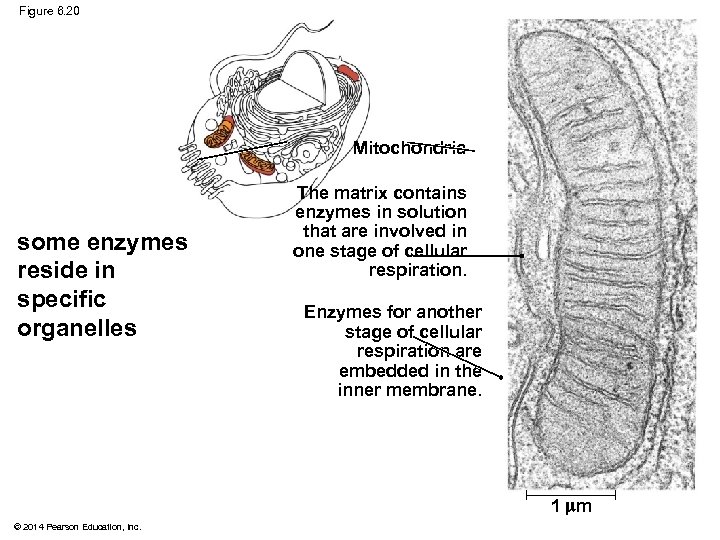
Figure 6. 20 Mitochondria some enzymes reside in specific organelles The matrix contains enzymes in solution that are involved in one stage of cellular respiration. Enzymes for another stage of cellular respiration are embedded in the inner membrane. 1 m © 2014 Pearson Education, Inc.

Exit Slip • Explain how enzymes regulate chemical reactions in the body by using the terms feedback, enzyme, active site, substrate, activation energy, activation and inhibition. Underline the words as you use them. © 2014 Pearson Education, Inc.
d749b2e7c6852b72dc8f3e37a4509149.ppt