6ebb2ee671ea4e57f37f147d1b5cccd9.ppt
- Количество слайдов: 33

California Clinical Laboratory Association New HIV Diagnostic Testing Algorithm November 8, 2012 Beatrice O’Keefe, Chief Laboratory Field Services California Department of Public Health

Good Morning

New HIV Algorithm l Rationale for New HIV Strategy l HIV Algorithm l Lab Issues l Challenges


Goals of the National HIV/ AIDS Strategy Reduce The Number of New HIV/AIDS Infections Increase access to care for people living with HIV/AIDS Reduce HIV related Health Disparities
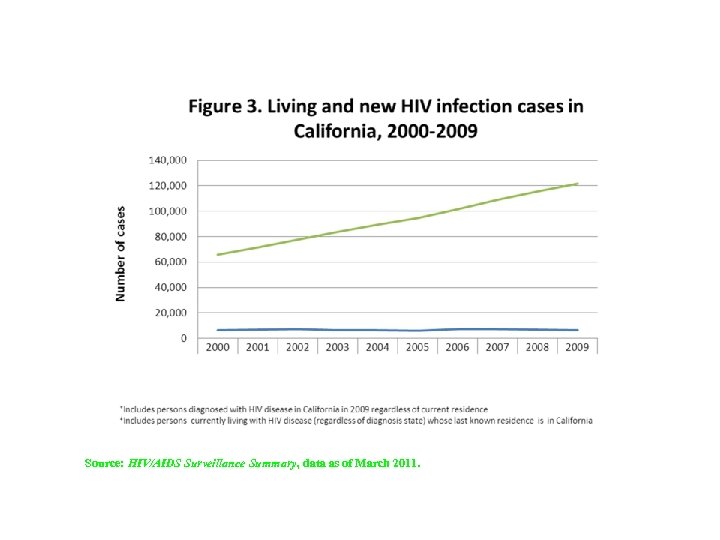
Source: HIV/AIDS Surveillance Summary, data as of March 2011.
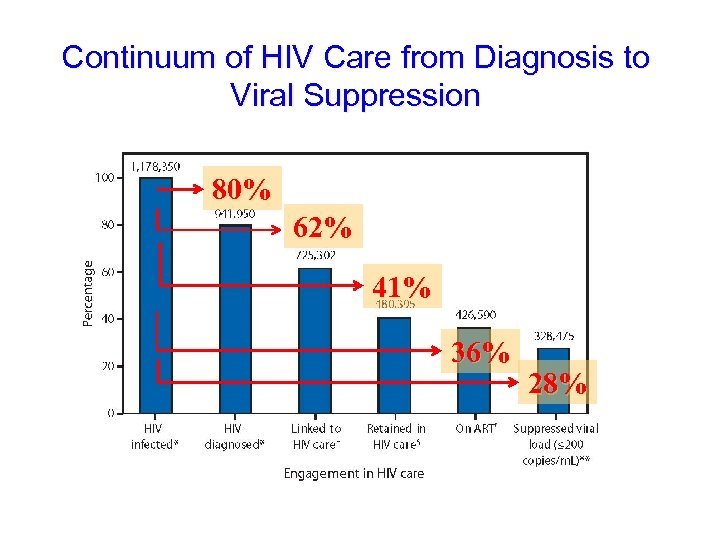
Continuum of HIV Care from Diagnosis to Viral Suppression 80% 62% 41% 36% 28%
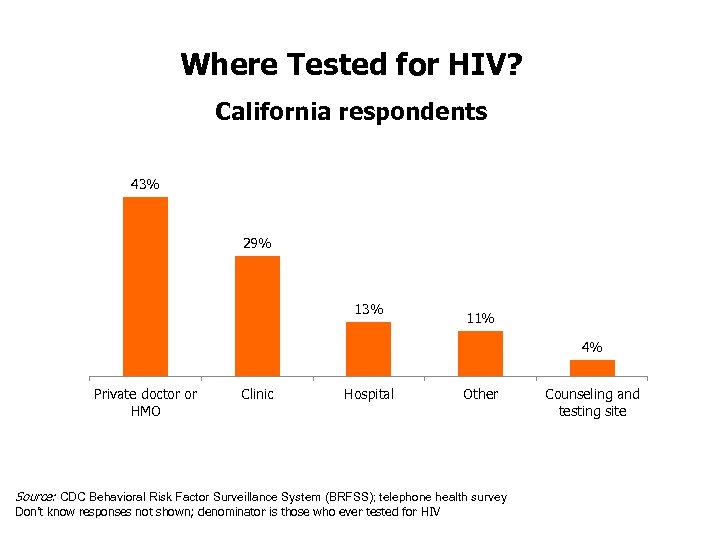
Where Tested for HIV? California respondents 43% 29% 13% 11% 4% Private doctor or HMO Clinic Hospital Other Source: CDC Behavioral Risk Factor Surveillance System (BRFSS); telephone health survey Don’t know responses not shown; denominator is those who ever tested for HIV Counseling and testing site
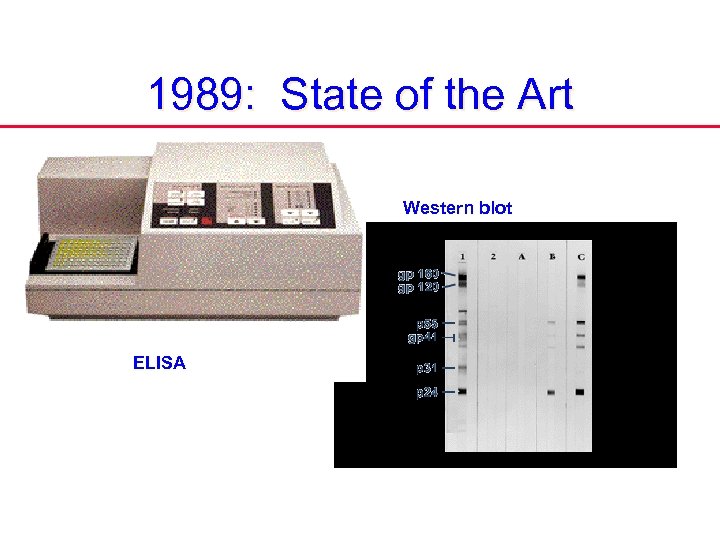
1989: State of the Art Western blot ELISA
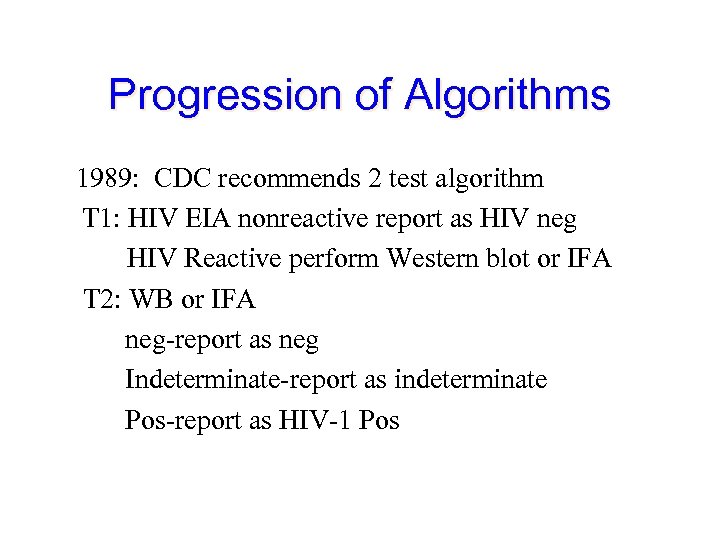
Progression of Algorithms 1989: CDC recommends 2 test algorithm T 1: HIV EIA nonreactive report as HIV neg HIV Reactive perform Western blot or IFA T 2: WB or IFA neg-report as neg Indeterminate-report as indeterminate Pos-report as HIV-1 Pos

1989 State of the Art
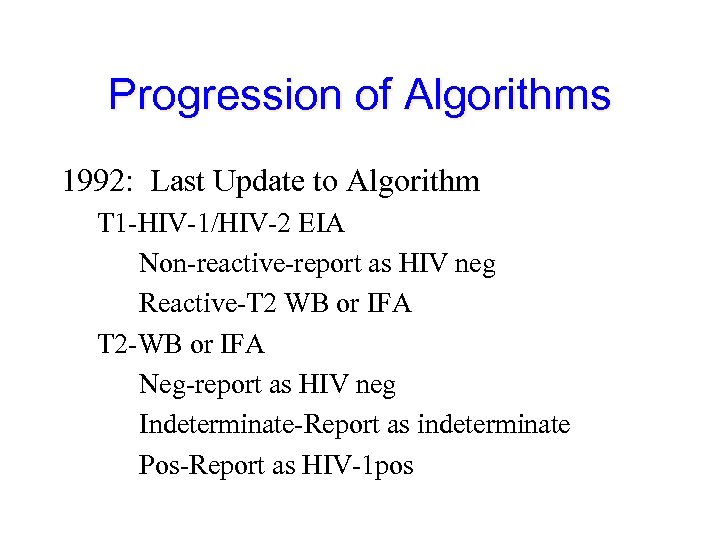
Progression of Algorithms 1992: Last Update to Algorithm T 1 -HIV-1/HIV-2 EIA Non-reactive-report as HIV neg Reactive-T 2 WB or IFA T 2 -WB or IFA Neg-report as HIV neg Indeterminate-Report as indeterminate Pos-Report as HIV-1 pos
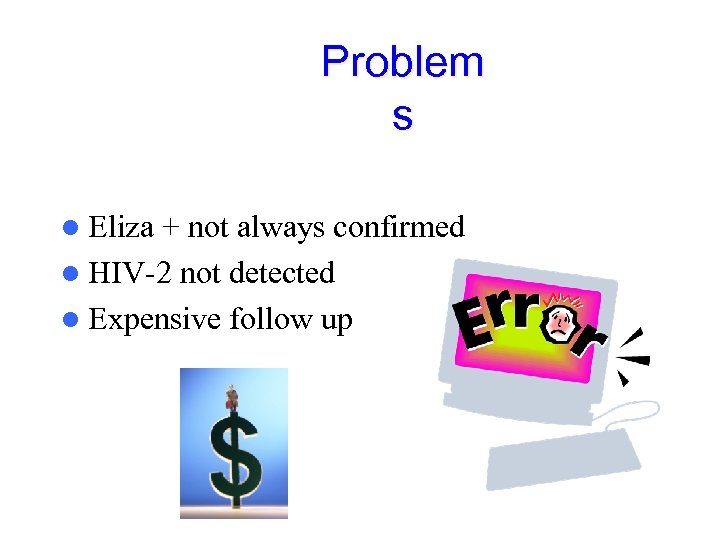
Problem s l Eliza + not always confirmed l HIV-2 not detected l Expensive follow up
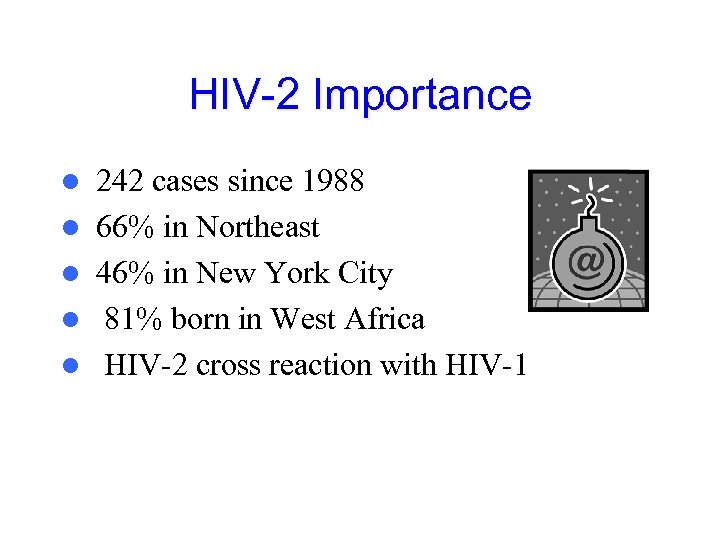
HIV-2 Importance l l l 242 cases since 1988 66% in Northeast 46% in New York City 81% born in West Africa HIV-2 cross reaction with HIV-1
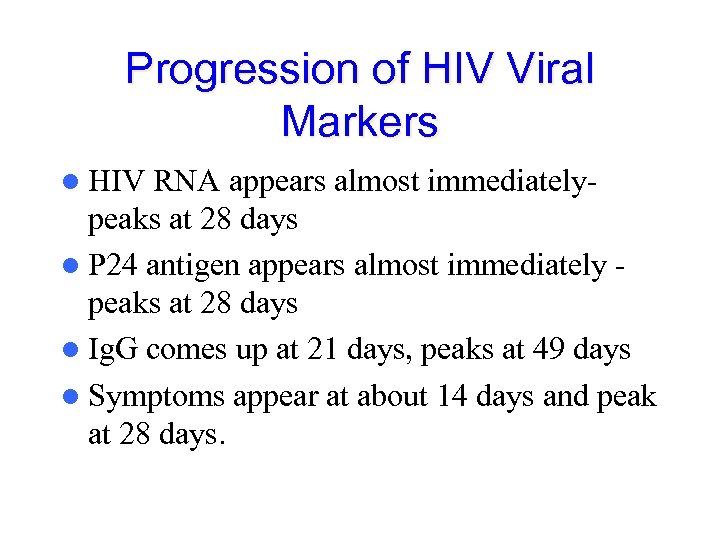
Progression of HIV Viral Markers l HIV RNA appears almost immediatelypeaks at 28 days l P 24 antigen appears almost immediately peaks at 28 days l Ig. G comes up at 21 days, peaks at 49 days l Symptoms appear at about 14 days and peak at 28 days.

New Strategy Needed 20% HIV infected persons unaware of status l Approx 52% of new sexually-transmitted infections involve infected persons unaware of HIV status l Includes 11% due to persons in acute phase of HIV infection l Early Detection beneficial for clinical care and prevention l

Strategic Plan Developed 2009 APHL & CDC status report l 2010 HIV Diagnostics conference l 2011 CLSI published criteria l
Rationale for New Algorithm l l l Expanded testing and earlier detection needed to reduce HIV infections New tests can detect acute & early HIV infection False positives occur with all types of initial tests WB is not able to confirm acute infections detected by 4 th generation Ag/Ab tests Existing algorithm does not provide good options for resolving discrepant or false-positive results HIV-2 cross-reactivity on HIV-1 WB confuses interpretation
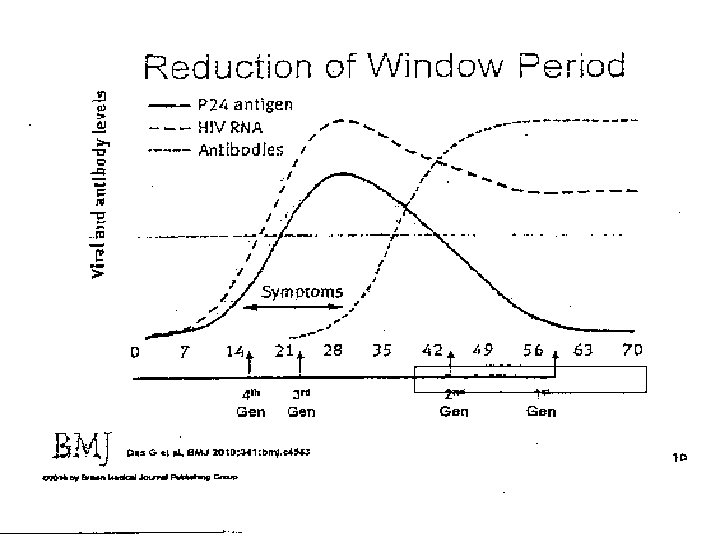
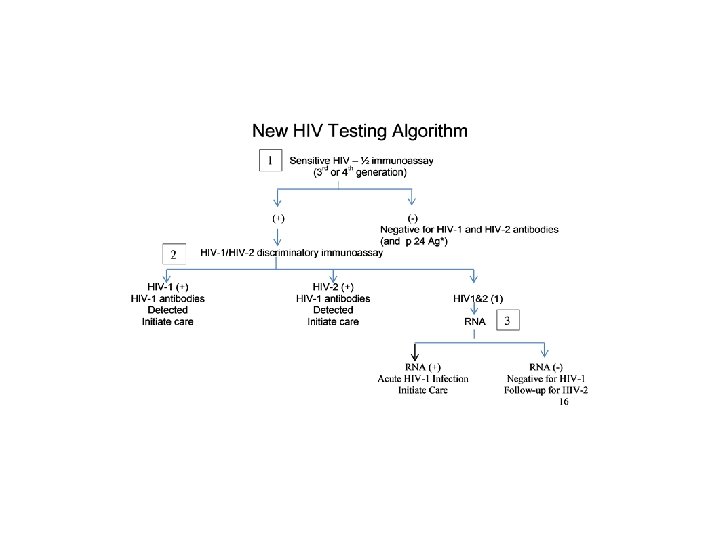
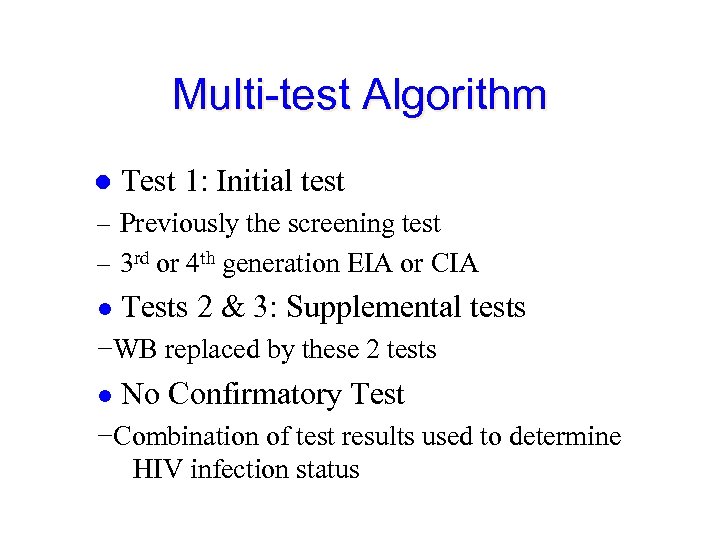
Multi-test Algorithm ● Test 1: Initial test – Previously the screening test – 3 rd or 4 th generation EIA or CIA ● Tests 2 & 3: Supplemental tests −WB replaced by these 2 tests ● No Confirmatory Test −Combination of test results used to determine HIV infection status
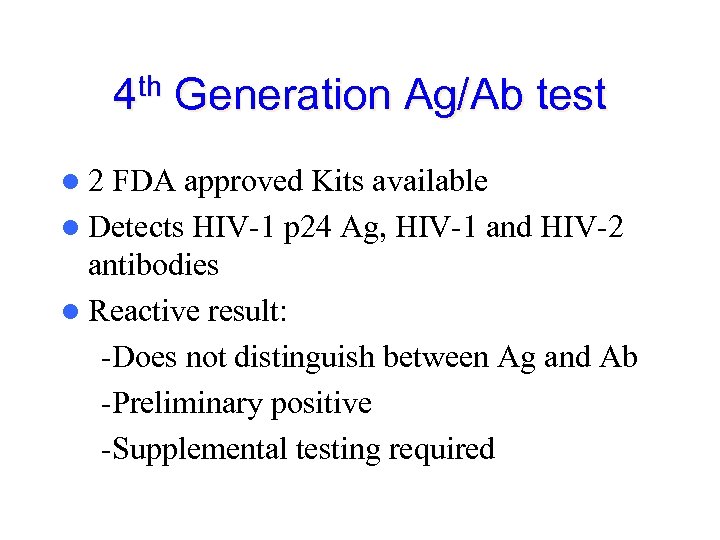
4 th Generation Ag/Ab test l 2 FDA approved Kits available l Detects HIV-1 p 24 Ag, HIV-1 and HIV-2 antibodies l Reactive result: -Does not distinguish between Ag and Ab -Preliminary positive -Supplemental testing required
Advantages of HIV Algorithm l HIV-1/HIV-2 l Timely assays must easier to use results l Reduced number of indeterminate results
Disadvantages of HIV Algorithm Cost of new HIV-1/2 Ag/Ab assays l NAAT testing for low volume of specimens l
State Law CCR 1230: HIV Screening Testing by Laboratories − Must use FDA approved test − Confirm all reactive or indeterminate HIV test results by the CDC confirmation protocol − Maintain a QA program that includes: Competency of testing personnel Assessment of test performance

Adoption in California l CCR 17. 1. 2. 9. 1. 1230 does not allow the adoption of new algorithms until published in the MMWR – Delay of MMWR due to HIV 1 / 2 differentiation test (Bio. Rad Multispot) not listed as a supplemental test in package insert. – Hope for MMWR by the end of the year. (HIV Diagnostics Conference, Dec 11 – 14, 2012, Atlanta, GA. )

Status of Recommendations Clinical and Laboratory Standards Institute Criteria for Laboratory Testing and Diagnosis of Human Immunodeficiency virus Infection; Approved guideline 2011 Special Edition of Journal of Clinical Virology Http: //www/sciencedirect. com/science/journal/138 66532/52/supp/S 1 CDC Recommendations Summer/Fall 2012 Will parallel CLSI document
Rapid HIV 1/2 Ag-Ab Test 29

Those Never Tested 1. 2 million have HIV l 240, 000 unaware of HIV status l 50, 000 new HIV infections each year occur from those not aware of HIV status ● At risk individuals would test more frequently if convenient test available l

Home Testing

Acknowledgements Bernard M. Branson, M. D. , Associate Director for Laboratory Diagnostics, Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention Monica Parker, Ph. D, Laboratory Chief, Bloodborne Diseases, New York State Department of Health, Albany, NY Michele Owen, Ph. D, Laboratory Branch/Division of HIV/AIDS Prevention, Centers for Disease Control and Prevention, Atlanta, GA

Questions? Bea. Okeefe@cdph. ca. gov LFS Web site: http: //cdph. ca. gov/programs/lfs
6ebb2ee671ea4e57f37f147d1b5cccd9.ppt