07738770506244396298dc1f2901d0e5.ppt
- Количество слайдов: 26
Calibration and Normalization of Protein Microarray Data Charlene Liang 1*, Virginia Espina 2, Julia Wulfkuhle 2, Emanuel F. Petricoin 3 III and Lance A. Liotta 2, Yuexia Li 1, Minzi Ruan 1, 1 Vigene. Tech Inc, 2 National Cancer Institute, Center for Cancer Research, Laboratory of Pathology, FDA-NCI Clinical Proteomics Group, 3 Food and Drug Administration, Office of Cellular and Gene Therapy, Center for Biologic Evaluation and Research, FDA-NCI Clinical Proteomics Group, Bethesda, MD * Correspondence: cliang@vigenetech. com

Abstract Reverse Phase Protein microarrays are a promising technology for characterization of cellular protein signaling networks. This platform has been shown to have high sensitivity and good reproducibility when used with validated antibodies. There has been a need in clinical research to quantify individual analytes across patient samples, as well as comparison of analytes before, during and after treatment. In addition, there has been a need to develop a method of normalization and calibration for the microarray. We have developed a method of normalization based on total protein per microarray spot by using a total protein stain on the microarray. This allows normalization of each spot to a known analyte, maximizing reproducibility. We also developed a reference lysate of known composition for quantification of spots based on a common unit, which we termed the ‘reference standard unit’. This reference lysate serves as a standard for compensation of spot-spot and day-to-day variation. Bioinformatic software capable of incorporating the normalization and calibration data is required for high throughput data analysis. We used Micro. Vigene software to quantify each analyte on the protein microarray, incorporating the reference lysate and total protein/spot data. A variety of automated curve fitting approaches are used to meet the coefficient of variation required for clinical trial research.

Reverse Phase Protein Array Coupling Laser Capture Microdissection With High Throughput Protein Arrays Patient biopsy tissue cells are microdissected: 30, 000 cells = 100 arrays Each patient sample is arrayed in a miniature dilution curve: Always in linear dynamic range of any antibody/ analyte pair Arrays probed with labeled amplified antibody: e. g. Ovarian cancer progression From one patient probed with Phospho-ERK antibody
Application Areas of the Technology Reserve Phase Protein Microarrays are applied to: 1. Clinical Research – utilized in clinical trials for assessing response to therapy and demonstrating protein molecular changes to therapy. 2. Disease Prognostics - utilized for determining which patient is likely to respond to a given therapy. 3. Personalized drug treatment – monitoring response to therapy before, during and after treatment.
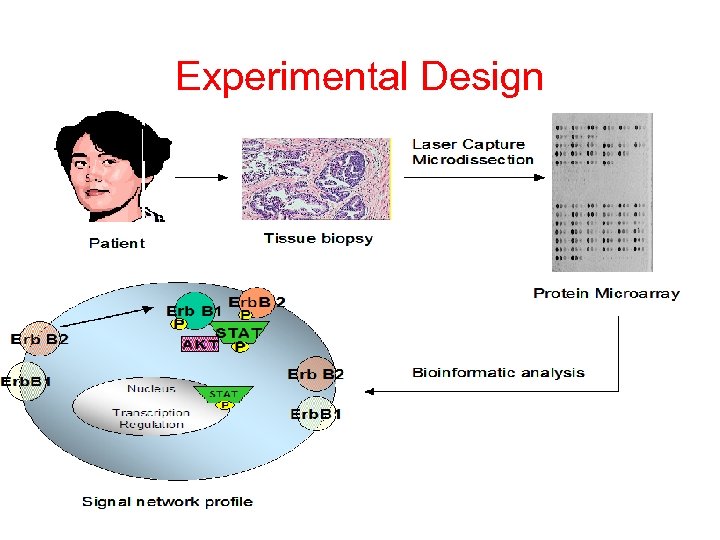
Experimental Design

Arrayer and Stainer used For Reverse-Phase Protein Arrays GMS 417 pin and ring arrayer Dako. Cytomation Autostainer for protein detection/signal development
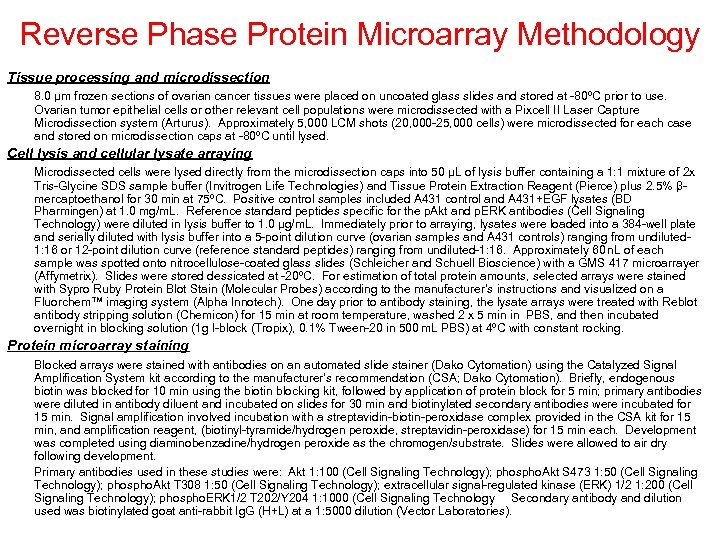
Reverse Phase Protein Microarray Methodology Tissue processing and microdissection 8. 0 μm frozen sections of ovarian cancer tissues were placed on uncoated glass slides and stored at -80ºC prior to use. Ovarian tumor epithelial cells or other relevant cell populations were microdissected with a Pixcell II Laser Capture Microdissection system (Arturus). Approximately 5, 000 LCM shots (20, 000 -25, 000 cells) were microdissected for each case and stored on microdissection caps at -80ºC until lysed. Cell lysis and cellular lysate arraying Microdissected cells were lysed directly from the microdissection caps into 50 μL of lysis buffer containing a 1: 1 mixture of 2 x Tris-Glycine SDS sample buffer (Invitrogen Life Technologies) and Tissue Protein Extraction Reagent (Pierce) plus 2. 5% βmercaptoethanol for 30 min at 75ºC. Positive control samples included A 431 control and A 431+EGF lysates (BD Pharmingen) at 1. 0 mg/m. L. Reference standard peptides specific for the p. Akt and p. ERK antibodies (Cell Signaling Technology) were diluted in lysis buffer to 1. 0 μg/m. L. Immediately prior to arraying, lysates were loaded into a 384 -well plate and serially diluted with lysis buffer into a 5 -point dilution curve (ovarian samples and A 431 controls) ranging from undiluted 1: 16 or 12 -point dilution curve (reference standard peptides) ranging from undiluted-1: 16. Approximately 60 n. L of each sample was spotted onto nitrocellulose-coated glass slides (Schleicher and Schuell Bioscience) with a GMS 417 microarrayer (Affymetrix). Slides were stored dessicated at -20ºC. For estimation of total protein amounts, selected arrays were stained with Sypro Ruby Protein Blot Stain (Molecular Probes) according to the manufacturer’s instructions and visualized on a Fluorchem™ imaging system (Alpha Innotech). One day prior to antibody staining, the lysate arrays were treated with Reblot antibody stripping solution (Chemicon) for 15 min at room temperature, washed 2 x 5 min in PBS, and then incubated overnight in blocking solution (1 g I-block (Tropix), 0. 1% Tween-20 in 500 m. L PBS) at 4ºC with constant rocking. Protein microarray staining Blocked arrays were stained with antibodies on an automated slide stainer (Dako Cytomation) using the Catalyzed Signal Amplification System kit according to the manufacturer’s recommendation (CSA; Dako Cytomation). Briefly, endogenous biotin was blocked for 10 min using the biotin blocking kit, followed by application of protein block for 5 min; primary antibodies were diluted in antibody diluent and incubated on slides for 30 min and biotinylated secondary antibodies were incubated for 15 min. Signal amplification involved incubation with a streptavidin-biotin-peroxidase complex provided in the CSA kit for 15 min, and amplification reagent, (biotinyl-tyramide/hydrogen peroxide, streptavidin-peroxidase) for 15 min each. Development was completed using diaminobenzadine/hydrogen peroxide as the chromogen/substrate. Slides were allowed to air dry following development. Primary antibodies used in these studies were: Akt 1: 100 (Cell Signaling Technology); phospho. Akt S 473 1: 50 (Cell Signaling Technology); phospho. Akt T 308 1: 50 (Cell Signaling Technology); extracellular signal-regulated kinase (ERK) 1/2 1: 200 (Cell Signaling Technology); phospho. ERK 1/2 T 202/Y 204 1: 1000 (Cell Signaling Technology Secondary antibody and dilution used was biotinylated goat anti-rabbit Ig. G (H+L) at a 1: 5000 dilution (Vector Laboratories).
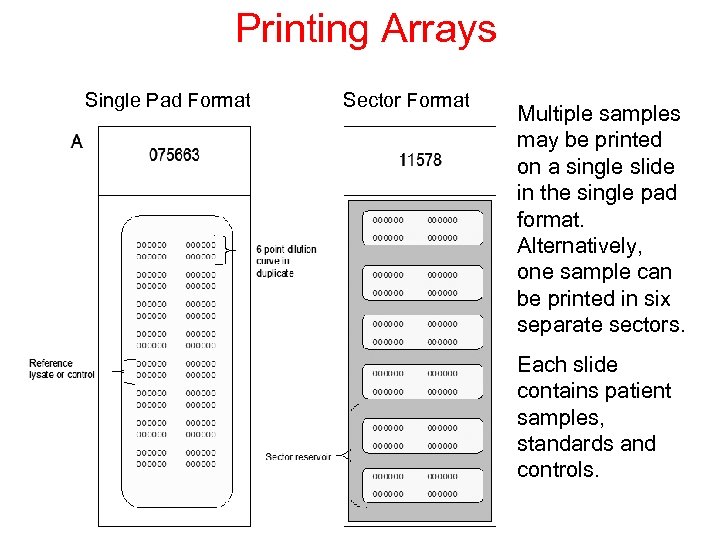
Printing Arrays Single Pad Format Sector Format Multiple samples may be printed on a single slide in the single pad format. Alternatively, one sample can be printed in six separate sectors. Each slide contains patient samples, standards and controls.
Normalization Using Total Protein • Why normalization? It is essential to account for differences in total protein concentration between each sample so that antibody staining between each tissue sample on the array can be compared directly. • How do we do it? One slide/printing is stained for total protein using a total protein stain such as Sypro Ruby™ blot stain or a colloidal gold stain. For estimation of total protein amounts, selected arrays were stained with Sypro Ruby™ Protein Blot Stain (Molecular Probes) according to the manufacturer’s instructions and visualized on a Fluorchem™ imaging system (Alpha Innotech). • Reproducibility Normalization is based on the total protein per microarray spot. Normalized intensity values are calculated by dividing the measured intensity value of the antibody by the corresponding measured intensity value of the total protein. This allows normalization of sample to a known analyte, maximizing reproducibility.

Negative Control Slides • Why negative slides? Serve as controls for non-specific binding of the secondary antibody to the array • How is it done? Arrays are probed with the labeled secondary antibody (biotinylated anti-rabbit or antimouse Ig. G) in the absence of the primary antibody against the analyte of interest and processed as all other slides in the experiment
Cross Sample Calibration using Reference Standard The reference standard is a pool of peptides. This pool is comprised of the peptides used as the immugen to produce the primary antibody. The reference standard dilution curve is printed on each microarray slide. Micro. Vigene™ software automatically finds RSU measurements for all samples through curve fitting and other mapping algorithms.

Image Quantification and Data Analysis Using Micro. Vigene™ • • • Quality Reproducibility Automation Dust and Contamination Control Performance for High Throughput
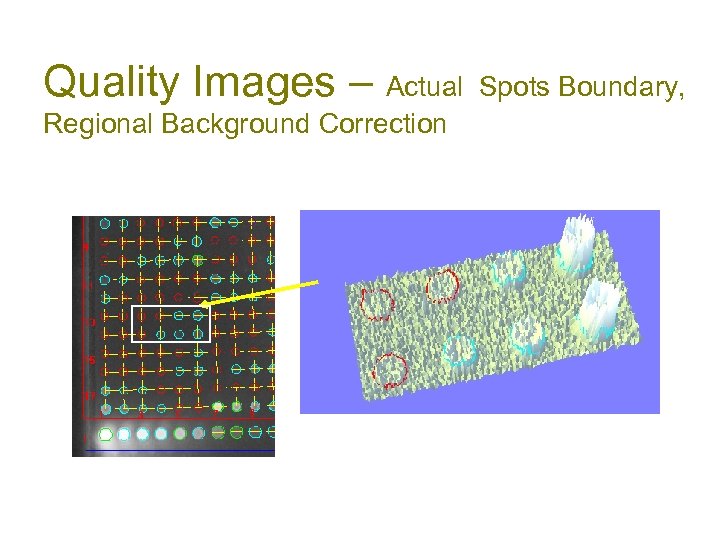
Quality Images – Actual Spots Boundary, Regional Background Correction
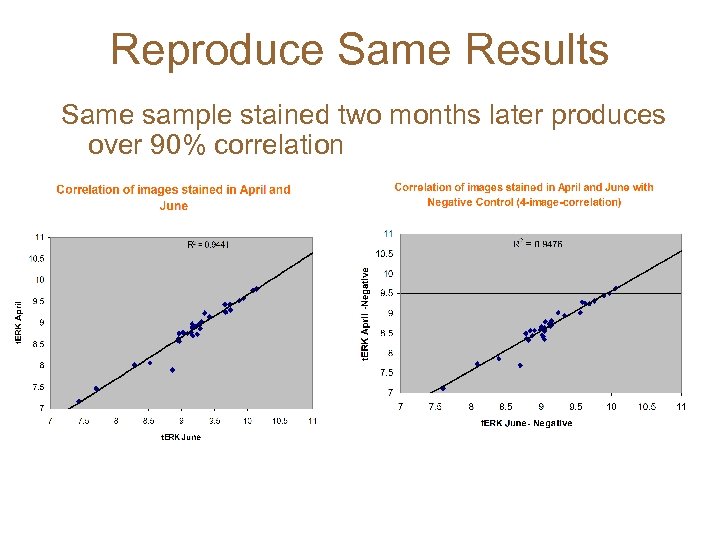
Reproduce Same Results Same sample stained two months later produces over 90% correlation
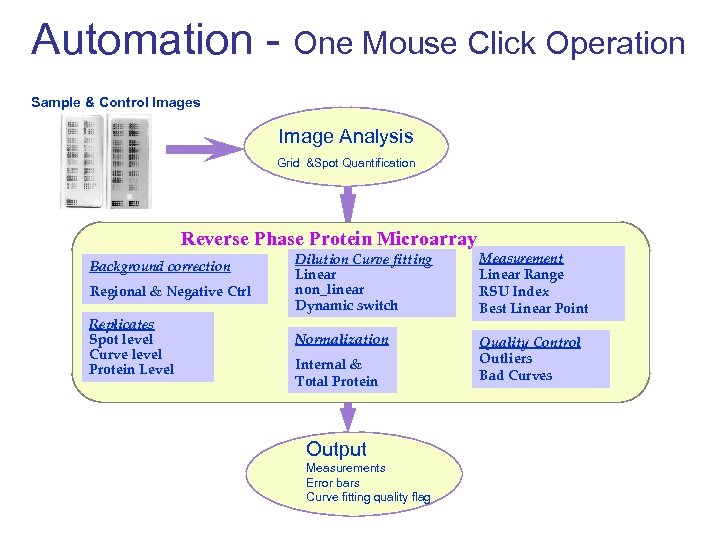
Automation - One Mouse Click Operation Sample & Control Images Image Analysis Grid &Spot Quantification Reverse Phase Protein Microarray Background correction Regional & Negative Ctrl Replicates Spot level Curve level Protein Level Dilution Curve fitting Linear non_linear Dynamic switch Measurement Linear Range RSU Index Best Linear Point Normalization Quality Control Outliers Bad Curves Internal & Total Protein Output Measurements Error bars Curve fitting quality flag
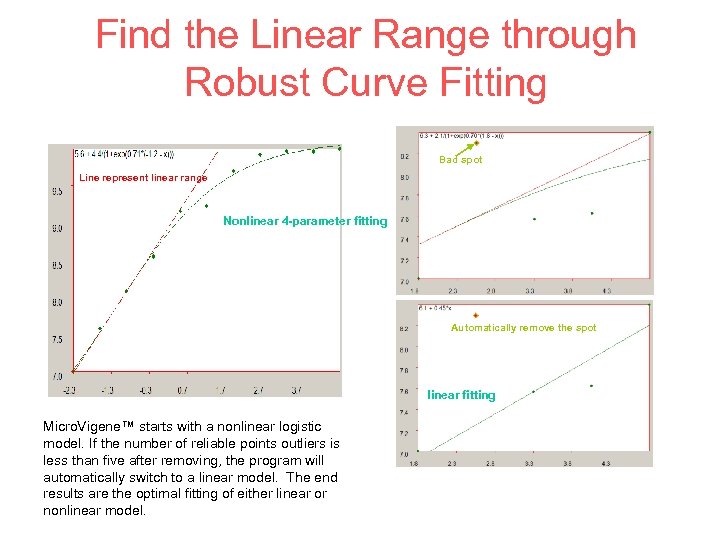
Find the Linear Range through Robust Curve Fitting Bad spot Line represent linear range Nonlinear 4 -parameter fitting Automatically remove the spot linear fitting Micro. Vigene™ starts with a nonlinear logistic model. If the number of reliable points outliers is less than five after removing, the program will automatically switch to a linear model. The end results are the optimal fitting of either linear or nonlinear model.
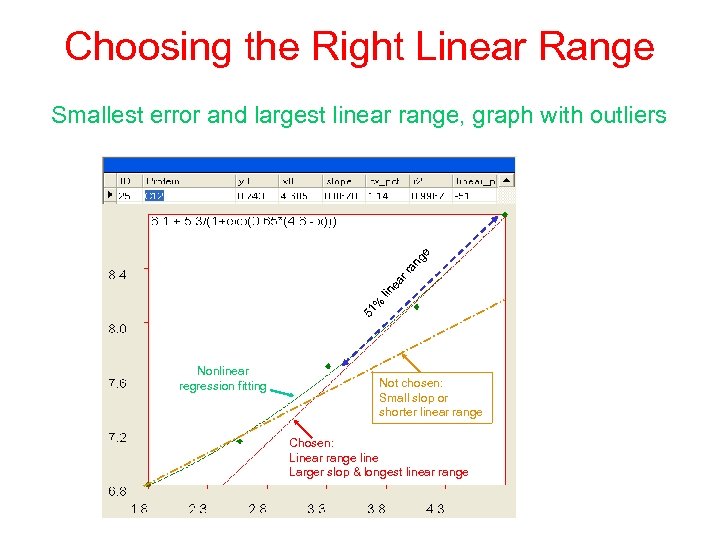
Choosing the Right Linear Range 51 % lin ea r r an g e Smallest error and largest linear range, graph with outliers Nonlinear regression fitting Not chosen: Small slop or shorter linear range Chosen: Linear range line Larger slop & longest linear range

Best Linear Point Measurements Taking Y intensity measurements at X 0 -average provides the least error due to extrapolation and offers means for sample comparison. Y X 0 -avg
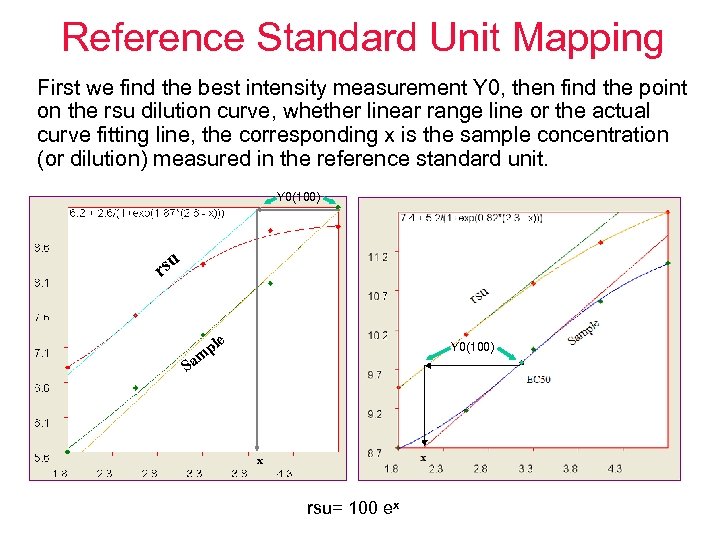
Reference Standard Unit Mapping First we find the best intensity measurement Y 0, then find the point on the rsu dilution curve, whether linear range line or the actual curve fitting line, the corresponding x is the sample concentration (or dilution) measured in the reference standard unit. Y 0(100) u rs e S pl m a Y 0(100) x rsu= 100 ex

Measurements for Control Types A 431 and A 431+EGF lysates are printed on each slide as process controls. Expected results are relative elevation of p. ERK and p. AKT in the A 431+EGF lysate as compared to the A 431 lysate.
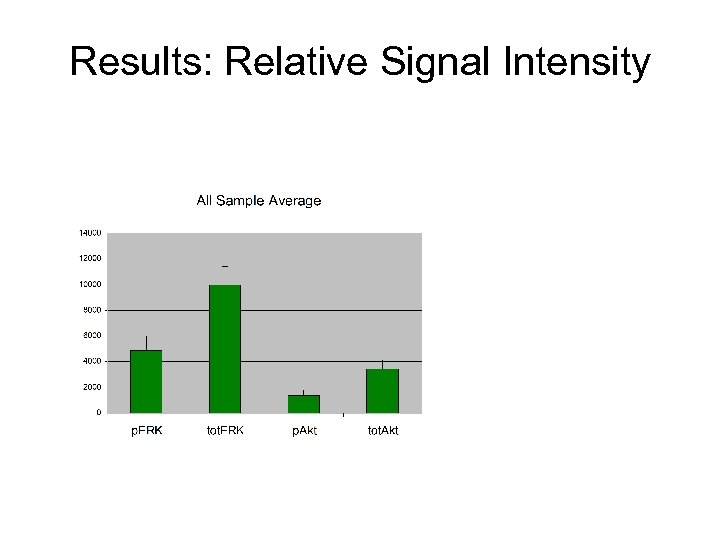
Results: Relative Signal Intensity
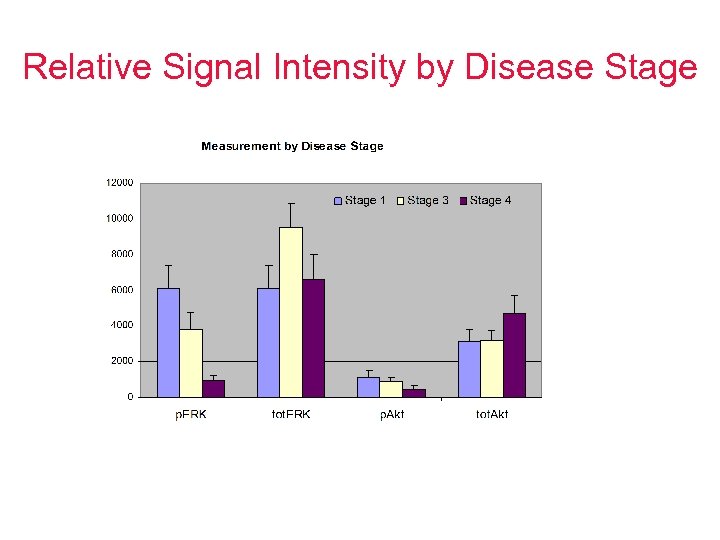
Relative Signal Intensity by Disease Stage
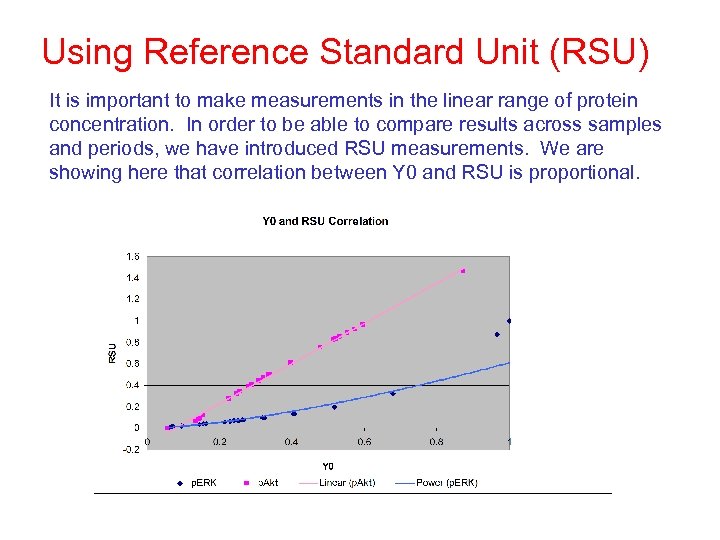
Using Reference Standard Unit (RSU) It is important to make measurements in the linear range of protein concentration. In order to be able to compare results across samples and periods, we have introduced RSU measurements. We are showing here that correlation between Y 0 and RSU is proportional.
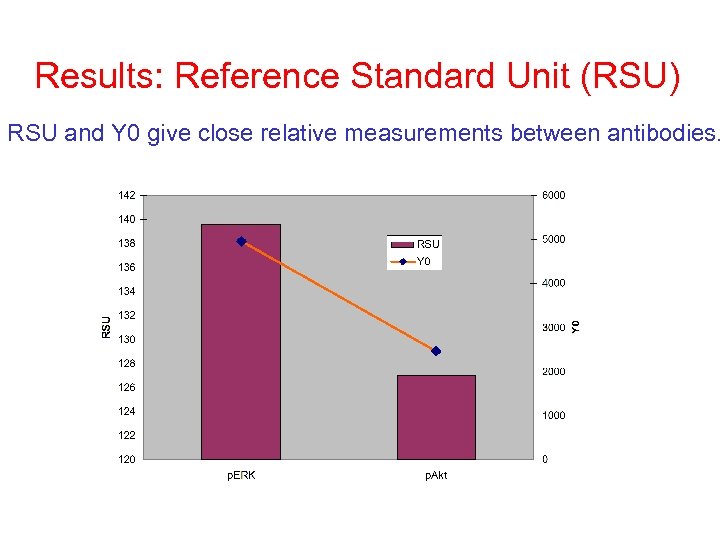
Results: Reference Standard Unit (RSU) RSU and Y 0 give close relative measurements between antibodies.
Conclusion The detection of changes in the activity of various signaling pathways in normal and tumor tissue in a patient is essential for understanding disease progression, appropriate treatment selection, and monitoring treatment efficacy. Reverse Phase Protein Microarray technology provides a means to detect, in a highly multiplex way, these changes. With the standardization on the total protein normalization and the incorporation of a reference standard, Reverse Phase Protein Microarrays make data comparison among different studies and clinical trials possible. This analysis is performed using Micro. Vigene™ software employing advanced algorithms for image analysis and novel data analysis processes for high quality, highly reproducible, end-to-end Reverse Phase Protein Microarray analysis solutions. Micro. Vigene™ also automates the Calibration, normalization and background correction analysis steps of the method.

ABOUT US • The goal of the FDA-NCI Clinical Proteomics Program is to invent and apply proteomics technology to patient care. New proteomics research technology is now being used for clinical studies ranging from cancer to cardiovascular disease and organ transplant. Researchers within the program are searching for proteins in the blood, urine, and diseased tissue that can be used as early biomarkers of disease, predict response to therapy, or the likelihood of relapse after treatment, or serve as new targets for therapy itself. • About Vigene. Tech, Inc. : Vigene. Tech provides novel scientific software, customized solutions and online services in the areas of image analysis, automation, and instrumentation. Vigene. Tech’s Micro. Vigene. TM for microarray image analysis delivers 100% reproducible, operator independent results; it is robust to handle various shifted and noisy images; and supports unattended batch process. For more information about Vigene. Tech, please visit http: //www. vigenetech. com.
07738770506244396298dc1f2901d0e5.ppt