e6eb2e73da928a3b25fecc44359ad70b.ppt
- Количество слайдов: 24
 CADTH SYMPOSIUM : HCV: NATURAL HISTORY AND THERAPEUTICS Alnoor Ramji Gastroenterology & Hepatology Clinical Associate Professor Division of Gastroenterology University Of British Columbia St. Paul’s Hospital Site ramji_a@hotmail. com
CADTH SYMPOSIUM : HCV: NATURAL HISTORY AND THERAPEUTICS Alnoor Ramji Gastroenterology & Hepatology Clinical Associate Professor Division of Gastroenterology University Of British Columbia St. Paul’s Hospital Site ramji_a@hotmail. com
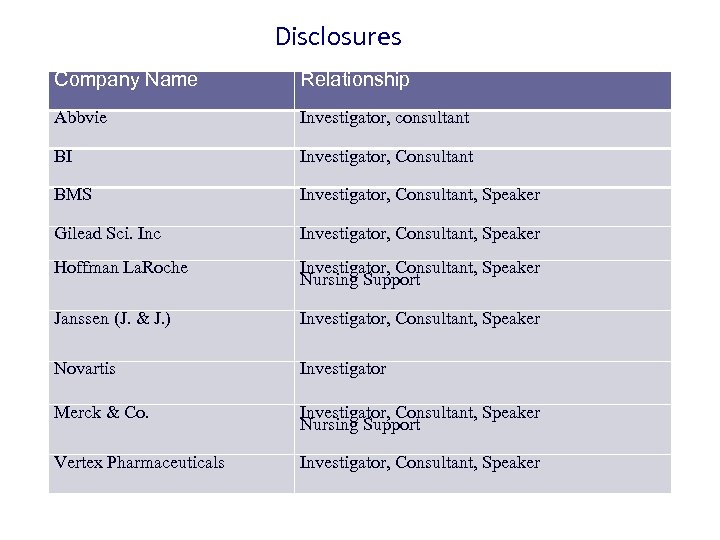 Disclosures Company Name Relationship Abbvie Investigator, consultant BI Investigator, Consultant BMS Investigator, Consultant, Speaker Gilead Sci. Inc Investigator, Consultant, Speaker Hoffman La. Roche Investigator, Consultant, Speaker Nursing Support Janssen (J. & J. ) Investigator, Consultant, Speaker Novartis Investigator Merck & Co. Investigator, Consultant, Speaker Nursing Support Vertex Pharmaceuticals Investigator, Consultant, Speaker
Disclosures Company Name Relationship Abbvie Investigator, consultant BI Investigator, Consultant BMS Investigator, Consultant, Speaker Gilead Sci. Inc Investigator, Consultant, Speaker Hoffman La. Roche Investigator, Consultant, Speaker Nursing Support Janssen (J. & J. ) Investigator, Consultant, Speaker Novartis Investigator Merck & Co. Investigator, Consultant, Speaker Nursing Support Vertex Pharmaceuticals Investigator, Consultant, Speaker
 Objectives • Review the natural history of hepatitis C and its complications. • Understand Treatment options for hepatitis C – Pegylated-interferon + ribavirin +/- Direct acting anti-virals (DAA’s). – Combination DAA’s
Objectives • Review the natural history of hepatitis C and its complications. • Understand Treatment options for hepatitis C – Pegylated-interferon + ribavirin +/- Direct acting anti-virals (DAA’s). – Combination DAA’s
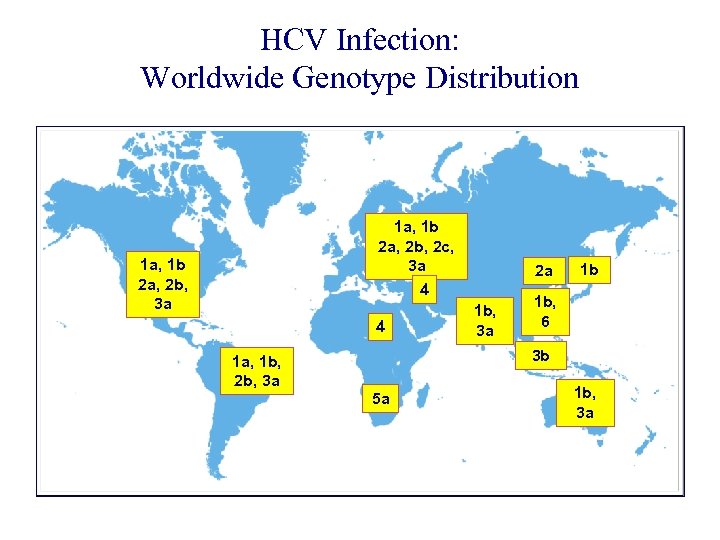 HCV Infection: Worldwide Genotype Distribution 1 a, 1 b 2 a, 2 b, 2 c, 3 a 1 a, 1 b 2 a, 2 b, 3 a 2 a 4 4 1 b, 3 a 1 b 1 b, 6 3 b 1 a, 1 b, 2 b, 3 a 5 a 1 b, 3 a Fang et al. Clin Liver Dis. 1997.
HCV Infection: Worldwide Genotype Distribution 1 a, 1 b 2 a, 2 b, 2 c, 3 a 1 a, 1 b 2 a, 2 b, 3 a 2 a 4 4 1 b, 3 a 1 b 1 b, 6 3 b 1 a, 1 b, 2 b, 3 a 5 a 1 b, 3 a Fang et al. Clin Liver Dis. 1997.
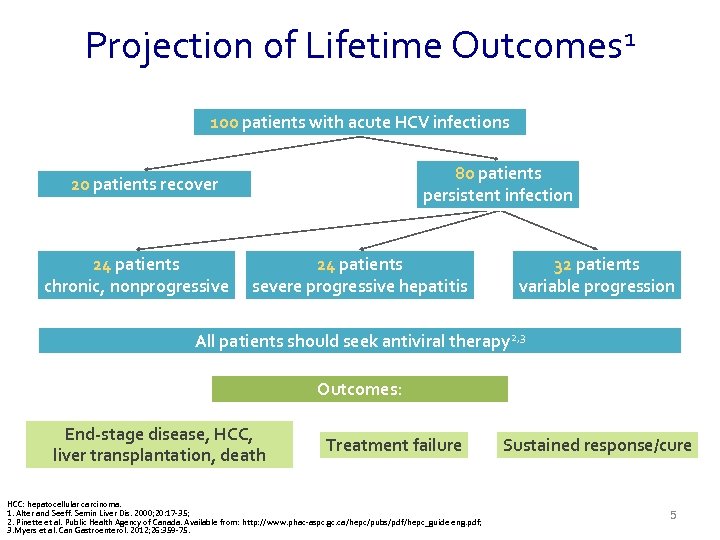 Projection of Lifetime Outcomes 1 100 patients with acute HCV infections 80 patients persistent infection 20 patients recover 24 patients chronic, nonprogressive 24 patients severe progressive hepatitis 32 patients variable progression All patients should seek antiviral therapy 2, 3 Outcomes: End-stage disease, HCC, liver transplantation, death Treatment failure HCC: hepatocellular carcinoma. 1. Alter and Seeff. Semin Liver Dis. 2000; 20: 17 -35; 2. Pinette et al. Public Health Agency of Canada. Available from: http: //www. phac-aspc. gc. ca/hepc/pubs/pdf/hepc_guide eng. pdf; 3. Myers et al. Can Gastroenterol. 2012; 26: 359 -75. Sustained response/cure 5
Projection of Lifetime Outcomes 1 100 patients with acute HCV infections 80 patients persistent infection 20 patients recover 24 patients chronic, nonprogressive 24 patients severe progressive hepatitis 32 patients variable progression All patients should seek antiviral therapy 2, 3 Outcomes: End-stage disease, HCC, liver transplantation, death Treatment failure HCC: hepatocellular carcinoma. 1. Alter and Seeff. Semin Liver Dis. 2000; 20: 17 -35; 2. Pinette et al. Public Health Agency of Canada. Available from: http: //www. phac-aspc. gc. ca/hepc/pubs/pdf/hepc_guide eng. pdf; 3. Myers et al. Can Gastroenterol. 2012; 26: 359 -75. Sustained response/cure 5
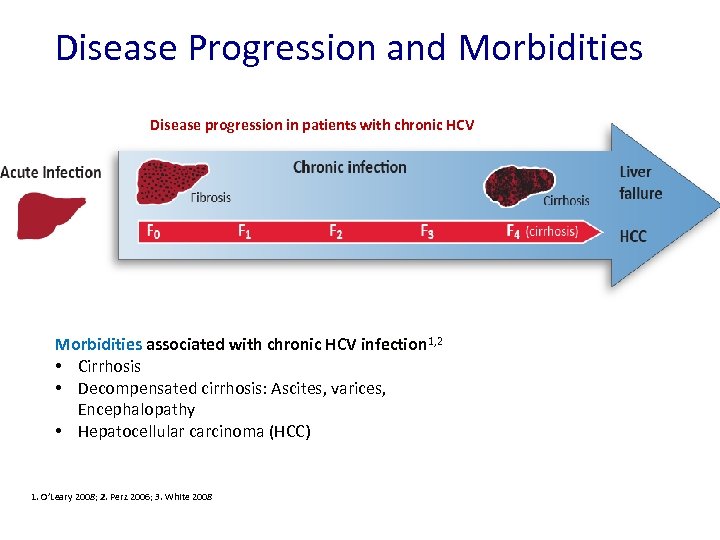 Disease Progression and Morbidities Disease progression in patients with chronic HCV Morbidities associated with chronic HCV infection 1, 2 • Cirrhosis • Decompensated cirrhosis: Ascites, varices, Encephalopathy • Hepatocellular carcinoma (HCC) 1. O’Leary 2008; 2. Perz 2006; 3. White 2008 Pre-Submission Briefing Meeting | July 2014 | Company Confidential © 2014 Abb. Vie 6
Disease Progression and Morbidities Disease progression in patients with chronic HCV Morbidities associated with chronic HCV infection 1, 2 • Cirrhosis • Decompensated cirrhosis: Ascites, varices, Encephalopathy • Hepatocellular carcinoma (HCC) 1. O’Leary 2008; 2. Perz 2006; 3. White 2008 Pre-Submission Briefing Meeting | July 2014 | Company Confidential © 2014 Abb. Vie 6
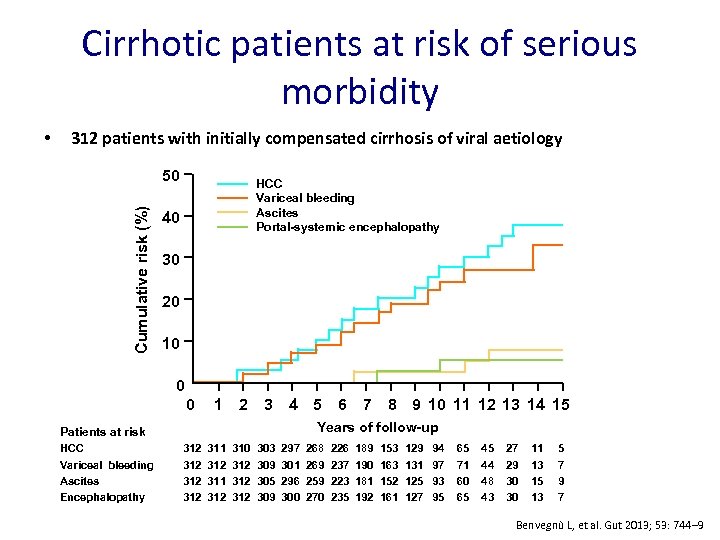 Cirrhotic patients at risk of serious morbidity 312 patients with initially compensated cirrhosis of viral aetiology 50 Cumulative risk (%) • HCC Variceal bleeding Ascites Portal-systemic encephalopathy 40 30 20 10 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Years of follow-up Patients at risk HCC Variceal bleeding Ascites Encephalopathy 312 312 311 312 310 312 312 303 309 305 309 297 301 296 300 268 269 259 270 226 237 223 235 189 190 181 192 153 163 152 161 129 131 125 127 94 97 93 95 65 71 60 65 45 44 48 43 27 29 30 30 11 13 15 13 5 7 9 7 Benvegnù L, et al. Gut 2013; 53: 744‒ 9
Cirrhotic patients at risk of serious morbidity 312 patients with initially compensated cirrhosis of viral aetiology 50 Cumulative risk (%) • HCC Variceal bleeding Ascites Portal-systemic encephalopathy 40 30 20 10 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Years of follow-up Patients at risk HCC Variceal bleeding Ascites Encephalopathy 312 312 311 312 310 312 312 303 309 305 309 297 301 296 300 268 269 259 270 226 237 223 235 189 190 181 192 153 163 152 161 129 131 125 127 94 97 93 95 65 71 60 65 45 44 48 43 27 29 30 30 11 13 15 13 5 7 9 7 Benvegnù L, et al. Gut 2013; 53: 744‒ 9
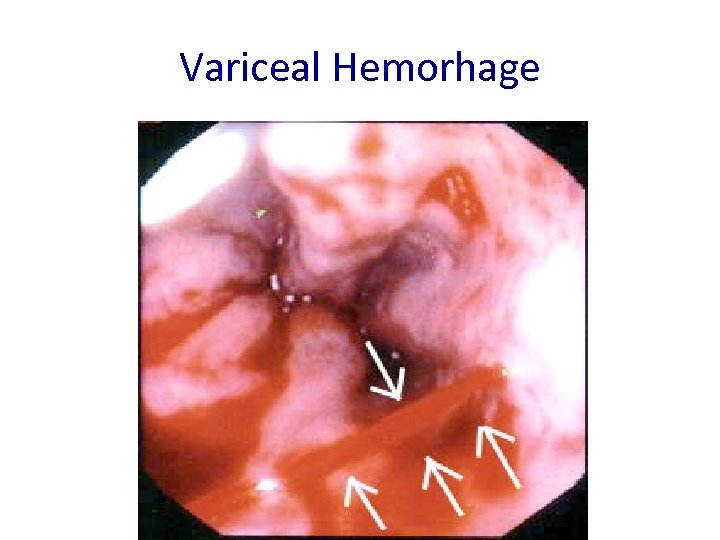 Variceal Hemorhage
Variceal Hemorhage
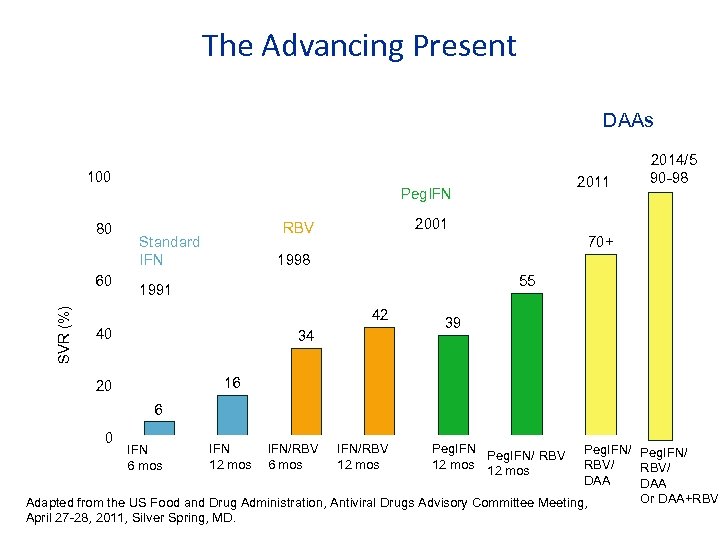 The Advancing Present DAAs 100 2011 Peg. IFN 80 SVR (%) 60 2001 RBV Standard IFN 2014/5 90 -98 70+ 1998 55 1991 42 40 34 39 16 20 6 0 IFN 12 mos IFN/RBV 6 mos IFN/RBV 12 mos Peg. IFN/ RBV/ DAA Or DAA+RBV Adapted from the US Food and Drug Administration, Antiviral Drugs Advisory Committee Meeting, April 27 -28, 2011, Silver Spring, MD. IFN 6 mos
The Advancing Present DAAs 100 2011 Peg. IFN 80 SVR (%) 60 2001 RBV Standard IFN 2014/5 90 -98 70+ 1998 55 1991 42 40 34 39 16 20 6 0 IFN 12 mos IFN/RBV 6 mos IFN/RBV 12 mos Peg. IFN/ RBV/ DAA Or DAA+RBV Adapted from the US Food and Drug Administration, Antiviral Drugs Advisory Committee Meeting, April 27 -28, 2011, Silver Spring, MD. IFN 6 mos
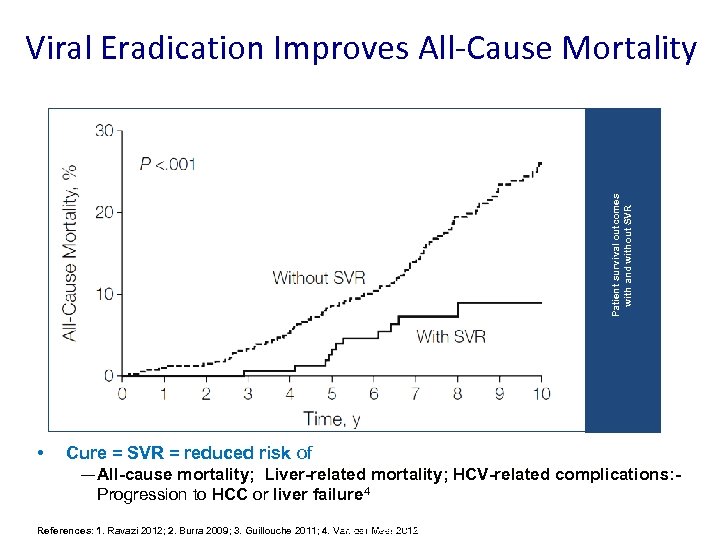 Patient survival outcomes with and without SVR Viral Eradication Improves All-Cause Mortality • Cure = SVR = reduced risk of — All-cause mortality; Liver-related mortality; HCV-related complications: Progression to HCC or liver failure 4 Pre-Submission Briefing References: 1. Ravazi 2012; 2. Burra 2009; 3. Guillouche 2011; 4. Van der Meer 2012 Meeting | July 2014 | Company Confidential © 2014 Abb. Vie 1 0
Patient survival outcomes with and without SVR Viral Eradication Improves All-Cause Mortality • Cure = SVR = reduced risk of — All-cause mortality; Liver-related mortality; HCV-related complications: Progression to HCC or liver failure 4 Pre-Submission Briefing References: 1. Ravazi 2012; 2. Burra 2009; 3. Guillouche 2011; 4. Van der Meer 2012 Meeting | July 2014 | Company Confidential © 2014 Abb. Vie 1 0
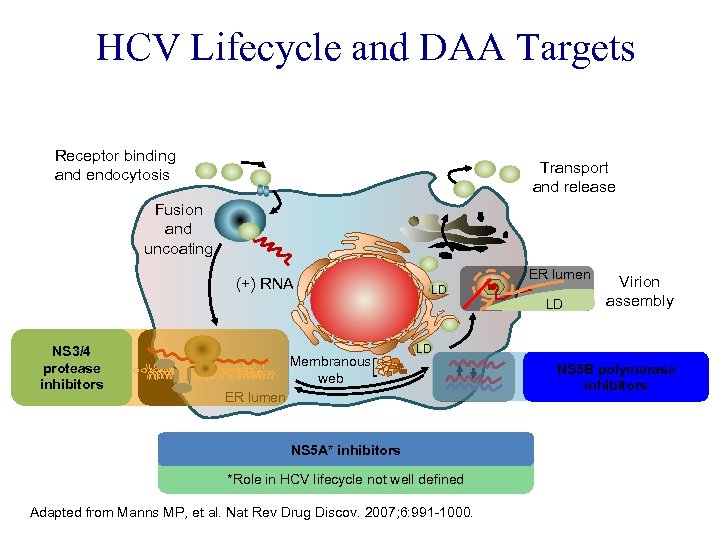 HCV Lifecycle and DAA Targets Receptor binding and endocytosis Transport and release Fusion and uncoating (+) RNA Translation and NS 3/4 protease polyprotein inhibitors processing Membranous web ER lumen LD LD Virion assembly LD ER lumen NS 5 A* inhibitors *Role in HCV lifecycle not well defined Adapted from Manns MP, et al. Nat Rev Drug Discov. 2007; 6: 991 -1000. NS 5 B polymerase RNA replication inhibitors
HCV Lifecycle and DAA Targets Receptor binding and endocytosis Transport and release Fusion and uncoating (+) RNA Translation and NS 3/4 protease polyprotein inhibitors processing Membranous web ER lumen LD LD Virion assembly LD ER lumen NS 5 A* inhibitors *Role in HCV lifecycle not well defined Adapted from Manns MP, et al. Nat Rev Drug Discov. 2007; 6: 991 -1000. NS 5 B polymerase RNA replication inhibitors
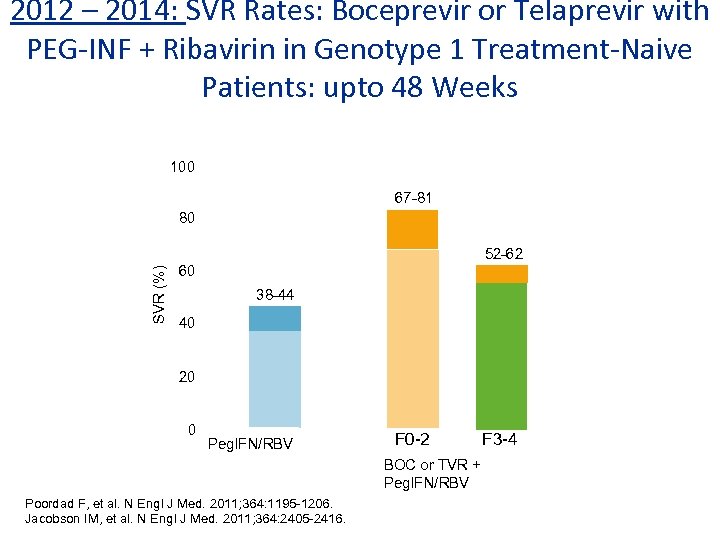 2012 – 2014: SVR Rates: Boceprevir or Telaprevir with PEG-INF + Ribavirin in Genotype 1 Treatment-Naive Patients: upto 48 Weeks 100 67 -81 80 SVR (%) 52 -62 60 38 -44 40 20 0 Peg. IFN/RBV F 0 -2 BOC or TVR + Peg. IFN/RBV Poordad F, et al. N Engl J Med. 2011; 364: 1195 -1206. Jacobson IM, et al. N Engl J Med. 2011; 364: 2405 -2416. F 3 -4
2012 – 2014: SVR Rates: Boceprevir or Telaprevir with PEG-INF + Ribavirin in Genotype 1 Treatment-Naive Patients: upto 48 Weeks 100 67 -81 80 SVR (%) 52 -62 60 38 -44 40 20 0 Peg. IFN/RBV F 0 -2 BOC or TVR + Peg. IFN/RBV Poordad F, et al. N Engl J Med. 2011; 364: 1195 -1206. Jacobson IM, et al. N Engl J Med. 2011; 364: 2405 -2416. F 3 -4
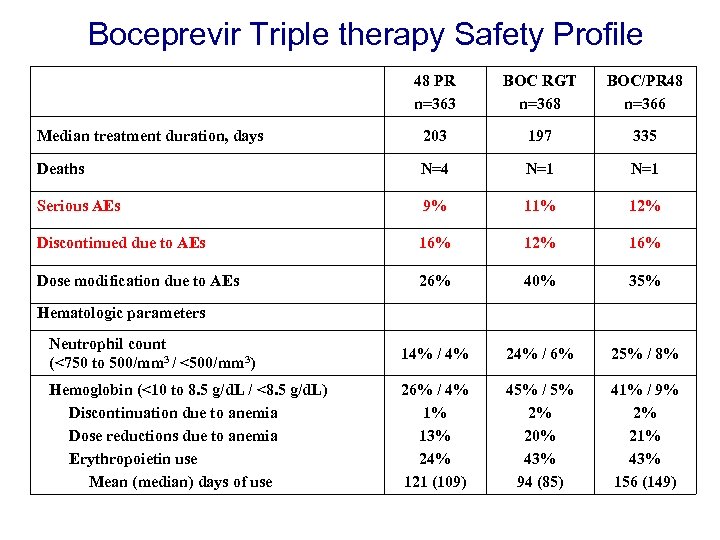 Boceprevir Triple therapy Safety Profile 48 PR n=363 BOC RGT n=368 BOC/PR 48 n=366 Median treatment duration, days 203 197 335 Deaths N=4 N=1 Serious AEs 9% 11% 12% Discontinued due to AEs 16% 12% 16% Dose modification due to AEs 26% 40% 35% Neutrophil count (<750 to 500/mm 3 / <500/mm 3) 14% / 4% 24% / 6% 25% / 8% Hemoglobin (<10 to 8. 5 g/d. L / <8. 5 g/d. L) Discontinuation due to anemia Dose reductions due to anemia Erythropoietin use Mean (median) days of use 26% / 4% 1% 13% 24% 121 (109) 45% / 5% 2% 20% 43% 94 (85) 41% / 9% 2% 21% 43% 156 (149) Hematologic parameters
Boceprevir Triple therapy Safety Profile 48 PR n=363 BOC RGT n=368 BOC/PR 48 n=366 Median treatment duration, days 203 197 335 Deaths N=4 N=1 Serious AEs 9% 11% 12% Discontinued due to AEs 16% 12% 16% Dose modification due to AEs 26% 40% 35% Neutrophil count (<750 to 500/mm 3 / <500/mm 3) 14% / 4% 24% / 6% 25% / 8% Hemoglobin (<10 to 8. 5 g/d. L / <8. 5 g/d. L) Discontinuation due to anemia Dose reductions due to anemia Erythropoietin use Mean (median) days of use 26% / 4% 1% 13% 24% 121 (109) 45% / 5% 2% 20% 43% 94 (85) 41% / 9% 2% 21% 43% 156 (149) Hematologic parameters
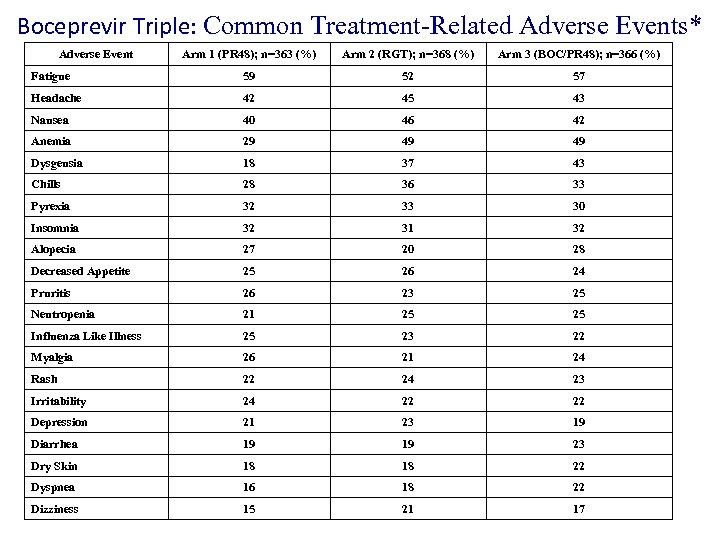 Boceprevir Triple: Common Treatment-Related Adverse Events* Adverse Event Arm 1 (PR 48); n=363 (%) Arm 2 (RGT); n=368 (%) Arm 3 (BOC/PR 48); n=366 (%) Fatigue 59 52 57 Headache 42 45 43 Nausea 40 46 42 Anemia 29 49 49 Dysgeusia 18 37 43 Chills 28 36 33 Pyrexia 32 33 30 Insomnia 32 31 32 Alopecia 27 20 28 Decreased Appetite 25 26 24 Pruritis 26 23 25 Neutropenia 21 25 25 Influenza Like Illness 25 23 22 Myalgia 26 21 24 Rash 22 24 23 Irritability 24 22 22 Depression 21 23 19 Diarrhea 19 19 23 Dry Skin 18 18 22 Dyspnea 16 18 22 Dizziness 15 21 17 *Reported in >20% of patients in any treatment arm and listed by decreasing overall frequency
Boceprevir Triple: Common Treatment-Related Adverse Events* Adverse Event Arm 1 (PR 48); n=363 (%) Arm 2 (RGT); n=368 (%) Arm 3 (BOC/PR 48); n=366 (%) Fatigue 59 52 57 Headache 42 45 43 Nausea 40 46 42 Anemia 29 49 49 Dysgeusia 18 37 43 Chills 28 36 33 Pyrexia 32 33 30 Insomnia 32 31 32 Alopecia 27 20 28 Decreased Appetite 25 26 24 Pruritis 26 23 25 Neutropenia 21 25 25 Influenza Like Illness 25 23 22 Myalgia 26 21 24 Rash 22 24 23 Irritability 24 22 22 Depression 21 23 19 Diarrhea 19 19 23 Dry Skin 18 18 22 Dyspnea 16 18 22 Dizziness 15 21 17 *Reported in >20% of patients in any treatment arm and listed by decreasing overall frequency
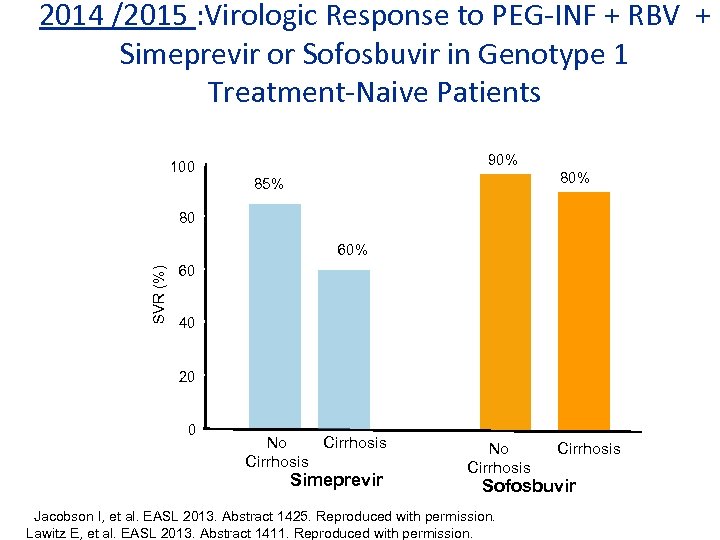 2014 /2015 : Virologic Response to PEG-INF + RBV + Simeprevir or Sofosbuvir in Genotype 1 Treatment-Naive Patients 90% 100 80% 85% 80 SVR (%) 60% 60 40 20 0 No Cirrhosis Simeprevir No Cirrhosis Sofosbuvir Jacobson I, et al. EASL 2013. Abstract 1425. Reproduced with permission. Lawitz E, et al. EASL 2013. Abstract 1411. Reproduced with permission.
2014 /2015 : Virologic Response to PEG-INF + RBV + Simeprevir or Sofosbuvir in Genotype 1 Treatment-Naive Patients 90% 100 80% 85% 80 SVR (%) 60% 60 40 20 0 No Cirrhosis Simeprevir No Cirrhosis Sofosbuvir Jacobson I, et al. EASL 2013. Abstract 1425. Reproduced with permission. Lawitz E, et al. EASL 2013. Abstract 1411. Reproduced with permission.
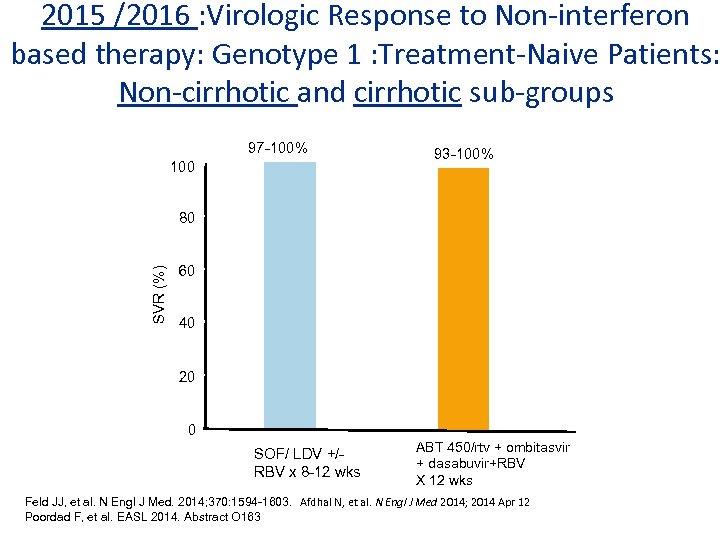 2015 /2016 : Virologic Response to Non-interferon based therapy: Genotype 1 : Treatment-Naive Patients: Non-cirrhotic and cirrhotic sub-groups 97 -100% 100 93 -100% SVR (%) 80 60 40 20 0 SOF/ LDV +/RBV x 8 -12 wks ABT 450/rtv + ombitasvir + dasabuvir+RBV X 12 wks Feld JJ, et al. N Engl J Med. 2014; 370: 1594 -1603. Afdhal N, et al. N Engl J Med 2014; 2014 Apr 12 Poordad F, et al. EASL 2014. Abstract O 163
2015 /2016 : Virologic Response to Non-interferon based therapy: Genotype 1 : Treatment-Naive Patients: Non-cirrhotic and cirrhotic sub-groups 97 -100% 100 93 -100% SVR (%) 80 60 40 20 0 SOF/ LDV +/RBV x 8 -12 wks ABT 450/rtv + ombitasvir + dasabuvir+RBV X 12 wks Feld JJ, et al. N Engl J Med. 2014; 370: 1594 -1603. Afdhal N, et al. N Engl J Med 2014; 2014 Apr 12 Poordad F, et al. EASL 2014. Abstract O 163
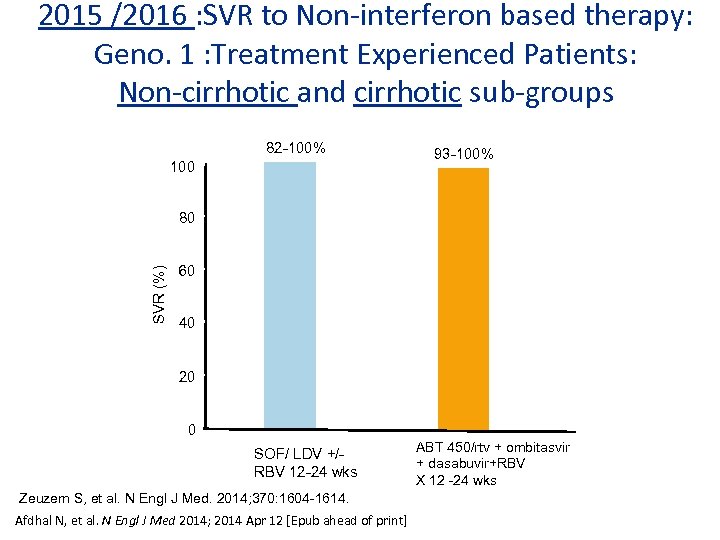 2015 /2016 : SVR to Non-interferon based therapy: Geno. 1 : Treatment Experienced Patients: Non-cirrhotic and cirrhotic sub-groups 82 -100% 100 93 -100% SVR (%) 80 60 40 20 0 SOF/ LDV +/RBV 12 -24 wks Zeuzem S, et al. N Engl J Med. 2014; 370: 1604 -1614. Afdhal N, et al. N Engl J Med 2014; 2014 Apr 12 [Epub ahead of print] ABT 450/rtv + ombitasvir + dasabuvir+RBV X 12 -24 wks
2015 /2016 : SVR to Non-interferon based therapy: Geno. 1 : Treatment Experienced Patients: Non-cirrhotic and cirrhotic sub-groups 82 -100% 100 93 -100% SVR (%) 80 60 40 20 0 SOF/ LDV +/RBV 12 -24 wks Zeuzem S, et al. N Engl J Med. 2014; 370: 1604 -1614. Afdhal N, et al. N Engl J Med 2014; 2014 Apr 12 [Epub ahead of print] ABT 450/rtv + ombitasvir + dasabuvir+RBV X 12 -24 wks
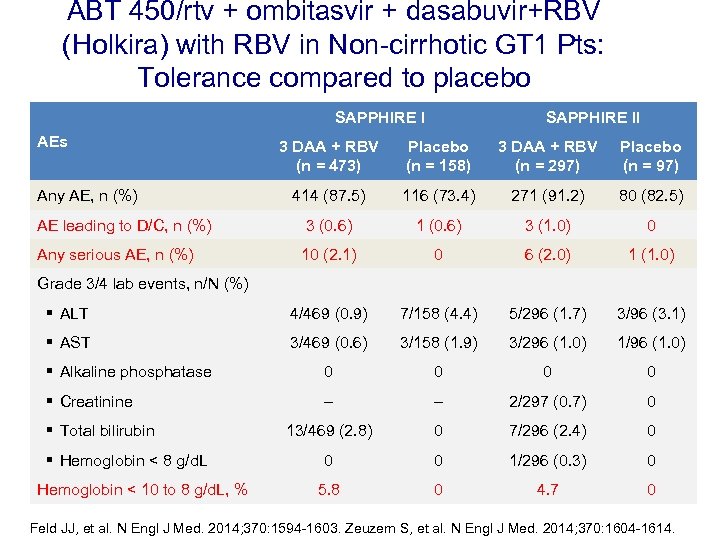 ABT 450/rtv + ombitasvir + dasabuvir+RBV (Holkira) with RBV in Non-cirrhotic GT 1 Pts: Tolerance compared to placebo SAPPHIRE I AEs SAPPHIRE II 3 DAA + RBV (n = 473) Placebo (n = 158) 3 DAA + RBV (n = 297) Placebo (n = 97) 414 (87. 5) 116 (73. 4) 271 (91. 2) 80 (82. 5) AE leading to D/C, n (%) 3 (0. 6) 1 (0. 6) 3 (1. 0) 0 Any serious AE, n (%) 10 (2. 1) 0 6 (2. 0) 1 (1. 0) § ALT 4/469 (0. 9) 7/158 (4. 4) 5/296 (1. 7) 3/96 (3. 1) § AST 3/469 (0. 6) 3/158 (1. 9) 3/296 (1. 0) 1/96 (1. 0) § Alkaline phosphatase 0 0 § Creatinine – – 2/297 (0. 7) 0 13/469 (2. 8) 0 7/296 (2. 4) 0 0 0 1/296 (0. 3) 0 5. 8 0 4. 7 0 Any AE, n (%) Grade 3/4 lab events, n/N (%) § Total bilirubin § Hemoglobin < 8 g/d. L Hemoglobin < 10 to 8 g/d. L, % Feld JJ, et al. N Engl J Med. 2014; 370: 1594 -1603. Zeuzem S, et al. N Engl J Med. 2014; 370: 1604 -1614.
ABT 450/rtv + ombitasvir + dasabuvir+RBV (Holkira) with RBV in Non-cirrhotic GT 1 Pts: Tolerance compared to placebo SAPPHIRE I AEs SAPPHIRE II 3 DAA + RBV (n = 473) Placebo (n = 158) 3 DAA + RBV (n = 297) Placebo (n = 97) 414 (87. 5) 116 (73. 4) 271 (91. 2) 80 (82. 5) AE leading to D/C, n (%) 3 (0. 6) 1 (0. 6) 3 (1. 0) 0 Any serious AE, n (%) 10 (2. 1) 0 6 (2. 0) 1 (1. 0) § ALT 4/469 (0. 9) 7/158 (4. 4) 5/296 (1. 7) 3/96 (3. 1) § AST 3/469 (0. 6) 3/158 (1. 9) 3/296 (1. 0) 1/96 (1. 0) § Alkaline phosphatase 0 0 § Creatinine – – 2/297 (0. 7) 0 13/469 (2. 8) 0 7/296 (2. 4) 0 0 0 1/296 (0. 3) 0 5. 8 0 4. 7 0 Any AE, n (%) Grade 3/4 lab events, n/N (%) § Total bilirubin § Hemoglobin < 8 g/d. L Hemoglobin < 10 to 8 g/d. L, % Feld JJ, et al. N Engl J Med. 2014; 370: 1594 -1603. Zeuzem S, et al. N Engl J Med. 2014; 370: 1604 -1614.
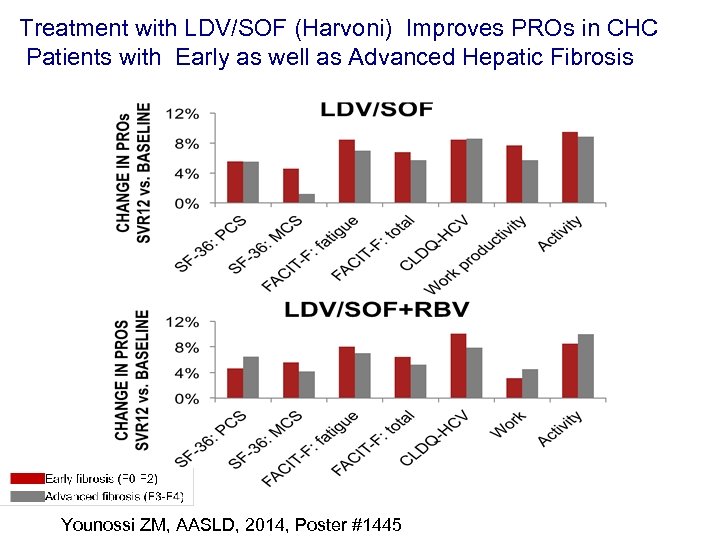 Treatment with LDV/SOF (Harvoni) Improves PROs in CHC Patients with Early as well as Advanced Hepatic Fibrosis Younossi ZM, AASLD, 2014, Poster #1445
Treatment with LDV/SOF (Harvoni) Improves PROs in CHC Patients with Early as well as Advanced Hepatic Fibrosis Younossi ZM, AASLD, 2014, Poster #1445
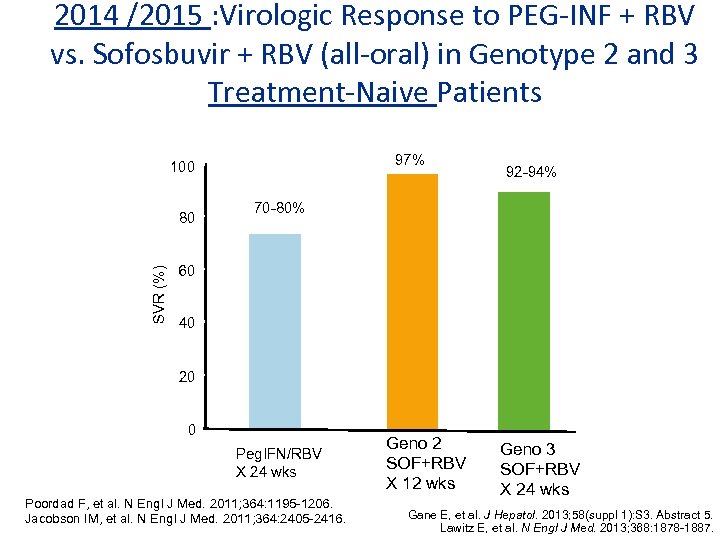 2014 /2015 : Virologic Response to PEG-INF + RBV vs. Sofosbuvir + RBV (all-oral) in Genotype 2 and 3 Treatment-Naive Patients 97% 100 SVR (%) 80 92 -94% 70 -80% 60 40 20 0 Peg. IFN/RBV X 24 wks Poordad F, et al. N Engl J Med. 2011; 364: 1195 -1206. Jacobson IM, et al. N Engl J Med. 2011; 364: 2405 -2416. Geno 2 SOF+RBV X 12 wks Geno 3 SOF+RBV X 24 wks Gane E, et al. J Hepatol. 2013; 58(suppl 1): S 3. Abstract 5. Lawitz E, et al. N Engl J Med. 2013; 368: 1878 -1887.
2014 /2015 : Virologic Response to PEG-INF + RBV vs. Sofosbuvir + RBV (all-oral) in Genotype 2 and 3 Treatment-Naive Patients 97% 100 SVR (%) 80 92 -94% 70 -80% 60 40 20 0 Peg. IFN/RBV X 24 wks Poordad F, et al. N Engl J Med. 2011; 364: 1195 -1206. Jacobson IM, et al. N Engl J Med. 2011; 364: 2405 -2416. Geno 2 SOF+RBV X 12 wks Geno 3 SOF+RBV X 24 wks Gane E, et al. J Hepatol. 2013; 58(suppl 1): S 3. Abstract 5. Lawitz E, et al. N Engl J Med. 2013; 368: 1878 -1887.
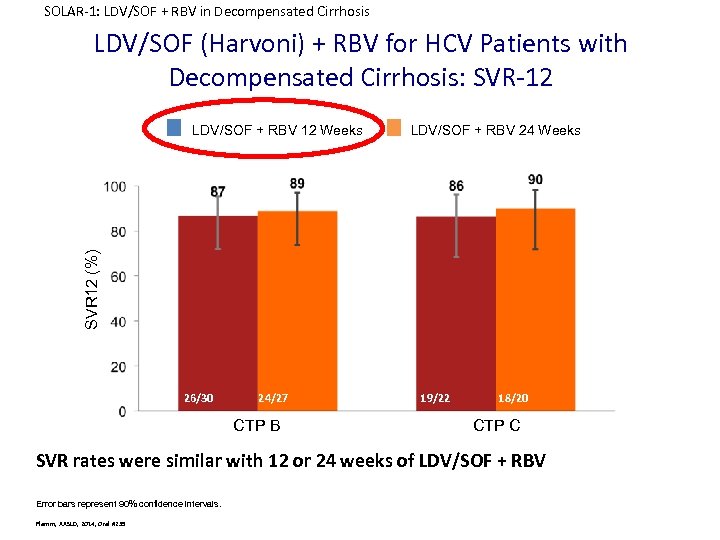 SOLAR-1: LDV/SOF + RBV in Decompensated Cirrhosis LDV/SOF (Harvoni) + RBV for HCV Patients with Decompensated Cirrhosis: SVR-12 LDV/SOF + RBV 24 Weeks SVR 12 (%) LDV/SOF + RBV 12 Weeks 26/30 24/27 CTP B 19/22 18/20 CTP C SVR rates were similar with 12 or 24 weeks of LDV/SOF + RBV Error bars represent 90% confidence intervals. Flamm, AASLD, 2014, Oral #239
SOLAR-1: LDV/SOF + RBV in Decompensated Cirrhosis LDV/SOF (Harvoni) + RBV for HCV Patients with Decompensated Cirrhosis: SVR-12 LDV/SOF + RBV 24 Weeks SVR 12 (%) LDV/SOF + RBV 12 Weeks 26/30 24/27 CTP B 19/22 18/20 CTP C SVR rates were similar with 12 or 24 weeks of LDV/SOF + RBV Error bars represent 90% confidence intervals. Flamm, AASLD, 2014, Oral #239
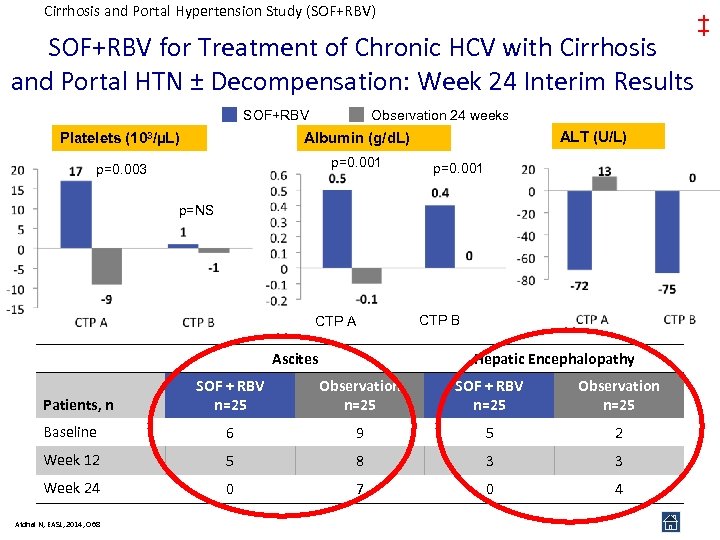 Cirrhosis and Portal Hypertension Study (SOF+RBV) SOF+RBV for Treatment of Chronic HCV with Cirrhosis and Portal HTN ± Decompensation: Week 24 Interim Results SOF+RBV Observation 24 weeks Platelets (103/µL) p=0. 003 p=0. 001 ALT (U/L) Albumin (g/d. L) p=0. 001 p=NS CTP A Ascites CTP B Hepatic Encephalopathy SOF + RBV n=25 Observation n=25 Baseline 6 9 5 2 Week 12 5 8 3 3 Week 24 0 7 0 4 Patients, n Afdhal N, EASL, 2014, O 68 ‡
Cirrhosis and Portal Hypertension Study (SOF+RBV) SOF+RBV for Treatment of Chronic HCV with Cirrhosis and Portal HTN ± Decompensation: Week 24 Interim Results SOF+RBV Observation 24 weeks Platelets (103/µL) p=0. 003 p=0. 001 ALT (U/L) Albumin (g/d. L) p=0. 001 p=NS CTP A Ascites CTP B Hepatic Encephalopathy SOF + RBV n=25 Observation n=25 Baseline 6 9 5 2 Week 12 5 8 3 3 Week 24 0 7 0 4 Patients, n Afdhal N, EASL, 2014, O 68 ‡
 Hepatitis C: Summary • HCV has a slowly progressive course to cirrhosis, with complications of decompensated disease. • Newer regimen: all-oral, efficacious, well tolerated • Viral eradication / cure in 70 -98% • Viral eradication has a mortality benefit
Hepatitis C: Summary • HCV has a slowly progressive course to cirrhosis, with complications of decompensated disease. • Newer regimen: all-oral, efficacious, well tolerated • Viral eradication / cure in 70 -98% • Viral eradication has a mortality benefit
 CADTH SYMPOSIUM : HCV: NATURAL HISTORY AND THERAPEUTICS Alnoor Ramji Gastroenterology & Hepatology Clinical Associate Professor Division of Gastroenterology University Of British Columbia St. Paul’s Hospital Site ramji_a@hotmail. com
CADTH SYMPOSIUM : HCV: NATURAL HISTORY AND THERAPEUTICS Alnoor Ramji Gastroenterology & Hepatology Clinical Associate Professor Division of Gastroenterology University Of British Columbia St. Paul’s Hospital Site ramji_a@hotmail. com


