83b923ee9ba6a836333a6997c216b73a.ppt
- Количество слайдов: 39

Building a safer NHS for patients IMPLEMENTING AN ORGANISATION WITH A MEMORY

Lapses in standards of care presented in different ways…. . . • As apparently unexpected adverse events • As poor or unsatisfactory outcomes of care • When patients are put at risk by a practitioner whose performance is impaired due to inadequate knowledge, skills, ill health or dysfunctional conduct

Contents • A New Focus on Patient Safety • When Something Goes Wrong: An Integrated Response from the NHS • A Blueprint for the New National System for Learning from Adverse Events and Near Misses • Specific Risks Targeted for Action • Key Questions in the Developing Patient Safety Research Agenda • Implementation Timetable

A New Focus on Patient Safety “There is simply no issue more important in health care than ensuring the safety of our patients” Dr Ken Kizer, President & CEO, National Forum for Health Care Quality Measurement and Reporting (NQF), USA

A New Focus on Patient Safety • Adverse events occur in around 10% of admissions, or at a rate of 850, 000 per year • Adverse events cost approx. £ 2 billion/year in hospital stay alone • Around 1150 people/year in recent contact with mental health services commit suicide • 400 people/year die or are seriously injured in adverse events involving medical devices • >£ 400 million clinical negligence settlements/year • Hospital acquired infections cost £ 1 billion/year around 15% of HAI may be avoidable

A New Focus on Patient Safety Discussions with colleagues in Australia and the USA have clearly demonstrated that: • the problems faced are very similar • there is enormous scope for collaboration in designing solutions for patient safety and finding effective ways of implementing them • there is a major need for international standardisation of terminology in the definition of different types of adverse event and in reporting

When something goes wrong: An integrated response from the NHS The handling of situations where there are potential risks to patients of poor outcomes of care or harm or where such events have already taken place will change. There will be a new integrated approach: • A new national system for reporting and analysing adverse events and near misses, including local investigations that focus on analysis of systems to identify underlying or root causes • Where the new national system reveals major service problems, CHI will be asked to consider whether to follow on with its own investigation

When something goes wrong: An integrated response from the NHS • Actual or potential risks to patients from poorly performing doctors that cannot be resolved locally will be referred to NCAA with emphasis on early intervention through retraining or education. Serious cases will still be referred to GMC • In assessing a doctor, if the NCAA discovers major service problems, CHI will be asked to consider whether to follow on with its own investigation • Regulation of other health professions is being strengthened and NCAA will eventually advise local NHS employers on dealing with poor clinical performance amongst other health professions

When something goes wrong: An integrated response from the NHS • For whole service failure, a seriously dysfunctional service, or major systems weaknesses, an independent investigation will be carried out by either the Department of Health (through one of Chief Officers or Regional Office) or CHI • No more multiple investigations into the same problem local internal inquiries will be limited to informing decision-making on what form of independent investigation or inquiry is needed (if any) • Inquiries into the mental health services (currently dealt with under a separate procedure) will be brought into the integrated Department of Health/CHI approach

When something goes wrong: An integrated response from the NHS • Where a service failure results in serious harm to larger numbers of patients, where there is serious national concern, or where a major issue of ethics or policy is raised for the first time by an incident, the Secretary of State for Health, using his statutory powers, may order a public inquiry • Complaints will be dealt with under a revised NHS complaints procedure, but patients and carers will have a role in the new adverse event reporting system

When something goes wrong: An integrated response from the NHS • Staff concerns about standards of care should be addressed by the new reporting system or as part of clinical governance more generally. Whistleblowing legislation will continue to protect staff (and patients) in very dysfunctional or repressive organisations • The NHS will work with stakeholders to explore how national audits and benchmarking can be developed and used to monitor and improve poor outcomes of clinical care • Detailed guidance will be issued to the NHS on investigations and inquiries

A Blueprint for the New National System for learning from Adverse Events and Near Misses • An adverse healthcare event is an event or omission arising during clinical care and causing physical or psychological injury to a patient. • A health care near miss is a situation in which an event or omission, or a sequence of events or omissions, arising during clinical care fails to develop further, whether or not as a result of compensating action, thus preventing injury to a patient. • Internationally agreed definitions for patient safety are being developed.

Key elements of the new system for learning from adverse events and near misses • Identifying, gathering information on, recording and reporting adverse events and near misses • Applying standardised root cause analysis methodologies to provide causal information to facilitate learning and action to minimise risk • Analysing patterns and trends • Reporting of standardised information on specified events and near misses to NPSA • Learning and disseminating lessons • Implementing effective change
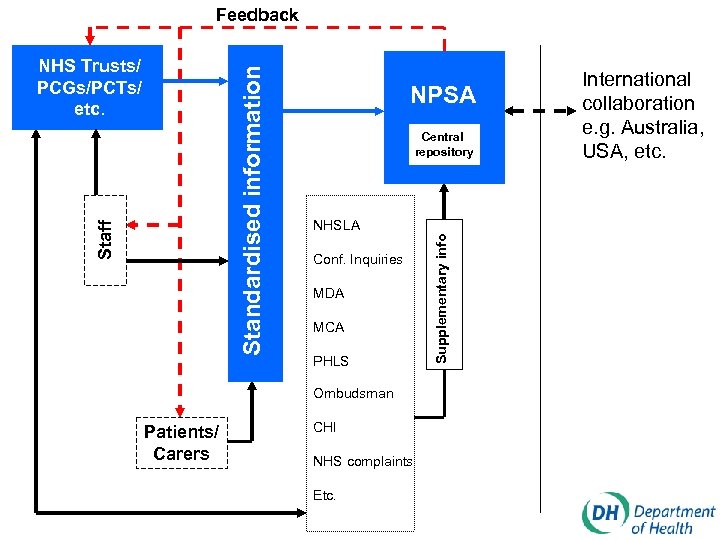
Feedback NPSA Central repository NHSLA Conf. Inquiries MDA MCA PHLS Ombudsman Patients/ Carers CHI NHS complaints Etc. Supplementary info Staff Standardised information NHS Trusts/ PCGs/PCTs/ etc. International collaboration e. g. Australia, USA, etc.

The National Patient Safety Agency (www. patientsafety. org. uk or www. npsa. org. uk) • Setting and maintaining reporting standards in conjunction with Department of Health • Collecting, collating, categorising and coding information on adverse events etc. • Assimilating other safety-related information • Analysing information and maintaining publicly available central repository • Examining and tracking patterns and trends • Providing feedback to organisations and individuals • Producing solutions to reduce risk and specifying national goals and targets • Promoting research • Promoting a reporting culture • Collaborating with relevant national and international bodies

Key questions for reporting systems • What makes reporting systems successful? • What data and other information should be collected and how? • How should data be categorised and aggregated to enable patterns of events and trends to be recognised? • How can confidentiality and discoverability be balanced with the need to inform in order to prevent harm to future patients? • What organisational cultures and leadership factors promote reporting as a means to improve patient safety?

Some barriers that need to be overcome • Lack of awareness of the need to report, what to report, and why • Lack of understanding of how to report • Staff feel they are too busy to make a report • Too much paperwork involved in reporting • Patient recovers from the event and the urgency goes out of the situation • Fear of ‘point scoring’ by colleagues, retribution by line management, disciplinary action or litigation • An assumption that someone else will make the report • No evidence of timely feedback and/or corrective action being taken resulting from making the report

A minimum data set for reporting? • What happened? (description, severity, people/equipment involved, etc. ) • Where did it happen? (speciality/location) • When did it happen? (date/time) • How did it happen? (immediate/proximate cause) • Why did it happen? (underlying/root cause) • What action was taken or proposed? • What impact did the event have? (financial and other harm to organisation, patient, etc. ) • What factors did, or could have minimised the impact of the event?
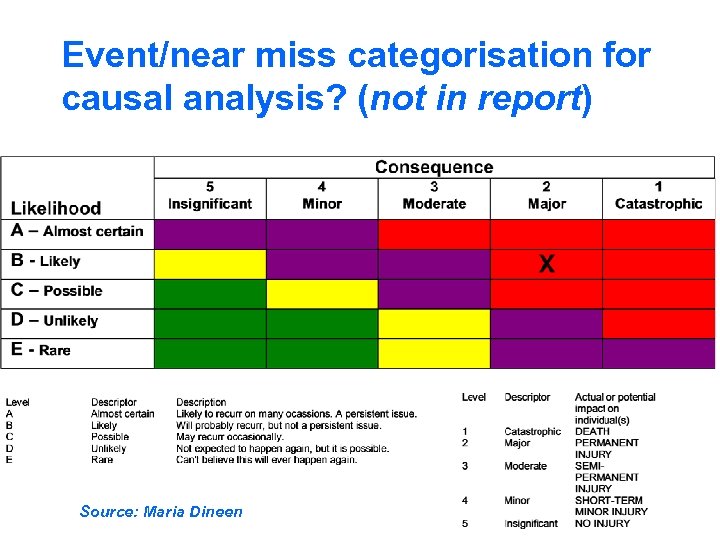
Event/near miss categorisation for causal analysis? (not in report) Source: Maria Dineen
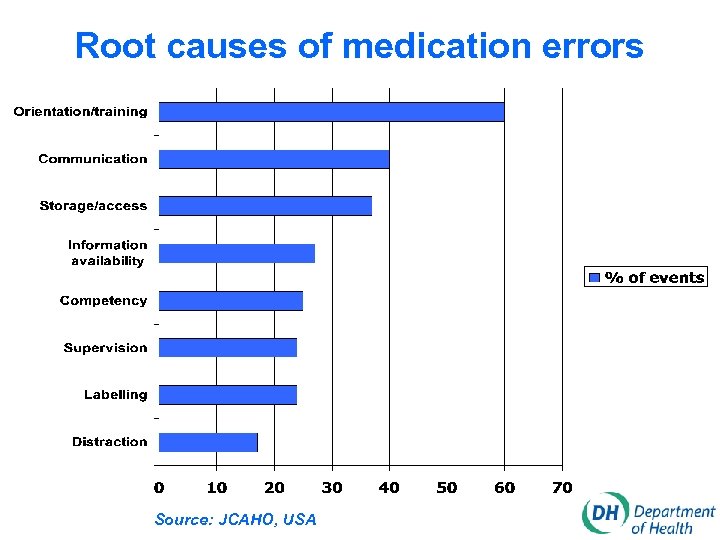
Root causes of medication errors Source: JCAHO, USA

The New National System. . “Improvement strategies that punish individual clinicians are misguided and do not work. Fixing dysfunctional systems on the other hand is the work that needs to be done” Saul Weingart, Harvard Executive, Session on Medical Error and Patient Safety
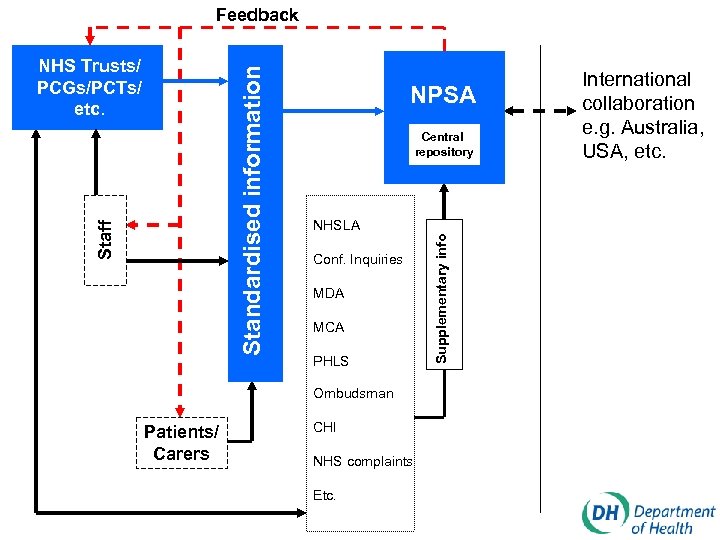
Feedback NPSA Central repository NHSLA Conf. Inquiries MDA MCA PHLS Ombudsman Patients/ Carers CHI NHS complaints Etc. Supplementary info Staff Standardised information NHS Trusts/ PCGs/PCTs/ etc. International collaboration e. g. Australia, USA, etc.

Standard forms for data capture ? • We’re committed to producing, as part of guidance on adverse event and near miss reporting, an exemplar form, or forms, for organisations, staff and patients/carers • We’re going to undertake a survey of forms already in use in NHS Trusts (please send a copy of yours to David Levy, Dept. of Health, Room 609, Richmond House, 79 Whitehall, London, SW 1 A) • We’re particularly interested in views on having a mandatory standard form for reporting of all adverse events and near misses

Software for incident reporting? • A software package is often a key component of a local incident reporting system • There a number of established software systems available • Some NHS organisations have developed their own. • Existing software systems will typically have capabilities/functionality exceeding that required for the new national system • Local organisations will be free to choose software that meets their needs. . . BUT must provide standardised information in accordance with minimum data set

Specific risks targeted for action From An Organisation with a Memory: • By the end of 2001, reduce to zero the number of patients dying or being paralysed by maladministered spinal injections • By 2005, reduce by 25% the incidence of harm in obstetrics & gynaecology which result in litigation • By 2005, reduce by 40% the number of serious errors in the use of prescribed drugs • By 2002, reduce to zero the number of suicides by mental health patients from non-collapsible bed or shower curtain rails on wards

Specific risks targeted for action Other areas being considered include: • Review of the care environment to identify environmental and care practice changes • Reviewing clinical practice with Royal Colleges, etc. • Building safety into purchasing policies • Seeking input from the world of design • Looking at potential for computers to reduce error • Identifying scope formal pre-procedure safety briefings in very high risk situations • Enhancing role of simulation laboratories • Creating a clear role for patients

Patient safety research “From past work in Human Factors a single standard emerges for judging success in research on error and safety. Research is successful to the degree that it helps recognise, anticipate and defend against paths to failure that arise as organisations and technology change, before any patient is injured” David Woods, Past President, Human Factors and Ergonomics Society

A coherent national research effort • The Department of Health will directly fund patient safety research and will indirectly work with relevant stakeholders, including both funders and users of research • A workshop will be held this year to bring together key stakeholders to drive forward the national strategy for patient safety research • The National Patient Safety Agency, once fully established, will help identify research needs and will take the lead in strengthening the relationship between the various stakeholders

A patient safety research strategy • Establishing the size and nature of the problem • Understanding the factors that cause harm and assessing the efficacy of intervention strategies • Developing and designing reporting systems to ensure their good use • Learning lessons and disseminating them • Changing individual and organisational behaviour • Involving patients

Patient safety research Examples of research questions include: • What are the main types of error and adverse event in different health care settings? • What methodologies would ensure effective patient and consumer involvement to enhance patient safety? • What strategies would ensure early detection of new risks before they result in a rare but catastrophic event? • How can organisational cultures be achieved that are safety conscious, ‘reporting-friendly’ and free of blame? • What methods can reduce error in particular specialist fields of healthcare (e. g. drug therapy)? • How can equipment acquisition and management policies reduce risk?

Patient safety research Examples of research questions cont. • What automated methods of data capture could be developed to reduce reliance on human reporting? • How can data collation, classification and analysis be enhanced to allow patterns of causation, presentation, detection and amelioration to be elucidated? • What are the characteristics of good leadership of clinical teams that have a good performance on patient safety? • Why does change to improve patient safety so often fail to be implemented despite widespread dissemination of strategies that have been show to work? • etc.

Implementation Headline Targets • By December 2001, 60% of NHS Trusts will be in a position to provide information to the national system and all NHS Trusts will be working towards this goal • By December 2002, all NHS Trusts and a significant proportion of primary care will be providing information to the national system • By December 2002, levels of reporting in the NHS will have doubled

Implementation Supporting Targets (1 of 6) • From March 2001 we will work with key partners to establish methods for implementing solutions from investigations and inquiries across the NHS • From April 2001 we will work closely with other reporting agencies • Work to take early action to reduce risk in specific areas and by influencing specific processes will be progressed from April 2001 onwards

Implementation Supporting Targets (2 of 6) • In April 2001 we will liaise with key stakeholders to progress the patient safety research agenda • In May 2001 we will issue a call for proposals as part of a funded programme of patient safety research • By July 2001, the NPSA will be established • By July 2001, guidance will be issued on procedure and criteria for establishing independent investigations and inquiries

Implementation Supporting Targets (3 of 6) • By August 2001, guidance will be issued on: – identifying and recording adverse events and near misses, including glossary of standardised terms and definitions – reporting of adverse events by organisations, including potential for use of IT – reporting by staff to the NPSA • From August 2001, the planned national reporting system will be tested through selective pilots and other evaluations

Implementation Supporting Targets (4 of 6) • From September 2001 we will link with education and training bodies to increase content of curricula and training programmes in relation to understanding error, systems thinking and patient safety • In October 2001 a strategy on building local capability will be produced • By November 2001, guidance will be issued on: – patient/carer reporting – root cause analysis

Implementation Supporting Targets (5 of 6) • By December 2001 a strategy for learning lessons, disseminating them, and implementing change will be developed • From December 2001 the national system will be progressively implemented across the NHS and into other organisations treating NHS patients
Implementation Supporting Targets (6 of 6) • We will deliver the specific targets identified in An Organisation with a Memory as follows: – – maladministered spinal injections by end 2001 harm in obs, gynae & midwifery care by end 2005 serious error in the use of medicines by end 2005 suicides by mental health patients as result of hanging from non-collapsible bed/shower curtain rails by March 2002

Thank you for your time and attention. . any questions ? www. patientsafety. org. uk www. npsa. org. uk
83b923ee9ba6a836333a6997c216b73a.ppt