baa509b688d12b863843a1269a2f570a.ppt
- Количество слайдов: 142

Bronchogenic carcinoma Prof. MUDr. Miloslav Marel, CSc. Pulm. Depth. of the 1 st Medical Faculty of Charles University, Prague

What is Lung Cancer? • An estimated 219, 440 people diagnosed in the United States in 2009 • The leading cause of cancer death among men and women • Begins when cells in the lung grow out of control and form a tumor • There are two main types of lung cancer: non-small cell and small cell
What is the Function of the Lungs? • The lungs consist of five lobes, three in the right lung and two in the left lung • Most cells in the lung are epithelial cells, which line the airways and produce mucus, which lubricates and protects the lungs • The main function of the lungs is to allow oxygen from the air to enter the bloodstream for delivery to the rest of the body

Lung Cancer Global situation in the world • The leading cause of cancer death in both women and men in USA, Canada and China • 997 000 death in men and 333 000 death in women in the world in 2000 • • An increase of adenocarcinoma 12, 3 % of all malignant tumors , 30% of cancer related death

Lung Cancer Global situation in the world European Union 29% of cancer death in men 9% in women The highest incidence in the world New Orleans 105/100 000 in men New Zeland 73/100 000 in women
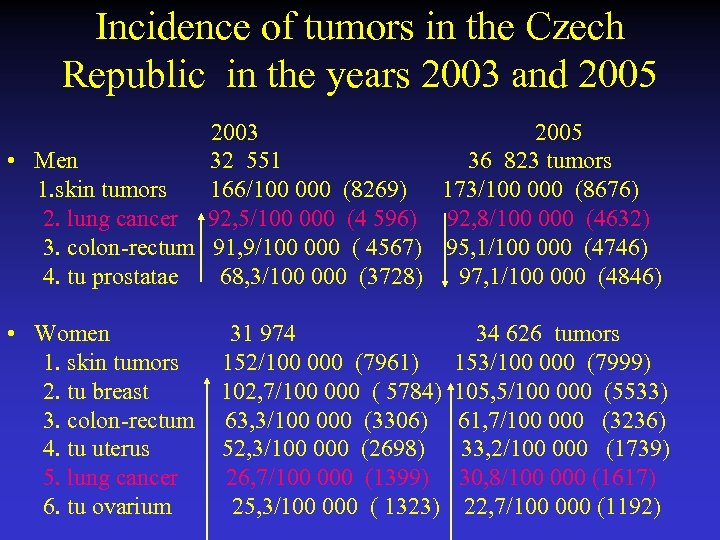
Incidence of tumors in the Czech Republic in the years 2003 and 2005 2003 2005 • Men 32 551 36 823 tumors 1. skin tumors 166/100 000 (8269) 173/100 000 (8676) 2. lung cancer 92, 5/100 000 (4 596) 92, 8/100 000 (4632) 3. colon-rectum 91, 9/100 000 ( 4567) 95, 1/100 000 (4746) 4. tu prostatae 68, 3/100 000 (3728) 97, 1/100 000 (4846) • Women 31 974 34 626 tumors 1. skin tumors 152/100 000 (7961) 153/100 000 (7999) 2. tu breast 102, 7/100 000 ( 5784) 105, 5/100 000 (5533) 3. colon-rectum 63, 3/100 000 (3306) 61, 7/100 000 (3236) 4. tu uterus 52, 3/100 000 (2698) 33, 2/100 000 (1739) 5. lung cancer 26, 7/100 000 (1399) 30, 8/100 000 (1617) 6. tu ovarium 25, 3/100 000 ( 1323) 22, 7/100 000 (1192)
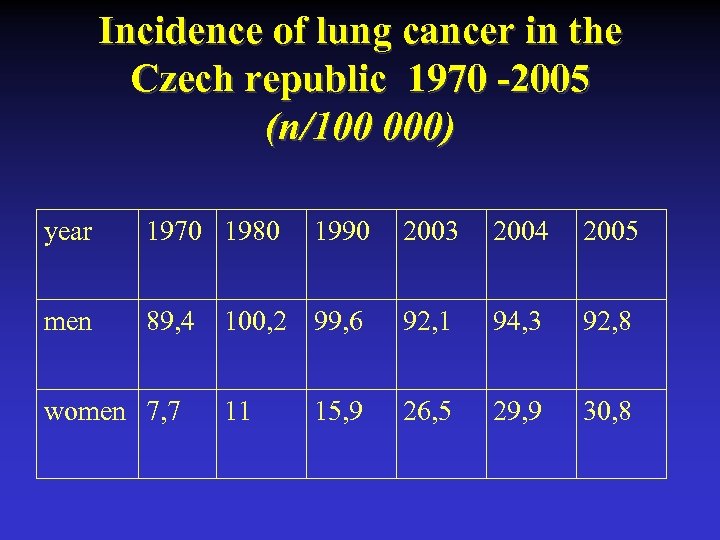
Incidence of lung cancer in the Czech republic 1970 -2005 (n/100 000) year 1970 1980 men 89, 4 women 7, 7 1990 2003 2004 2005 100, 2 99, 6 92, 1 94, 3 92, 8 11 26, 5 29, 9 30, 8 15, 9
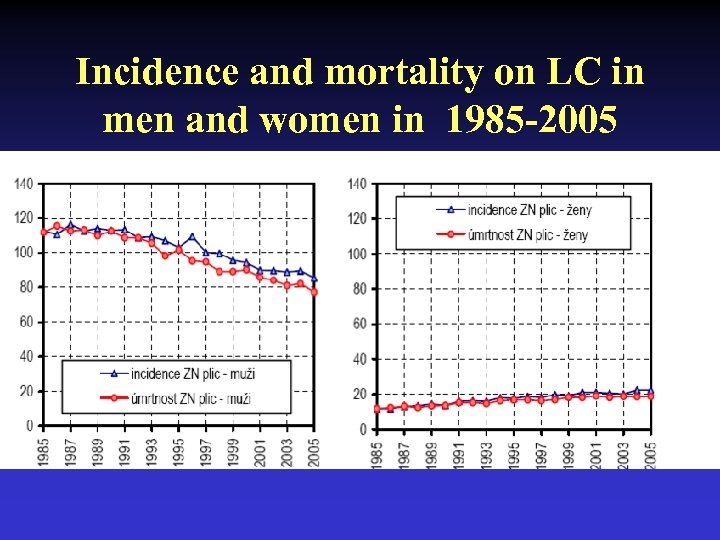
Incidence and mortality on LC in men and women in 1985 -2005
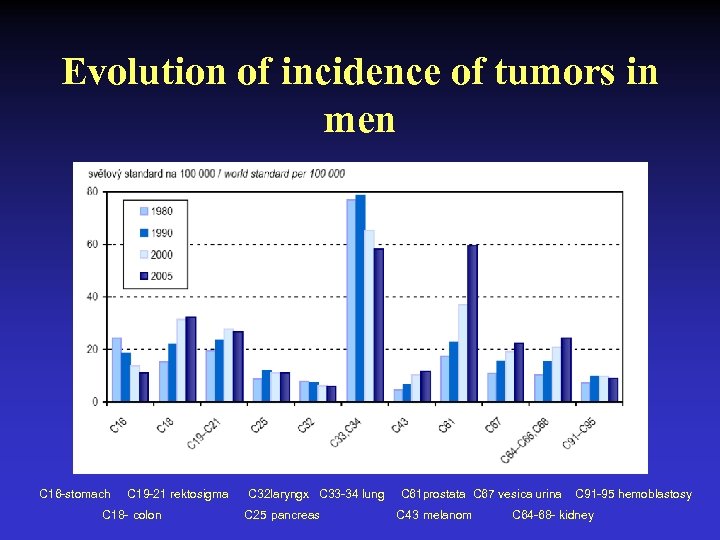
Evolution of incidence of tumors in men C 16 -stomach C 19 -21 rektosigma C 18 - colon C 32 laryngx C 33 -34 lung C 25 pancreas C 61 prostata C 67 vesica urina C 43 melanom C 91 -95 hemoblastosy C 64 -68 - kidney
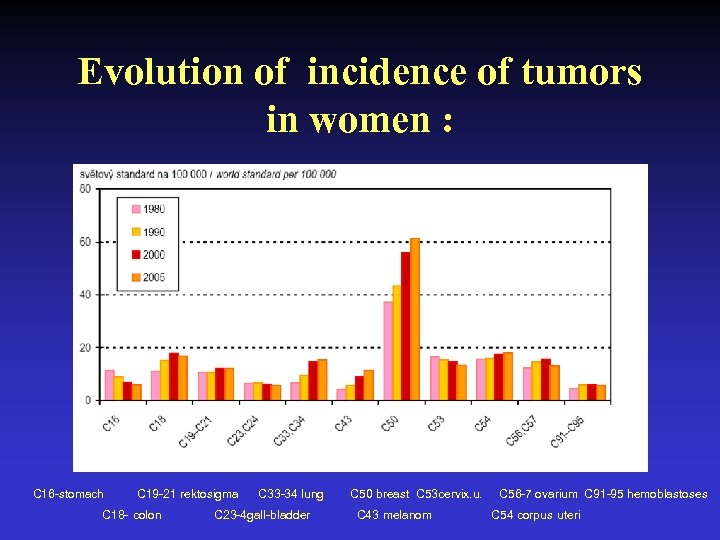
Evolution of incidence of tumors in women : C 16 -stomach C 19 -21 rektosigma C 18 - colon C 33 -34 lung C 23 -4 gall-bladder C 50 breast C 53 cervix. u. C 43 melanom C 56 -7 ovarium C 91 -95 hemoblastoses C 54 corpus uteri
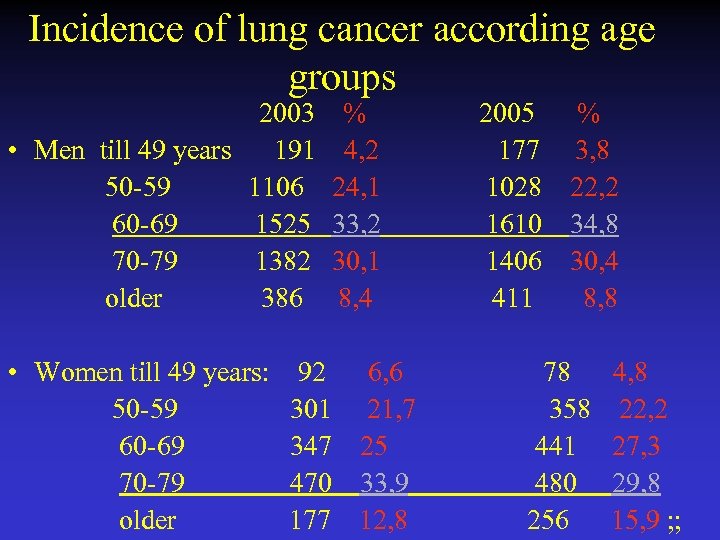
Incidence of lung cancer according age groups 2003 % 2005 % • Men till 49 years 191 4, 2 177 3, 8 50 -59 1106 24, 1 1028 22, 2 60 -69 1525 33, 2 1610 34, 8 70 -79 1382 30, 1 1406 30, 4 older 386 8, 4 411 8, 8 • Women till 49 years: 92 6, 6 78 4, 8 50 -59 301 21, 7 358 22, 2 60 -69 347 25 441 27, 3 70 -79 470 33, 9 480 29, 8 older 177 12, 8 256 15, 9 ; ;
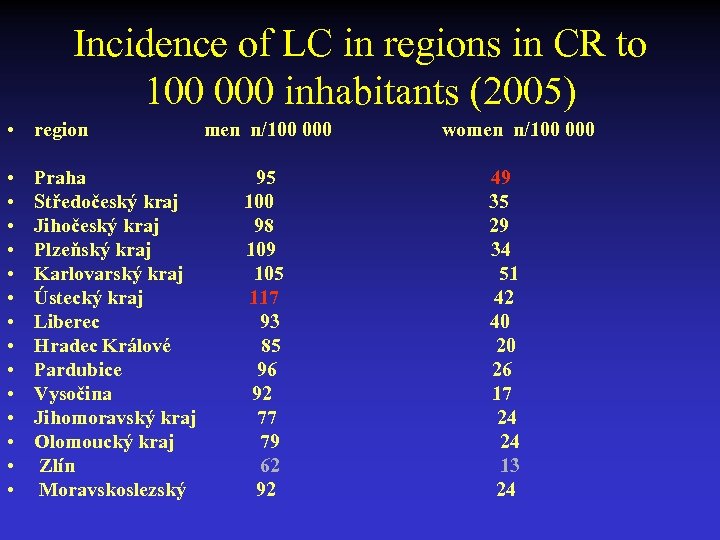
Incidence of LC in regions in CR to 100 000 inhabitants (2005) • region • • • • Praha Středočeský kraj Jihočeský kraj Plzeňský kraj Karlovarský kraj Ústecký kraj Liberec Hradec Králové Pardubice Vysočina Jihomoravský kraj Olomoucký kraj Zlín Moravskoslezský men n/100 000 95 100 98 109 105 117 93 85 96 92 77 79 62 92 women n/100 000 49 35 29 34 51 42 40 20 26 17 24 24 13 24
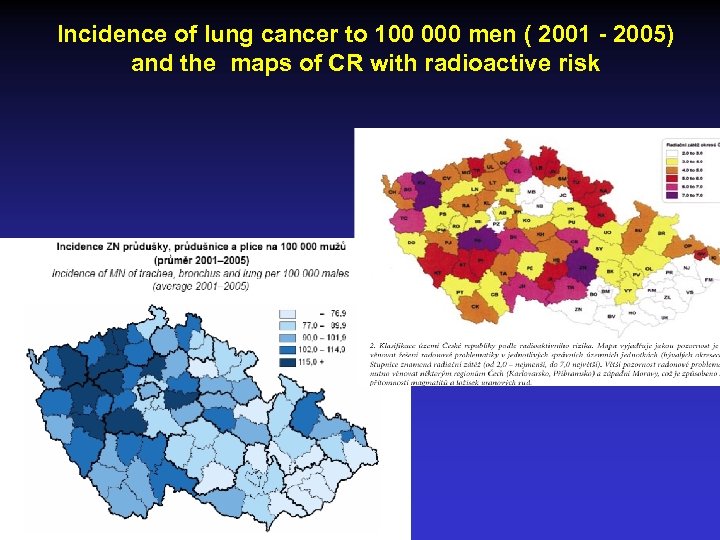
Incidence of lung cancer to 100 000 men ( 2001 - 2005) and the maps of CR with radioactive risk
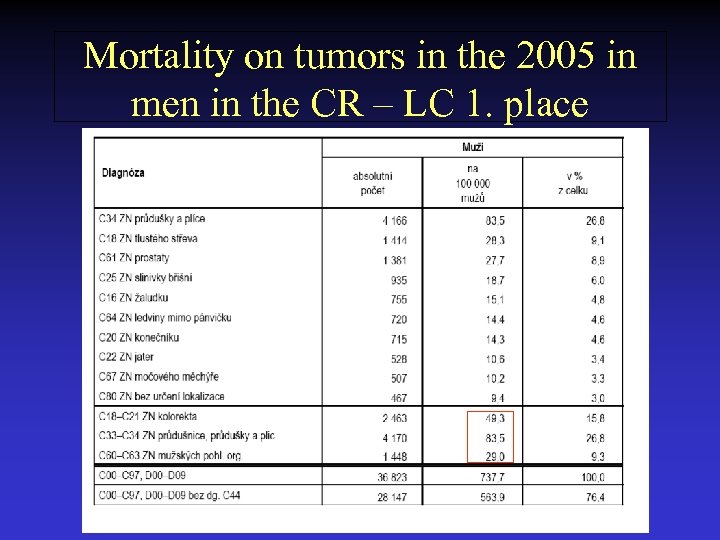
Mortality on tumors in the 2005 in men in the CR – LC 1. place
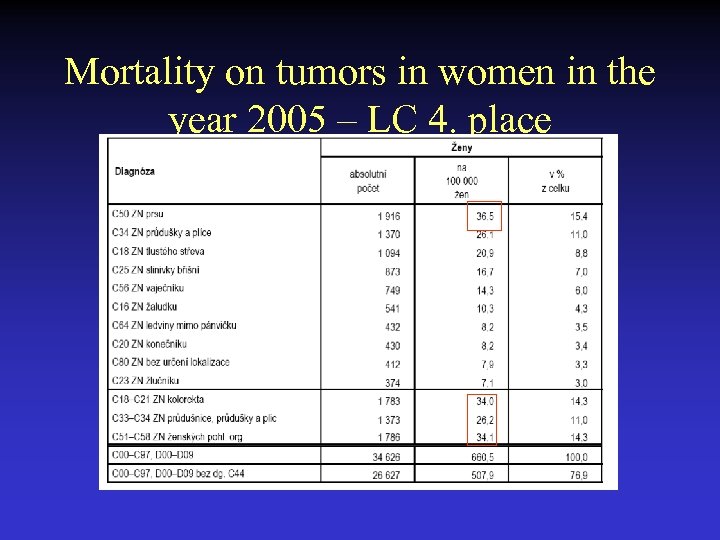
Mortality on tumors in women in the year 2005 – LC 4. place
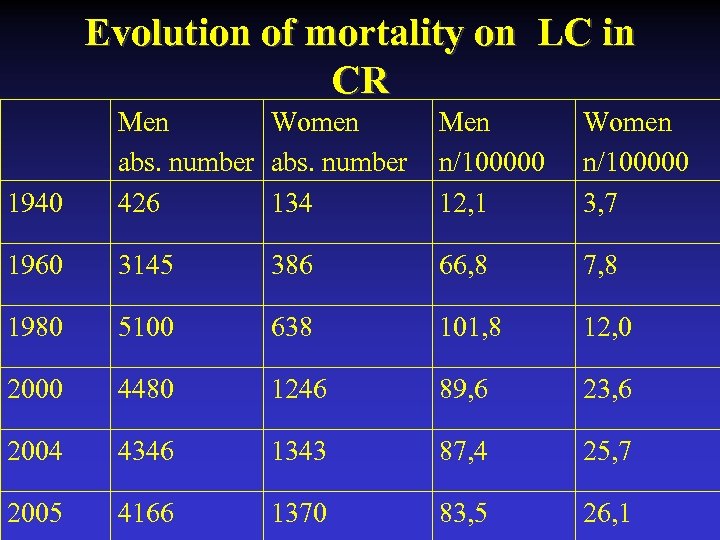
Evolution of mortality on LC in CR 1940 Men Women abs. number 426 134 Men n/100000 12, 1 Women n/100000 3, 7 1960 3145 386 66, 8 7, 8 1980 5100 638 101, 8 12, 0 2000 4480 1246 89, 6 23, 6 2004 4346 1343 87, 4 25, 7 2005 4166 1370 83, 5 26, 1
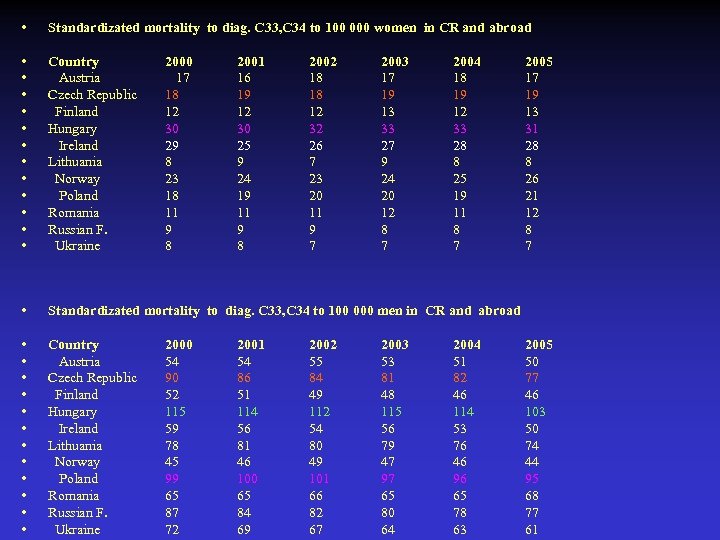
• Standardizated mortality to diag. C 33, C 34 to 100 000 women in CR and abroad • • • Country Austria Czech Republic Finland Hungary Ireland Lithuania Norway Poland Romania Russian F. Ukraine • Standardizated mortality to diag. C 33, C 34 to 100 000 men in CR and abroad • • • Country Austria Czech Republic Finland Hungary Ireland Lithuania Norway Poland Romania Russian F. Ukraine 2000 17 18 12 30 29 8 23 18 11 9 8 2000 54 90 52 115 59 78 45 99 65 87 72 2001 16 19 12 30 25 9 24 19 11 9 8 2001 54 86 51 114 56 81 46 100 65 84 69 2002 18 18 12 32 26 7 23 20 11 9 7 2002 55 84 49 112 54 80 49 101 66 82 67 2003 17 19 13 33 27 9 24 20 12 8 7 2003 53 81 48 115 56 79 47 97 65 80 64 2004 18 19 12 33 28 8 25 19 11 8 7 2004 51 82 46 114 53 76 46 96 65 78 63 2005 17 19 13 31 28 8 26 21 12 8 7 2005 50 77 46 103 50 74 44 95 68 77 61
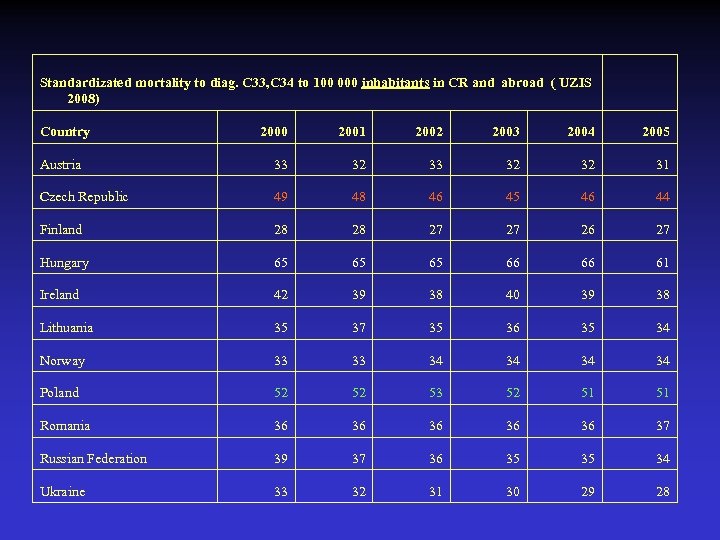
Standardizated mortality to diag. C 33, C 34 to 100 000 inhabitants in CR and abroad ( UZIS 2008) Country 2000 2001 2002 2003 2004 2005 Austria 33 32 32 31 Czech Republic 49 48 46 45 46 44 Finland 28 28 27 27 26 27 Hungary 65 65 65 66 66 61 Ireland 42 39 38 40 39 38 Lithuania 35 37 35 36 35 34 Norway 33 33 34 34 Poland 52 52 53 52 51 51 Romania 36 36 36 37 Russian Federation 39 37 36 35 35 34 Ukraine 33 32 31 30 29 28
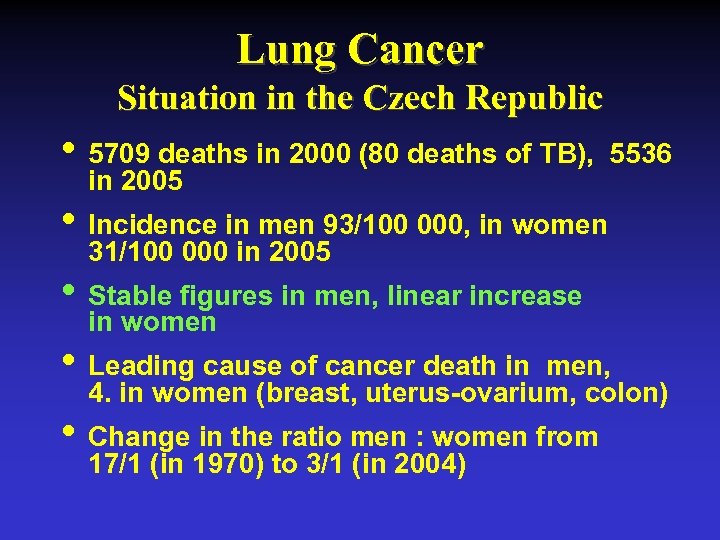
Lung Cancer Situation in the Czech Republic • 5709 deaths in 2000 (80 deaths of TB), 5536 in 2005 • Incidence in men 93/100 000, in women 31/100 000 in 2005 • Stable figures in men, linear increase in women • Leading cause of cancer death in men, 4. in women (breast, uterus-ovarium, colon) • Change in the ratio men : women from 17/1 (in 1970) to 3/1 (in 2004)

What are the Risk Factors for Lung Cancer? • Tobacco and secondhand smoke • Asbestos • Radon • Most people who develop lung cancer today have either stopped smoking years earlier or have never smoked

Lung cancer epidemic = Smoking epidemic • LC incidence follows the smoking incidence with the latency of 20 -30 years • USA smokers in 1965 - 42%, in 1995 - 25% (men) • WHO in 1998 47% men and 12% women are smokers in the world • Smoking related LC - 83 -94% in men, 57 -80% in women

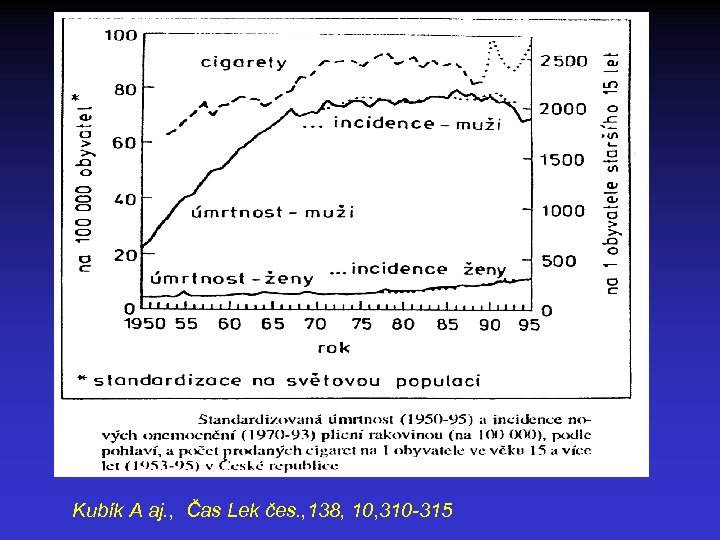
Kubík A aj. , Čas Lek čes. , 138, 10, 310 -315

Smoking and other risk factors • Smoking caused lung cancer in 94% men and 52% women (Kubík et al: Cancer, 1995, 7, 2452 -60) • CR: 40 % men and 25 % women in th age 30 - 60 years are smokers (UZIS ČR 2004) • 10 – 18 % of smokers will suffer from lung cancer • • • coincidence with chronic lung diseases genetic predisposition cumulative effect of risks !!



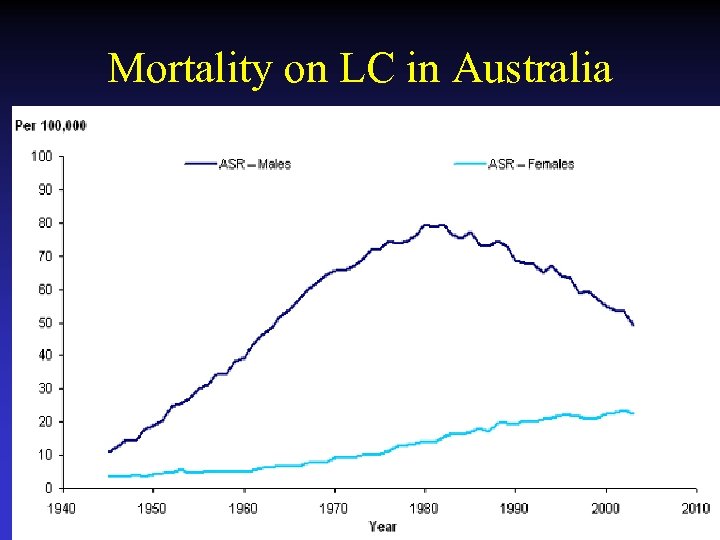
Mortality on LC in Australia
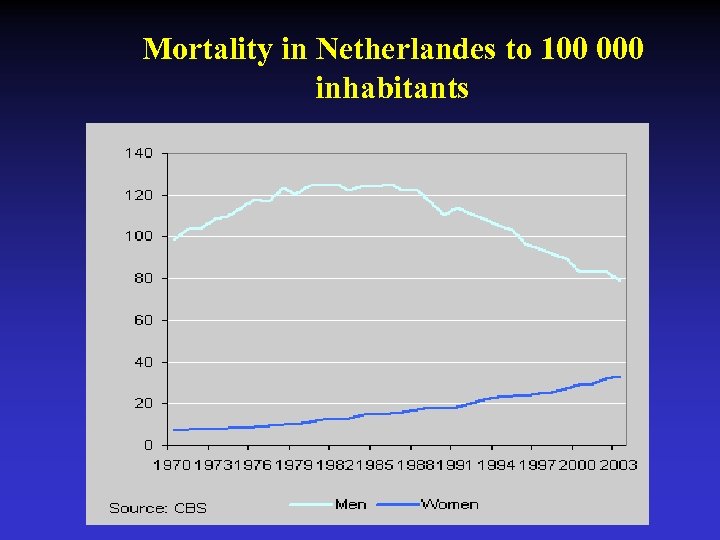
Mortality in Netherlandes to 100 000 inhabitants

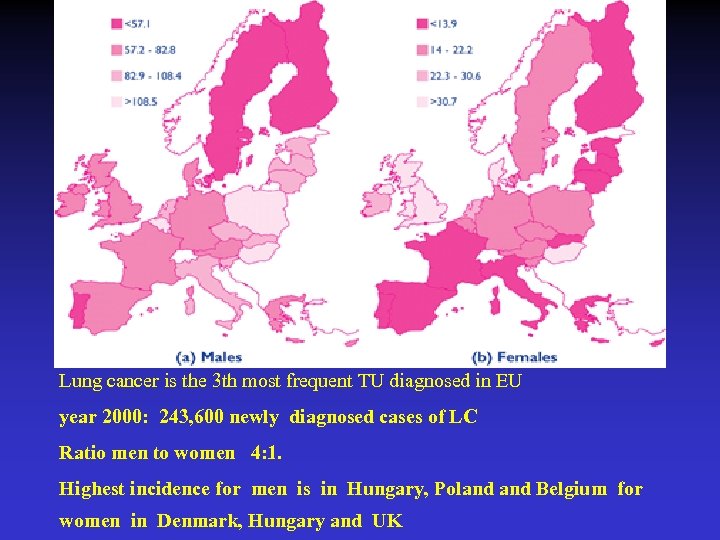
Lung cancer is the 3 th most frequent TU diagnosed in EU year 2000: 243, 600 newly diagnosed cases of LC Ratio men to women 4: 1. Highest incidence for men is in Hungary, Poland Belgium for women in Denmark, Hungary and UK
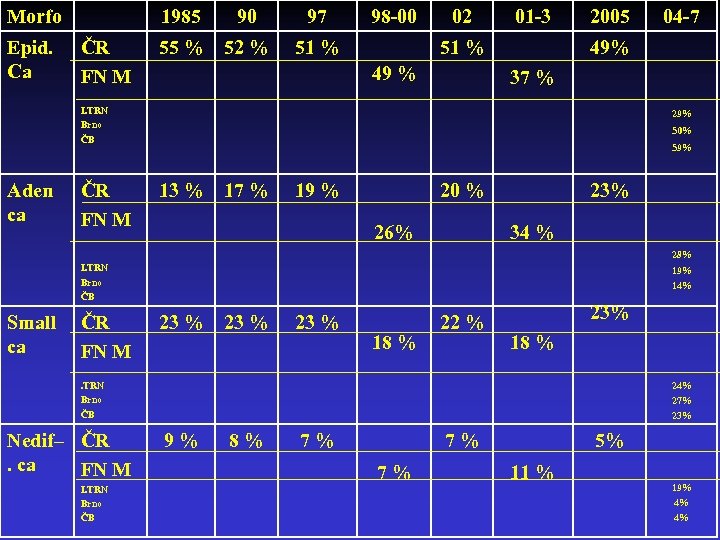
Morfo Epid. Ca 1985 ČR FN M 90 97 55 % 52 % 98 -00 51 % 02 01 -3 51 % 49 % 2005 49% 37 % I. TRN Brno ČB Aden ca ČR FN M 04 -7 29% 50% 59% 13 % 17 % 19 % 20 % 26% 23% 34 % 28% I. TRN Brno ČB Small ca ČR FN M 19% 14% 23 % 18 % 22 % 23% 18 % . TRN Brno ČB Nedif– ČR. ca FN M I. TRN Brno ČB 24% 27% 23% 9% 8% 7% 7% 7% 5% 11 % 19% 4% 4%
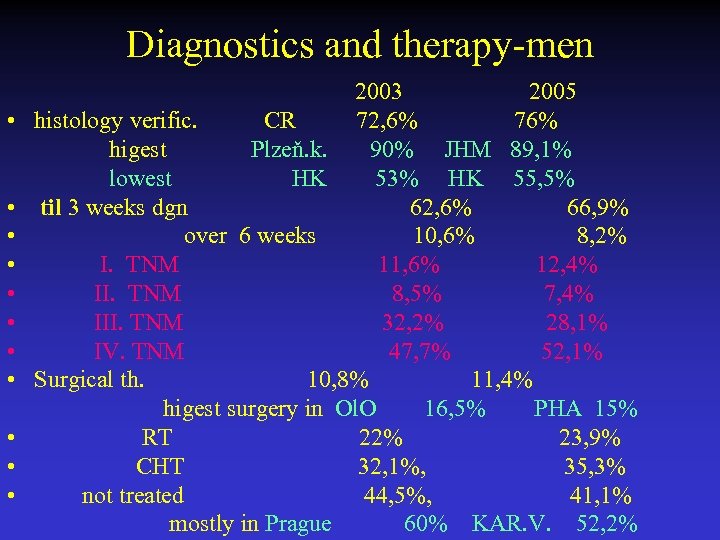
Diagnostics and therapy-men 2003 2005 • histology verific. CR 72, 6% 76% higest Plzeň. k. 90% JHM 89, 1% lowest HK 53% HK 55, 5% • til 3 weeks dgn 62, 6% 66, 9% • over 6 weeks 10, 6% 8, 2% • I. TNM 11, 6% 12, 4% • II. TNM 8, 5% 7, 4% • III. TNM 32, 2% 28, 1% • IV. TNM 47, 7% 52, 1% • Surgical th. 10, 8% 11, 4% higest surgery in Ol. O 16, 5% PHA 15% • RT 22% 23, 9% • CHT 32, 1%, 35, 3% • not treated 44, 5%, 41, 1% mostly in Prague 60% KAR. V. 52, 2%
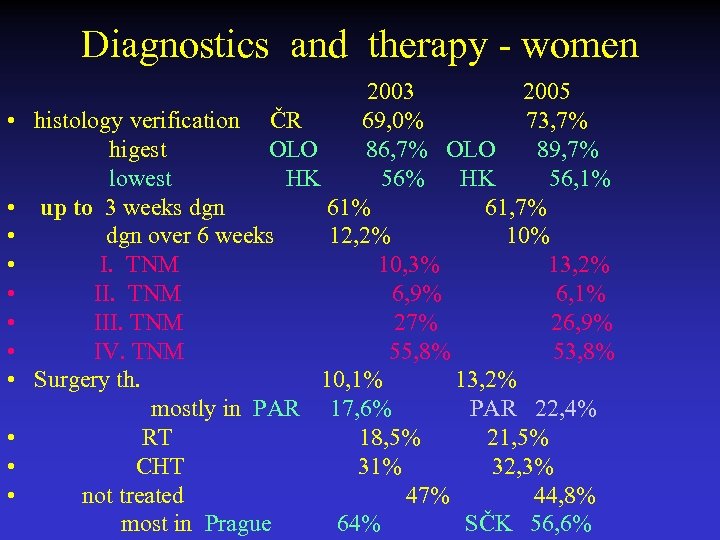
Diagnostics and therapy - women 2003 2005 • histology verification ČR 69, 0% 73, 7% higest OLO 86, 7% OLO 89, 7% lowest HK 56% HK 56, 1% • up to 3 weeks dgn 61% 61, 7% • dgn over 6 weeks 12, 2% 10% • I. TNM 10, 3% 13, 2% • II. TNM 6, 9% 6, 1% • III. TNM 27% 26, 9% • IV. TNM 55, 8% 53, 8% • Surgery th. 10, 1% 13, 2% mostly in PAR 17, 6% PAR 22, 4% • RT 18, 5% 21, 5% • CHT 31% 32, 3% • not treated 47% 44, 8% most in Prague 64% SČK 56, 6%
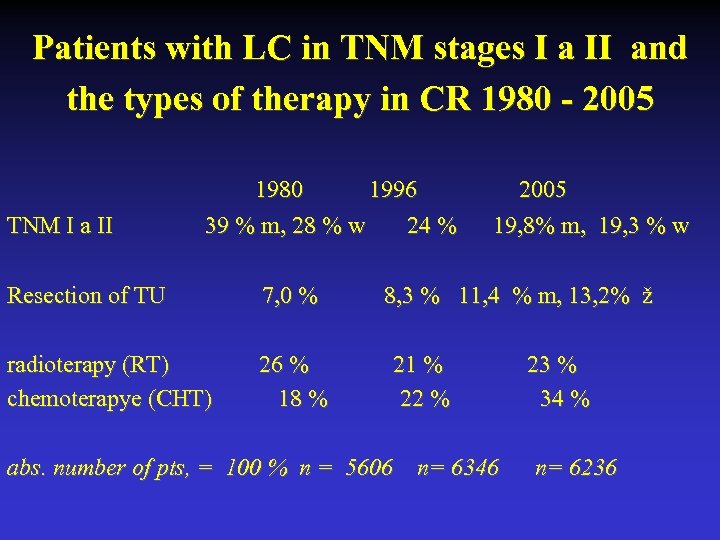
Patients with LC in TNM stages I a II and the types of therapy in CR 1980 - 2005 1980 1996 2005 TNM I a II 39 % m, 28 % w 24 % 19, 8% m, 19, 3 % w Resection of TU 7, 0 % 8, 3 % 11, 4 % m, 13, 2% ž radioterapy (RT) 26 % 21 % 23 % chemoterapye (CHT) 18 % 22 % 34 % abs. number of pts, = 100 % n = 5606 n= 6346 n= 6236
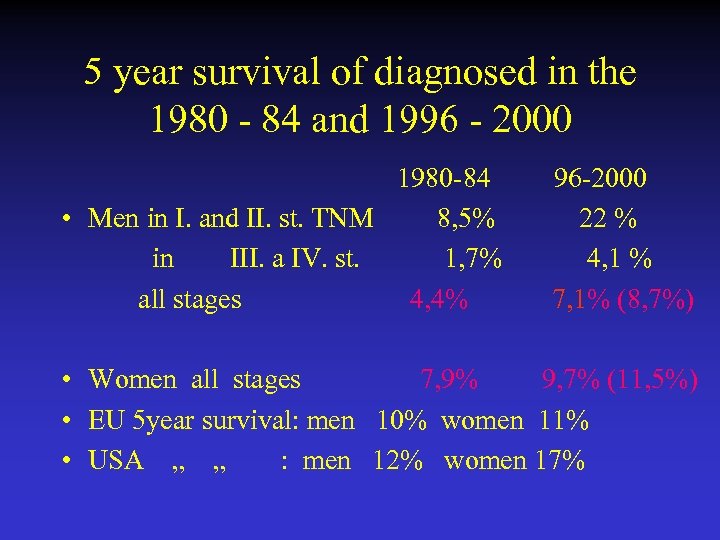
5 year survival of diagnosed in the 1980 - 84 and 1996 - 2000 1980 -84 96 -2000 • Men in I. and II. st. TNM 8, 5% 22 % in III. a IV. st. 1, 7% 4, 1 % all stages 4, 4% 7, 1% (8, 7%) • Women all stages 7, 9% 9, 7% (11, 5%) • EU 5 year survival: men 10% women 11% • USA „ „ : men 12% women 17%

Epidemiological conclusions • decreasing incidence of LC in men, plateau point of incidence of women is nearing…hopefully • problems - high incidence of LC in men - late diagnostics - operability only 11, 4% in men and 13, 2 in women - in 2003 5995 new cases , died 5568 !! - number of „ NO therapy“ didnt change in the past 20 years • pozitive data - we did not reached the highest EU incidence in women - increased 5 year survival - higher level of verification of LC - lower late diagnosed pts over 6 weeks •
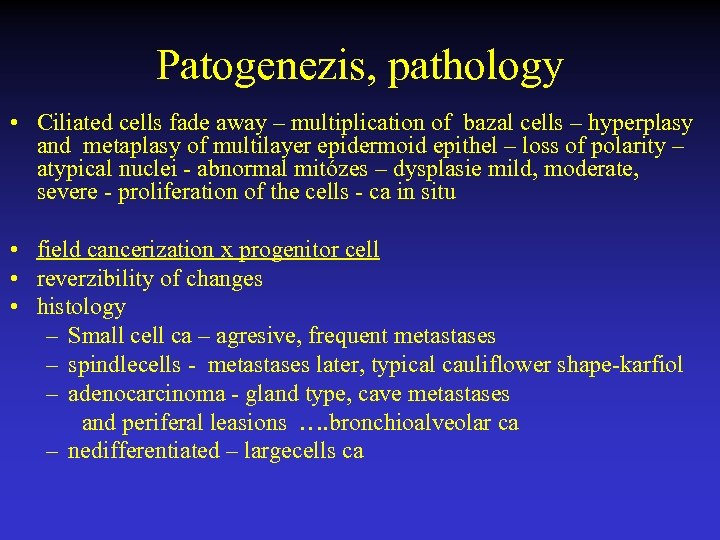
Patogenezis, pathology • Ciliated cells fade away – multiplication of bazal cells – hyperplasy and metaplasy of multilayer epidermoid epithel – loss of polarity – atypical nuclei - abnormal mitózes – dysplasie mild, moderate, severe - proliferation of the cells - ca in situ • field cancerization x progenitor cell • reverzibility of changes • histology – Small cell ca – agresive, frequent metastases – spindlecells - metastases later, typical cauliflower shape-karfiol – adenocarcinoma - gland type, cave metastases and periferal leasions …. bronchioalveolar ca – nedifferentiated – largecells ca

Pathology- 2 • Development of cancer in the mucosa up to 15 years • doubling time • death - 1 kg tumoros mass doubling timeí years to diagnozes to death • Small cell ca 29 days squamous ca 88 days adenoca 161 days 2, 8 3, 2 8, 4 9, 6 15, 4 17, 6 • central, peripheral Metastases to - liver, suprarenal glands, bone, brain • Direct invasion • lymphatica • hematogenes
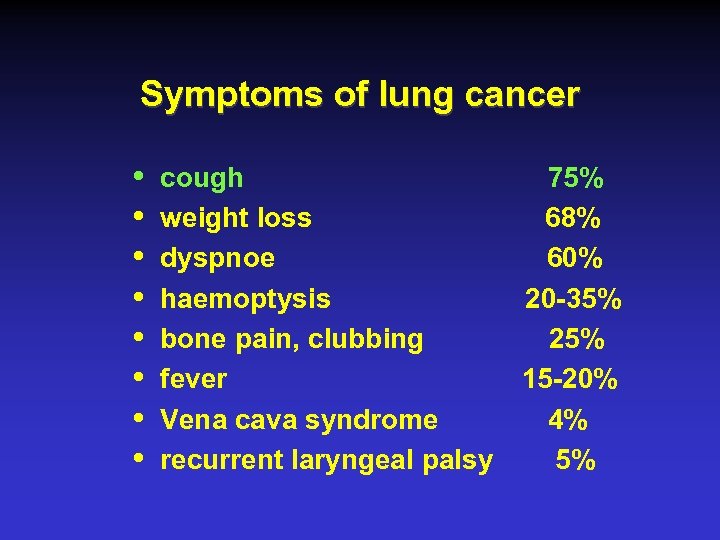
Symptoms of lung cancer i i i i cough 75% weight loss 68% dyspnoe 60% haemoptysis 20 -35% bone pain, clubbing 25% fever 15 -20% Vena cava syndrome 4% recurrent laryngeal palsy 5%

What are the Symptoms of Lung Cancer? • • Fatigue (tiredness) Cough Shortness of breath Chest pain Loss of appetite Coughing up phlegm Hemoptysis (coughing up blood) If cancer has spread, symptoms include bone pain, difficulty breathing, abdominal or back pain, headache, weakness, and speech difficulties

Paraneoplastic syndrome Endocrine syndromes • Cushing´s sy (ACTH) 2 -7%, SCLC 30 -50%, • Nonmetastatic hypercalcemia - squamous ca 15% • Inappropriate antidiuretic hormone in SCLC , hyponatremia, urine osmolarity over 500 m. Osm. kg-1 • Gynecomastia (HCG)

Paraneoplastic syndrome Neurological syndrome • Symptoms peripheral neuropathy, encephalomyelitis • Lambert Eaton myasthenic syndrome Cutaneous • Erythema gyratum repens, acanthosis nigrans Haematological • microcytic anemia in 20%, haemostatic disturbance Clubbing Anorexia, nausea, vomiting
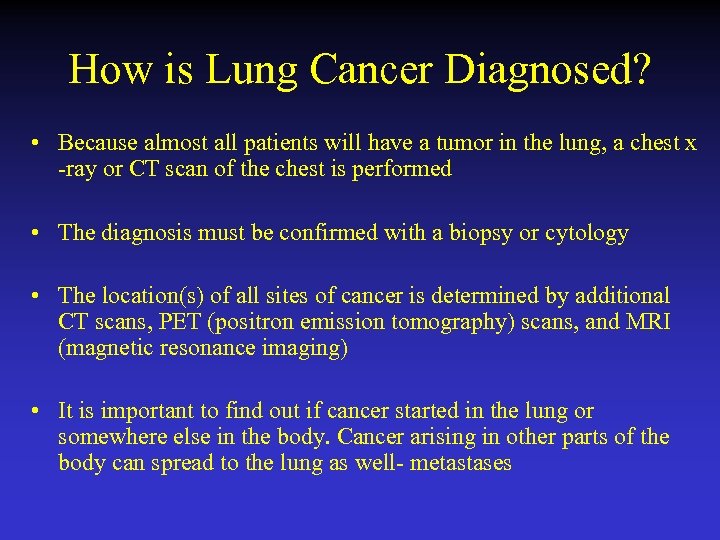
How is Lung Cancer Diagnosed? • Because almost all patients will have a tumor in the lung, a chest x -ray or CT scan of the chest is performed • The diagnosis must be confirmed with a biopsy or cytology • The location(s) of all sites of cancer is determined by additional CT scans, PET (positron emission tomography) scans, and MRI (magnetic resonance imaging) • It is important to find out if cancer started in the lung or somewhere else in the body. Cancer arising in other parts of the body can spread to the lung as well- metastases
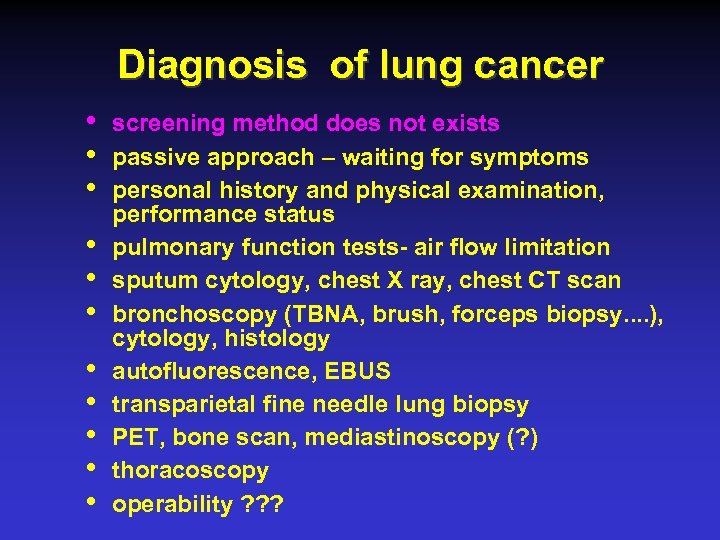
Diagnosis of lung cancer • • • screening method does not exists passive approach – waiting for symptoms personal history and physical examination, performance status pulmonary function tests- air flow limitation sputum cytology, chest X ray, chest CT scan bronchoscopy (TBNA, brush, forceps biopsy. . ), cytology, histology autofluorescence, EBUS transparietal fine needle lung biopsy PET, bone scan, mediastinoscopy (? ) thoracoscopy operability ? ? ?

Staging of lung cancer prognosis therapy NSCLC – SCLC
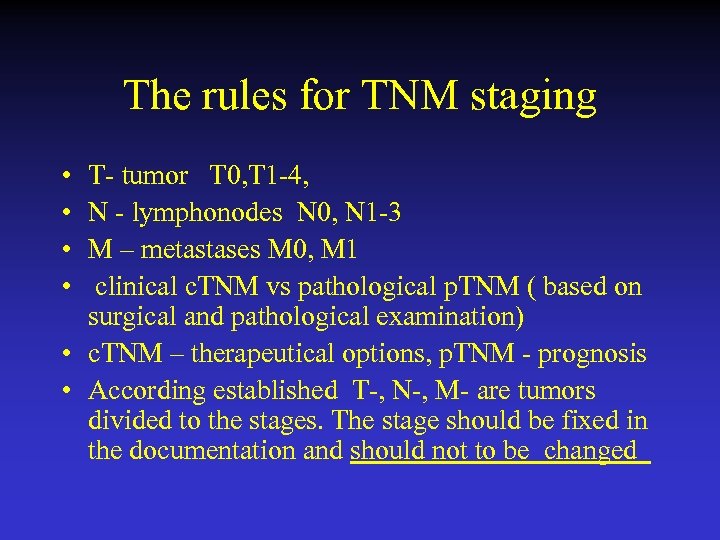
The rules for TNM staging • • T- tumor T 0, T 1 -4, N - lymphonodes N 0, N 1 -3 M – metastases M 0, M 1 clinical c. TNM vs pathological p. TNM ( based on surgical and pathological examination) • c. TNM – therapeutical options, p. TNM - prognosis • According established T-, N-, M- are tumors divided to the stages. The stage should be fixed in the documentation and should not to be changed
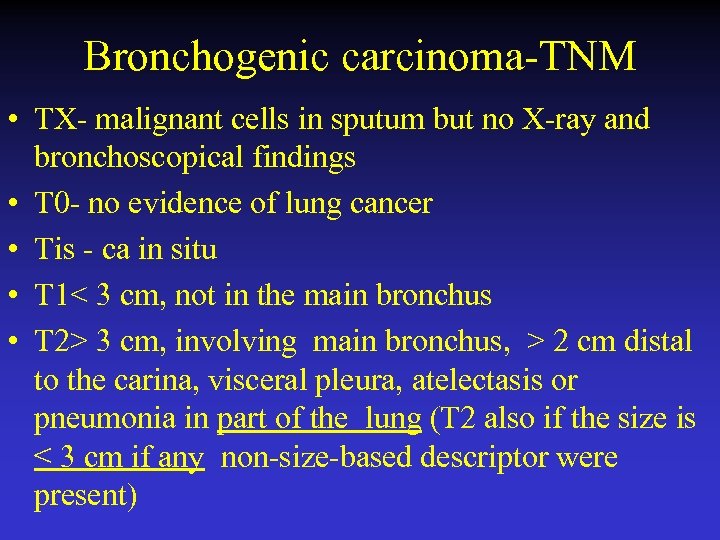
Bronchogenic carcinoma-TNM • TX- malignant cells in sputum but no X-ray and bronchoscopical findings • T 0 - no evidence of lung cancer • Tis - ca in situ • T 1< 3 cm, not in the main bronchus • T 2> 3 cm, involving main bronchus, > 2 cm distal to the carina, visceral pleura, atelectasis or pneumonia in part of the lung (T 2 also if the size is ≤ 3 cm if any non-size-based descriptor were present)
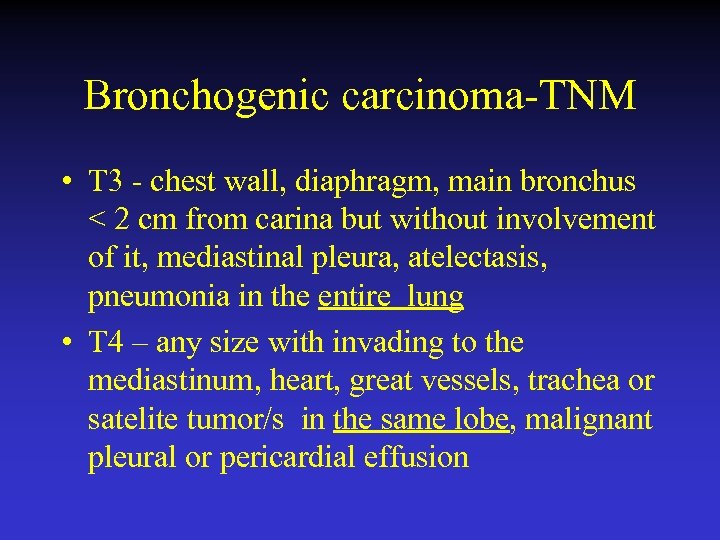
Bronchogenic carcinoma-TNM • T 3 - chest wall, diaphragm, main bronchus < 2 cm from carina but without involvement of it, mediastinal pleura, atelectasis, pneumonia in the entire lung • T 4 – any size with invading to the mediastinum, heart, great vessels, trachea or satelite tumor/s in the same lobe, malignant pleural or pericardial effusion
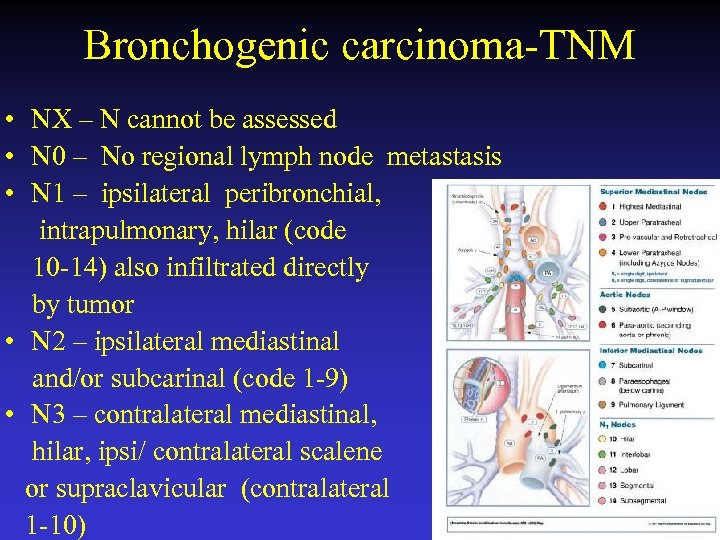
Bronchogenic carcinoma-TNM • NX – N cannot be assessed • N 0 – No regional lymph node metastasis • N 1 – ipsilateral peribronchial, intrapulmonary, hilar (code 10 -14) also infiltrated directly by tumor • N 2 – ipsilateral mediastinal and/or subcarinal (code 1 -9) • N 3 – contralateral mediastinal, hilar, ipsi/ contralateral scalene or supraclavicular (contralateral 1 -10)
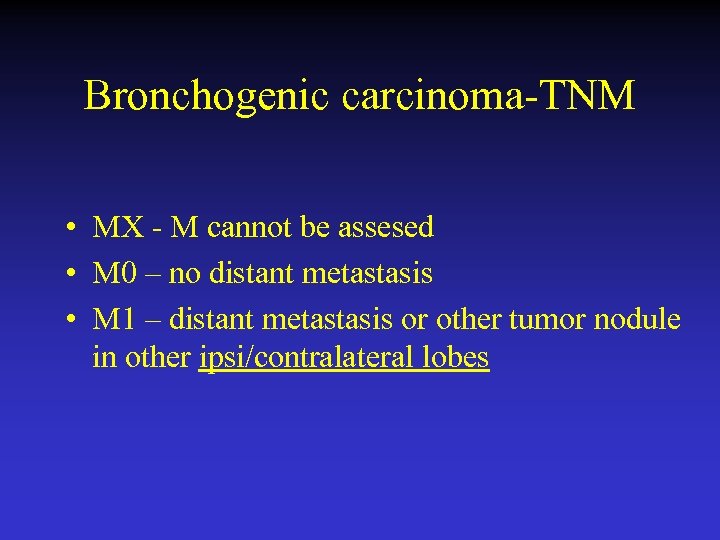
Bronchogenic carcinoma-TNM • MX - M cannot be assesed • M 0 – no distant metastasis • M 1 – distant metastasis or other tumor nodule in other ipsi/contralateral lobes
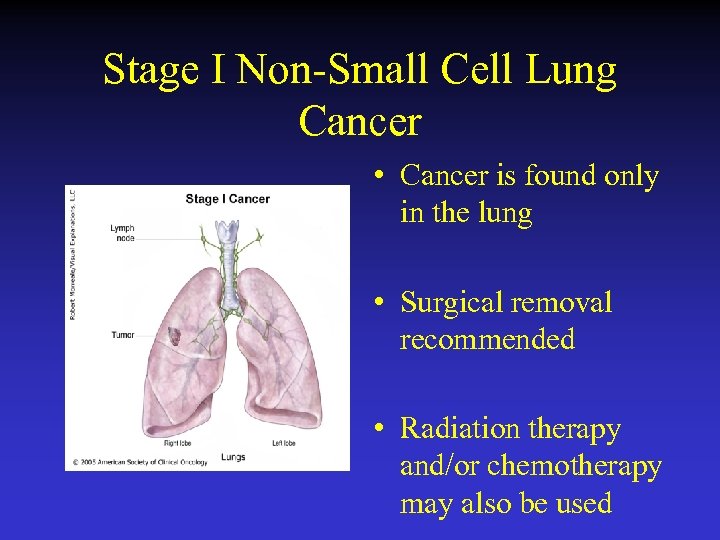
Stage I Non-Small Cell Lung Cancer • Cancer is found only in the lung • Surgical removal recommended • Radiation therapy and/or chemotherapy may also be used
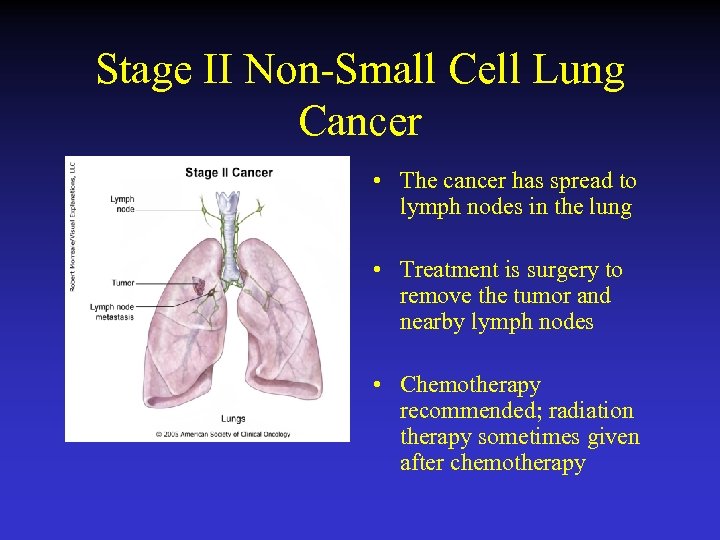
Stage II Non-Small Cell Lung Cancer • The cancer has spread to lymph nodes in the lung • Treatment is surgery to remove the tumor and nearby lymph nodes • Chemotherapy recommended; radiation therapy sometimes given after chemotherapy
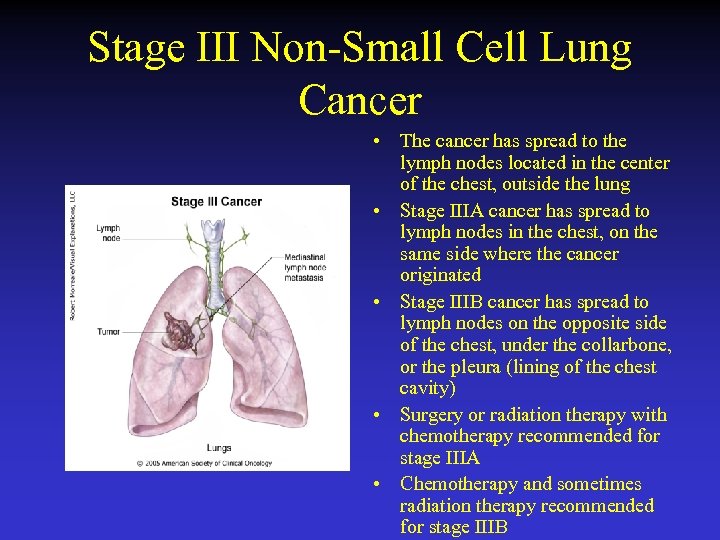
Stage III Non-Small Cell Lung Cancer • The cancer has spread to the lymph nodes located in the center of the chest, outside the lung • Stage IIIA cancer has spread to lymph nodes in the chest, on the same side where the cancer originated • Stage IIIB cancer has spread to lymph nodes on the opposite side of the chest, under the collarbone, or the pleura (lining of the chest cavity) • Surgery or radiation therapy with chemotherapy recommended for stage IIIA • Chemotherapy and sometimes radiation therapy recommended for stage IIIB
Stage IV Non-Small Cell Lung Cancer • The cancer has spread to different lobes of the lung or to other organs, such as the brain, bones, and liver • Stage IV non-small cell lung cancer is treated with chemotherapy • More information can be found in the What to Know: ASCO’s Guideline on Advanced Lung Cancer

Other characteristic of tumors • G- histopathological grading GX not assesed G 1 well differentiated G 2 middle grade of differentiation G 3 poorly differentiated G 4 non differentiated • Resection of tumor under 2 cm in 160 pts with stage I TNM. In every pts were checked clinicopathological features : sex, age, smoking habits, CEA, and histopathological grade. • Results: pts with poorly differentiated carcinomas showed significantly unfavorable survival p< 0, 001 compared with pts with well-moderately differentiated carcinomas. Kobayashi N et al: J Thorac Oncol 2007, September, 2(9): 808 -12 • R- residual tumor after treatment RX residual tumor not evaluated R 0 without residual tumor R 1 microscopic residue of tumor R 2 macroscopic residue of tumor
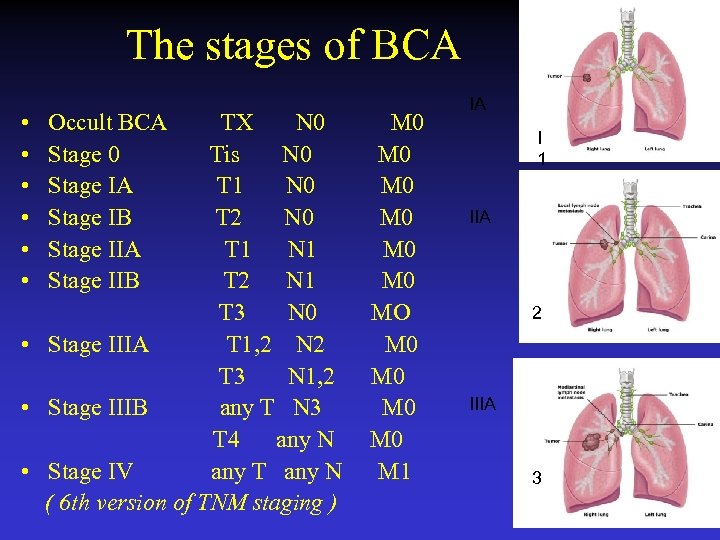
The stages of BCA • Occult BCA TX N 0 M 0 • Stage 0 Tis N 0 M 0 • Stage IA T 1 N 0 M 0 • Stage IB T 2 N 0 M 0 • Stage IIA T 1 N 1 M 0 • Stage IIB T 2 N 1 M 0 T 3 N 0 MO • Stage IIIA T 1, 2 N 2 M 0 T 3 N 1, 2 M 0 • Stage IIIB any T N 3 M 0 T 4 any N M 0 • Stage IV any T any N M 1 ( 6 th version of TNM staging ) IA I 1 IIA 2 IIIA 3

Lung Cancer Staging • Staging is a way of describing a cancer, such as the size of a tumor and if or where it has spread • Staging is the most important tool doctors have to determine a patient’s prognosis • The type of treatment a person receives depends on the stage of the cancer • Staging is different for non-small cell lung cancer and small cell lung cancer • Recurrent cancer is cancer that comes back after treatment

Small Cell Lung Cancer: All Stages • Classified as limited stage (confined to one area of the chest) or extensive stage (not confined to one area of the chest) • Patients with limited stage small cell lung cancer are best treated with simultaneous radiation therapy and chemotherapy • Patients with extensive stage small cell lung cancer are treated with chemotherapy • In patients whose tumors have shrunk after chemotherapy, preventive radiation therapy to the head cuts the risk that the cancer will spread to the brain and extends patients’ the lives
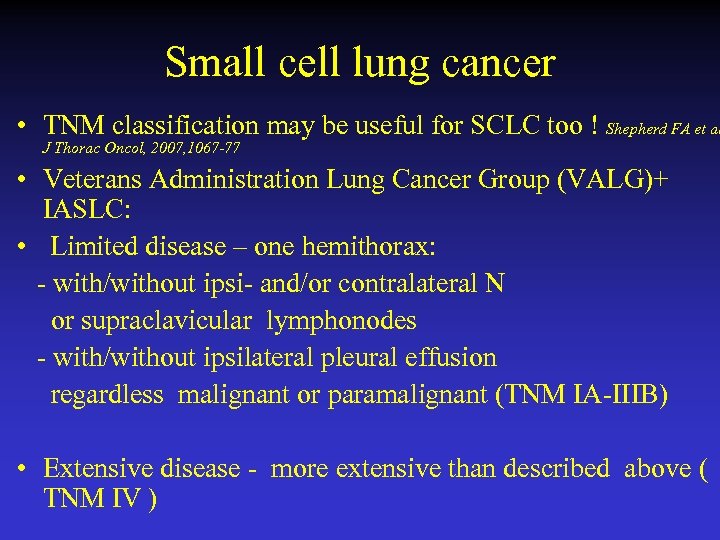
Small cell lung cancer • TNM classification may be useful for SCLC too ! Shepherd FA et al J Thorac Oncol, 2007, 1067 -77 • Veterans Administration Lung Cancer Group (VALG)+ IASLC: • Limited disease – one hemithorax: - with/without ipsi- and/or contralateral N or supraclavicular lymphonodes - with/without ipsilateral pleural effusion regardless malignant or paramalignant (TNM IA-IIIB) • Extensive disease - more extensive than described above ( TNM IV )
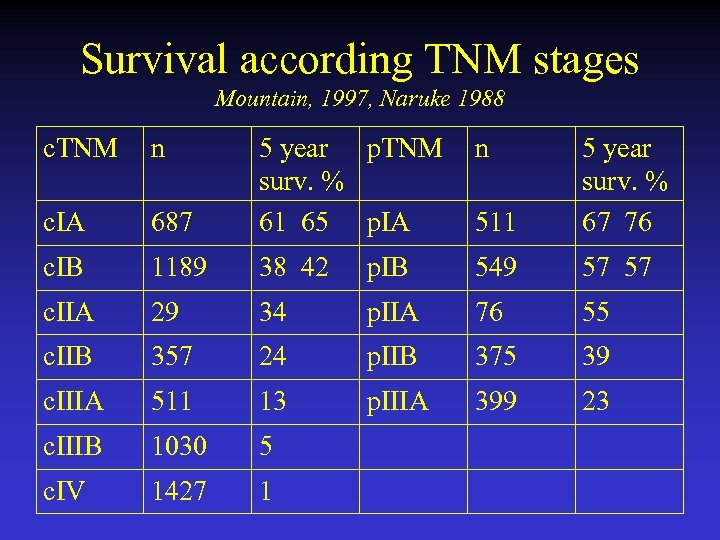
Survival according TNM stages Mountain, 1997, Naruke 1988 c. TNM n n 687 5 year p. TNM surv. % 61 65 p. IA 511 5 year surv. % 67 76 c. IA c. IB 1189 38 42 p. IB 549 57 57 c. IIA 29 34 p. IIA 76 55 c. IIB 357 24 p. IIB 375 39 c. IIIA 511 13 p. IIIA 399 23 c. IIIB 1030 5 c. IV 1427 1
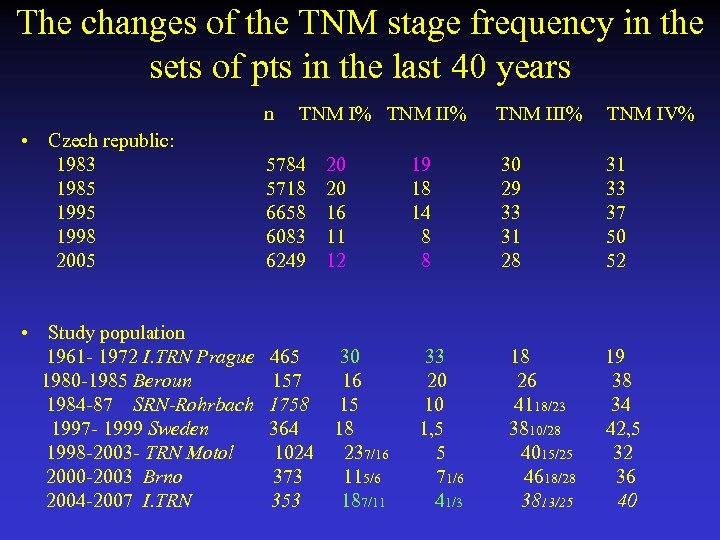
The changes of the TNM stage frequency in the sets of pts in the last 40 years n TNM I% TNM II% TNM IV% • Czech republic: 1983 5784 20 19 30 31 1985 5718 20 18 29 33 1995 6658 16 14 33 37 1998 6083 11 8 31 50 2005 6249 12 8 28 52 • Study population 1961 - 1972 I. TRN Prague 465 30 33 18 19 1980 -1985 Beroun 157 16 20 26 38 1984 -87 SRN-Rohrbach 1758 15 10 4118/23 34 1997 - 1999 Sweden 364 18 1, 5 3810/28 42, 5 1998 -2003 - TRN Motol 1024 237/16 5 4015/25 32 2000 -2003 Brno 373 115/6 71/6 4618/28 36 2004 -2007 I. TRN 353 187/11 41/3 3813/25 40
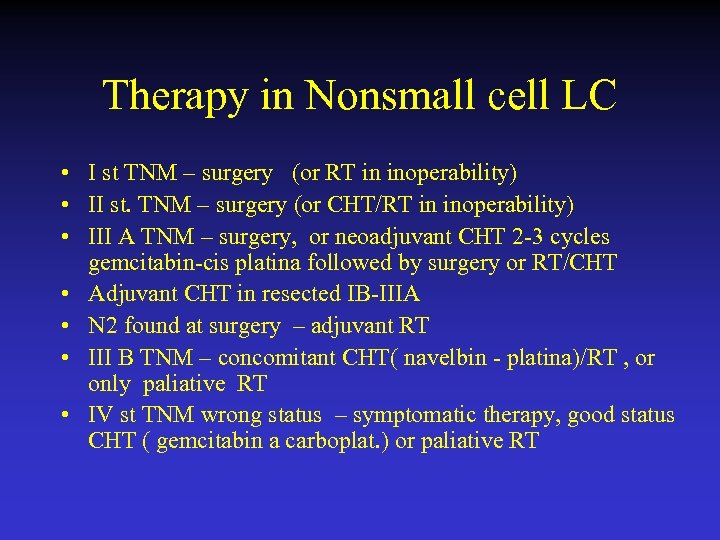
Therapy in Nonsmall cell LC • I st TNM – surgery (or RT in inoperability) • II st. TNM – surgery (or CHT/RT in inoperability) • III A TNM – surgery, or neoadjuvant CHT 2 -3 cycles gemcitabin-cis platina followed by surgery or RT/CHT • Adjuvant CHT in resected IB-IIIA • N 2 found at surgery – adjuvant RT • III B TNM – concomitant CHT( navelbin - platina)/RT , or only paliative RT • IV st TNM wrong status – symptomatic therapy, good status CHT ( gemcitabin a carboplat. ) or paliative RT

Therapy in Small Cell Lung Cancer • Limited disease: good general condition - CHT ( cis platin + etoposide) and concomitant normo/ hyperfractionated radiotherapy (RT) from 1. cycle 45 -55 Gy • I stage surgery and adj CHT, • LD SCLC wrong general status, polymorbidity, sequence CHT-5 cycles/RT up to 60 Gy • Extensive diseases – 6 x CHT ( etoposid-carboplatin) • Relaps till 3 months - other CHT ( gemcitabin, taxany, ifosf. , topotecan aj. ) • Relaps over 3 months the same CHT as in 1. line

How is Lung Cancer Treated? • Treatment depends on the stage and type of lung cancer • Surgery • Radiation therapy • Chemotherapy (options include a combination of drugs) • Targeted therapy • Lung cancer is usually treated with a combination of therapies

Cancer Treatment: Surgery • The tumor and the nearby lymph nodes in the chest are typically removed to offer the best chance for cure • For non-small cell lung cancer, a lobectomy (removal of the entire lobe where the tumor is located), has shown to be most effective • Surgery may not be possible in some patients
Cancer Treatment: Adjuvant Therapy • Treatment given after surgery to lower the risk of the cancer returning • May include chemotherapy, radiation therapy, and targeted therapy • More information may be found in the What to Know: ASCO’s Guideline on Adjuvant Treatment for Lung Cancer

Cancer Treatment: Radiation Therapy • The use of high-energy x-rays to destroy cancer cells • Side effects include fatigue, malaise (feeling unwell), loss of appetite, and skin irritation at the treatment site • Radiation pneumonitis is the irritation and inflammation of the lung; occurs in 15% of patients • It is important that the radiation treatments avoid the healthy parts of the lung

Cancer Treatment: Chemotherapy • Use of drugs to kill cancer cells • A combination of medications is often used • May be prescribed before or after surgery, or before, during, or after radiation therapy • Can improve survival and lessen lung cancer symptoms in all patients, even those with widespread lung cancer

Cancer Treatment: Targeted Therapy • Treats lung cancer by stopping the action of abnormal proteins that cause cells to grow and divide out of control • Bevacizumab (Avastin) prevents the formation of new blood vessels, which help feed the growth and spread of a tumor; given with chemotherapy • Erlotinib (Tarceva) approved for locally advanced and metastatic non-small cell lung cancer • Cetuximab (Erbitux) (monocklonal AB Ig. G 1, which bind to receptor of epidermal growth factor (EGFR). Cetuximab is highly specific with higher afinity to this receptor than - epidermal growth factor (EGF) and transforming growth factor alfa (TGF-alfa)) may be given with chemotherapy in situations where bevacizumab may be unsafe

The Role of Clinical Trials for the Treatment of Lung Cancer • Clinical trials are research studies involving people • They test new treatment and prevention methods to determine whether they are safe, effective, and better than the standard treatment • The purpose of a clinical trial is to answer a specific medical question in a highly structured, controlled process • Clinical trials can evaluate methods of cancer prevention, screening, diagnosis, treatment, and/or quality of life

Clinical Trials: Patient Safety • Informed consent: participants should understand why they are being offered entry into a clinical trial and the potential benefits and risks; informed consent is an ongoing process • Participation is always voluntary, and patients can leave the trial at any time • Other safeguards exist to ensure ongoing patient safety

Clinical Trials: Phases • Phase I trials determine the safety and dose of a new treatment in a small group of people • Phase II trials provide more detail about the safety of the new treatment and determine how well it works for treating a specific type of cancer • Phase III trials take a new treatment that has shown promising results when used to treat a small number of patients with cancer and compare it with the standard treatment for that disease; phase III trials involve a large number of patients

Clinical Trials Resources • Coalition of Cancer Cooperative Groups (www. Cancer. Trials. Help. org) • Center. Watch (www. centerwatch. com) • National Cancer Institute (www. cancer. gov/clinical_trials) • Emerging. Med (www. emergingmed. com)

Living With Lung Cancer • Many people with lung cancer feel that they will not receive as much support or help from people around them because they believe others will think that their behavior caused the disease • Doctors and other members of the health care team can help patients and families cope with a diagnosis of lung cancer • Patients can take comfort knowing that the advances being made in the diagnosis and treatment of lung cancer will provide more and more patients with a chance for cure

Coping with Side Effects • • Side effects are treatable; talk with the doctor or nurse Fatigue is a common, treatable side effect Pain is treatable; non-narcotic pain-relievers are available Antiemetic drugs can reduce or prevent nausea and vomiting • Medications and extra oxygen can improve breathing • Radiation therapy or surgery can be used to treat metastases that are causing pain or other symptoms • For more information, visit www. cancer. net/sideeffects

After Treatment • Patients with lung cancer face the risk of cancer growing back or the development of a new lung cancer. All patients must follow up with their doctors for regular x-rays, scans, and check-ups • Quitting smoking helps recovery and health. Patients who have developed lung cancer who then stop live longer. It is never too late to stop smoking • Many survivors are at high risk for heart disease, stroke, emphysema, and chronic bronchitis; some cancer treatments increase this risk • Walking for 15 to 30 minutes each day can improve lung and heart functioning

Where to Find More Information Cancer. Net Guide to Lung Cancer (www. cancer. net/lung) • • • Overview Medical Illustrations Risk Factors Symptoms Diagnosis Staging With Illustrations • Treatment • Clinical Trials • Living With Lung Cancer • Side Effects • After Treatment • Current Research • Questions to Ask the Doctor • Patient Information Resources

Cancer. Net (www. cancer. net) • Comprehensive, oncologist-approved cancer information • Guides to more than 120 types of cancer and cancer-related syndromes • Coping resources • Survivorship information • Cancer information in Spanish • Weekly feature articles • The latest cancer news • For patient information resources, please call 888651 -3038

Adjuvant Chemotherapy and Adjuvant Radiation Therapy for Stages I-IIIA Resectable Non-Small Cell Lung Cancer Guideline Cancer Care Ontario and American Society of Clinical Oncology

Introduction • The Cancer Care Ontario (CCO) Program in Evidencebased Care (PEBC) and the American Society of Clinical Oncology (ASCO) convened an expert panel in August 2006 to review the evidence and draft recommendations on the role of adjuvant chemotherapy and adjuvant radiation therapy for completely resected stages I-IIIA non-small cell lung cancer (NSCLC). • CCO originally published guidelines in 1997 and updated them in 2004 -2006. • Both CCO-PEBC and ASCO conducted external reviews of the current guidelines.

2007 Recommendations for Adjuvant Treatment of Stages I-IIIA NSCLC Clinical Questions 1. What is the benefit in terms of overall survival of adjuvant chemotherapy in patients with completely resected stages I – IIIA non-small cell lung cancer? 2. What is the benefit in terms of overall survival of adjuvant radiation therapy in patients with completely resected stages I – IIIA non-small cell lung cancer? 3. What roles should adjuvant chemotherapy and adjuvant radiation therapy play in completely resected stages I – IIIA non-small cell lung cancer?

2007 Recommendations for Adjuvant Treatment of Stages I-IIIA NSCLC Adjuvant Cisplatin-Based Chemotherapy • Stage IA: Adjuvant chemotherapy is not recommended • Stage IB: Adjuvant cisplatin-based chemotherapy is not recommended for routine use. • Stage IIA: Adjuvant cisplatin-based chemotherapy is recommended. • Stage IIB: Adjuvant cisplatin-based chemotherapy is recommended. • Stage IIIA: Adjuvant cisplatin-based chemotherapy is recommended. • The use of adjuvant chemotherapy regimens that include alkylating agents is not recommended as these agents have been found to be detrimental to survival. v Recommendations apply only to completely resected tumors.

Recommended Dose: Adjuvant Chemotherapy for Stages IIA-IIIA NSCLC Cisplatin-Vinorelbine • Cisplatin: 50 mg/m 2 on days 1 and 8 every four weeks for four cycles, and • Vinorelbine: 25 mg/m 2 weekly for 16 weeks for four cycles • Considerations: – Convenience for patients – Patients’ resource constraints – The use of one cisplatin-based chemotherapy regimen consistently in order to ensure familiarity and optimize patient safety

2007 Recommendations for Adjuvant Treatment of Stages I-IIIA NSCLC: Adjuvant Radiotherapy • Stages IA/B and IIA/B: Adjuvant radiation is not recommended. • Stage IIIA: Adjuvant radiation therapy is not recommended for routine use because of the lack of prospective, randomized clinical trial data evaluating its efficacy. A clinical trial is underway to determine the advisability of its routine use. v Recommendations apply only to completely resected tumors.

2007 Recommendations for Adjuvant Treatment of Stages I-IIIA NSCLC Special Considerations • Patients with poor performance status • Patients with advanced age

Strategies to Improve Doctor-Patient Communication • Therapeutic nihilism towards adjuvant chemotherapy for stages II-III NSCLC should now be abandoned • Recognize that unique issues face people with lung cancer • Offer a session devoted solely to discussing patient’s prognosis and the risks and benefits of adjuvant chemotherapy **This section is consensus-based, rather than evidence-based

Strategies to Improve Doctor-Patient Communication • Patients with cancer generally prefer shareddecision making • Present patients with individualized descriptions of their risks and benefits • Graphs included in guideline to help physicians communicate the absolute risk and benefit of adjuvant chemotherapy for the various stages of NSCLC **This section is consensus-based, rather than evidencebased

Strategies to Improve Doctor-Patient Communication, cont’d • With the physician providing immediate guidance and interpretation, a graph may help patients achieve a better understanding of absolute risk and benefit. • Graphical Representations* • Source: LACE meta-analysis • Using LACE data to estimate absolute benefit, adjuvant chemotherapy raises 5 -year survival from 64% to 67% for stage IB NSCLC, from 39% to 49% for stage II NSCLC, and from 26% to 39% for stage III NSCLC
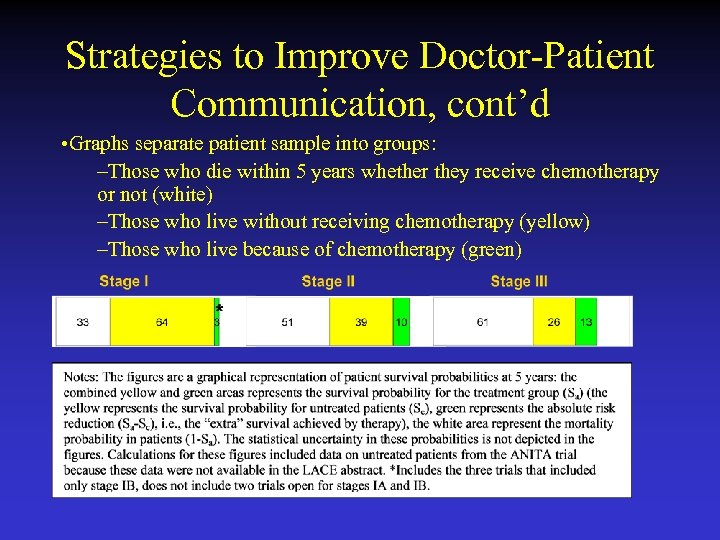
Strategies to Improve Doctor-Patient Communication, cont’d • Graphs separate patient sample into groups: –Those who die within 5 years whether they receive chemotherapy or not (white) –Those who live without receiving chemotherapy (yellow) –Those who live because of chemotherapy (green)

Selective Review of Molecular Markers in NSCLC • Panel undertook selective review of the literature pertaining to seven molecular markers • The majority were investigated for their possible ability to predict cisplatin resistance • Currently there is a lack of conclusive evidence showing that any marker is significantly related to clinical outcome
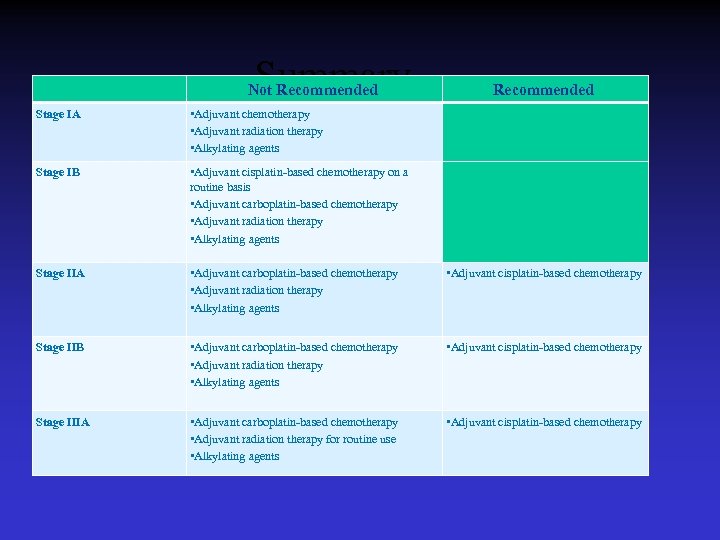
Summary Not Recommended Stage IA • Adjuvant chemotherapy • Adjuvant radiation therapy • Alkylating agents Stage IB • Adjuvant cisplatin-based chemotherapy on a routine basis • Adjuvant carboplatin-based chemotherapy • Adjuvant radiation therapy • Alkylating agents Stage IIA • Adjuvant carboplatin-based chemotherapy • Adjuvant radiation therapy • Alkylating agents • Adjuvant cisplatin-based chemotherapy Stage IIB • Adjuvant carboplatin-based chemotherapy • Adjuvant radiation therapy • Alkylating agents • Adjuvant cisplatin-based chemotherapy Stage IIIA • Adjuvant carboplatin-based chemotherapy • Adjuvant radiation therapy for routine use • Alkylating agents • Adjuvant cisplatin-based chemotherapy

Additional ASCO Resources • The full text of the guideline, this slide set, a Decision Aid Tool*, and additional resources are available at: http: //www. asco. org/guidelines/adjuvantnsclc • A Patient Guide on Adjuvant Treatment for Lung Cancer can be found at http: //www. cancer. net • *A version of Adjuvant! has been produced to make estimates of NSCLC patient outcomes with and without adjuvant therapy (1, 2, 3). We have for the publication of these guidelines produced our own version of such a tool. 1) Ravdin PM, Davis GJ. Prognosis of patients with resected non-small cell lung cancer: Impact of clinical and pathologic variables. Lung Cancer. 2006 May; 52(2): 207 -12. 2) A computer program designed to assist in NSCLC adjuvant therapy decision making. P. M. Ravdin Abstract - No. 7230. 2006 ASCO Annual Meeting 3) www. adjuvantonline. com

ASCO Guidelines

• Stage IV NSCLC • Therapy • ASCO recommendation

2009 Recommendations First-Line Chemotherapy • Recommendation A 1. Evidence supports the use of chemotherapy in patients with stage IV* non-small cell lung cancer with ECOG/Zubrod performance status 0, 1, and possibly 2. *Stage IV as defined by the International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Project, for the 7 th Edition of the TNM Classification of Malignant Tumors {Goldstraw P, J Thorac Onc , 2007}

2009 Recommendations First-Line Chemotherapy • Recommendation A 2. In patients with performance status 0 or 1, evidence supports using a combination of two cytotoxic drugs for first-line therapy. Platinum combinations are preferred over nonplatinum combinations because they are superior in response rate, and marginally superior in overall survival. Nonplatinum therapy combinations are reasonable in patients who have contraindications to platinum therapy. Recommendations A 8 and A 9 address whether to add bevacizumab or cetuximab to first-line cytotoxic therapy.

2009 Recommendations First-Line Chemotherapy • Recommendation A 3. Available data support the use of single-agent chemotherapy in patients with a performance status of 2. Data are insufficient to make a recommendation for or against using a combination of two cytotoxic drugs in patients with performance status 2. • Recommendation A 4. The evidence does not support the selection of a specific first-line chemotherapy drug or combination based on age alone.

2009 Recommendations First-Line Chemotherapy • Recommendation A 5. The choice of either cisplatin or carboplatin is acceptable. Drugs that may be combined with platinum include third-generation cytotoxic drugs docetaxel, gemcitabine, irinotecan, paclitaxel, pemetrexed, and vinorelbine. The evidence suggests that cisplatin combinations have a higher response rate than carboplatin and may improve survival when combined with thirdgeneration agents. Carboplatin is less likely to cause nausea, nephrotoxicity, and neurotoxicity than cisplatin, but more likely to cause thrombocytopenia.

2009 Recommendations First-Line Chemotherapy • Recommendation A 6. In patients with stage IV NSCLC, first-line cytotoxic chemotherapy should be stopped at disease progression or after four cycles in patients whose disease is not responding to treatment. Two-drug cytotoxic combinations should be administered for no more than six cycles. For patients who have stable disease or who respond to first-line therapy, evidence does not support the continuation of cytotoxic chemotherapy until disease progression, or the initiation of a different chemotherapy prior to disease progression.

2009 Recommendations First-Line Chemotherapy • Recommendation A 7. In unselected patients, erlotinib or gefitinib should not be used in combination with cytotoxic chemotherapy as first-line therapy. In unselected patients, evidence is insufficient to recommend single-agent erlotinib or gefitinib as firstline therapy. The first-line use of gefitinib may be recommended for patients with activating EGFR mutations. If EGFR mutation status is negative, or unknown, then cytotoxic chemotherapy is preferred (see Recommendation A 2).

2009 Recommendations First-Line Chemotherapy • Recommendation A 8. Based on the results of one large phase III randomized controlled trial, the Update Committee recommends the addition of bevacizumab, 15 mg/kg every three weeks, to carboplatin-paclitaxel, except for those patients with squamous cell carcinoma histologic type, brain metastases, clinically significant hemoptysis, inadequate organ function, ECOG performance status >1, therapeutic anticoagulation, clinically significant cardiovascular disease, or medically uncontrolled hypertension. (Based on exclusion criteria for Sandler et al. registration trial) Bevacizumab may be continued, as tolerated, until disease progression.

2009 Recommendations First-Line Chemotherapy • Recommendation A 9. Based on the results of one large phase III randomized controlled trial, clinicians may consider the addition of cetuximab to cisplatin-vinorelbine in first-line therapy in patients with an EGFR positive tumor as measured by immunohistochemistry. Cetuximab may be continued, as tolerated, until disease progression.

2009 Recommendations Second-Line Chemotherapy • Recommendation B 1. Docetaxel, erlotinib, gefitinib, or pemetrexed is acceptable as secondline therapy for patients with advanced non-small cell lung cancer with adequate performance status when the disease has progressed during or after first-line, platinum-based therapy. • Recommendation B 2. The evidence does not support the selection of a specific second-line chemotherapy drug or combination based on age alone.

2009 Recommendations Third-Line Chemotherapy • Recommendation C 1. When disease progresses on or after second-line chemotherapy, treatment with erlotinib may be recommended as third-line therapy for patients with performance status 0 to 3 who have not received prior erlotinib or gefitinib. • Recommendation C 2. The data are not sufficient to make a recommendation for or against using a cytotoxic drug as third-line therapy. These patients should consider clinical trials, experimental treatment, and best supportive care.
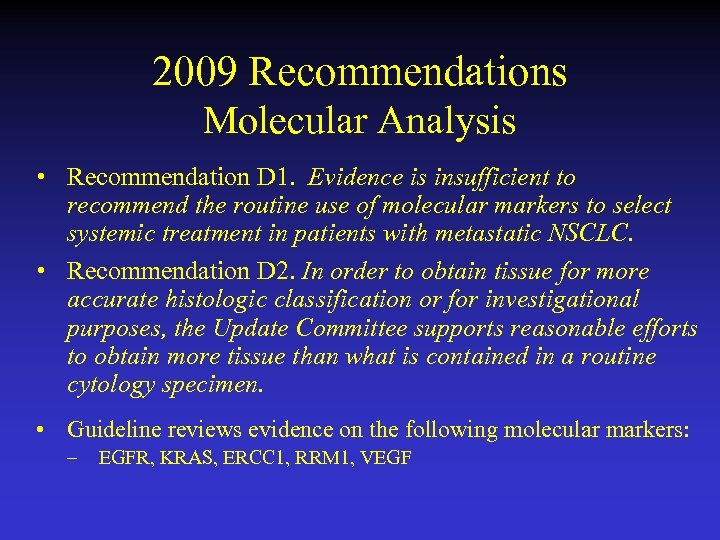
2009 Recommendations Molecular Analysis • Recommendation D 1. Evidence is insufficient to recommend the routine use of molecular markers to select systemic treatment in patients with metastatic NSCLC. • Recommendation D 2. In order to obtain tissue for more accurate histologic classification or for investigational purposes, the Update Committee supports reasonable efforts to obtain more tissue than what is contained in a routine cytology specimen. • Guideline reviews evidence on the following molecular markers: – EGFR, KRAS, ERCC 1, RRM 1, VEGF

2009 Recommendations Future Directions of Research • Research needed with participants who: – – are elderly (≥ 65 or ≥ 70) have ECOG Performance Status ≥ 2 (distinguish those with PS ≥ 2 from NSCLC from those impaired by co-morbidities) • Enrich trial population with participants with tumors with recently discovered prognostic markers and clinical characteristics e. g. : – – Histology Molecular characteristics Number and time receiving prior therapies With known smoking status

2009 Recommendations Future Directions of Research (cont’d) • Stratify trials by the prognostic factors listed above • Treatments which improve only PFS require greater scrutiny for toxicity, side effects, quality of life, and cost effectiveness • Establish more data on biologic factors of NSCLC in parallel with drug discovery • Research on strategies to improve patient-clinician communication Encourage patients to participate in clinical research trials at any time during the course of their disease.

Patient-Physician Communication in NSCLC Treatment • Research specific to NSCLC has found: – Missed opportunities for expressing empathy – Observantions of blaming words ( obviňování) – Lack of discussion on prognosis (n. b. approximately 20% of patients may not want discussion of prognostic information) – Lack of information-exchange and trust between patients and clinicians of different racial/ethnic backgrounds – Intensive training for clinicians can help, as can presence of a caregiver at appointment(s)

Patient-Physician Communication in NSCLC Treatment (cont’d) • Patients with lung cancer may overestimate the survival benefits of potentially toxic treatment • Suggested language: – – “Tell me what you know about your lung cancer? ” “How much do you want to know? ” “Sounds like what you are telling me is” “It sounds like you were really frightened when you got that news about the cancer. ”

Patient-Physician Communication in NSCLC Treatment (cont’d) • Qualitative statements, e. g. “chances are you will live longer if you take this chemotherapy versus another, or no chemotherapy. ” • Quantitative statements, e. g. “Chemotherapy will improve your chance of being alive in one year from 10 -20% up to 30 -50%. ” • “Without any chemotherapy, the average person will live about 4 and a half months. With chemotherapy most will live longer and some will live a shorter time. More recent chemotherapy trials have shown that people live about 3 months longer than if they did not get chemotherapy…” (Continued on next slide)

Patient-Physician Communication in NSCLC Treatment (cont’d) • (Continued from previous slide) “…Even with chemotherapy , the chance of being alive at one year is about 30 -50%; the chance of dying within this year is 50 -70%. ” • State at least one pessimistic aspect, e. g. “…the chance of dying is…. ; ” • If asked “can you cure me? ” a suggested answer is “No, I can’t, but we have good chances of prolonging your life and keeping you comfortable and we will always be here to help you and your family. ”

Guideline Methodology: Update Committee Members Christopher G. Azzoli, MD, Co-Chair Memorial-Sloan Kettering Cancer Center Giuseppe Giaccone, MD, Co-Chair National Cancer Institute Reily Smith, Patient Representative Bakersfield, CA John R. Strawn, MD, Patient Representative Timothy Aliff, MD Houston, TX Sherman Baker, Jr. , MD Virginia Commonwealth University - Massey Cancer Center Julie Brahmer, MD Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University Northwest Oncology & Hematology Associates David H. Johnson, MD, Co-Chair 2003 Vanderbilt-Ingram Cancer Center Update and current panelist Janessa L. Laskin, MD British Columbia Cancer Agency

Guideline Methodology: Update Committee Members (cont’d) Gregory Masters, MD Helen F. Graham Cancer Center Daniel Milton, MD Hematology/Oncology of Indiana, PC Luke Nordquist, MD William Pao, MD, Ph. D David G. Pfister, MD, Co-Chair 2003 Update and current panelist Nebraska Cancer Specialists, PC Vanderbilt-Ingram Cancer Center Memorial-Sloan Kettering Cancer Center Steven Piantadosi, MD, Ph. D Samuel Oschin Comprehensive Cancer Center Institute Joan H. Schiller, MD University of Texas, Southwestern medical Center Virginia Commonwealth University - Massey Cancer Center Thomas J. Smith, MD David Trent, MD, Ph. D Virginia Cancer Center

Additional ASCO Resources • The full text and an abridged version of the guideline, this slide set, and a set of Clinician. Patient Decision Aids can be found at: http: //www. asco. org/guidelines/nsclc • A patient guide, “What to Know” about this guideline, is available at http: //www. cancer. net

ASCO Guidelines
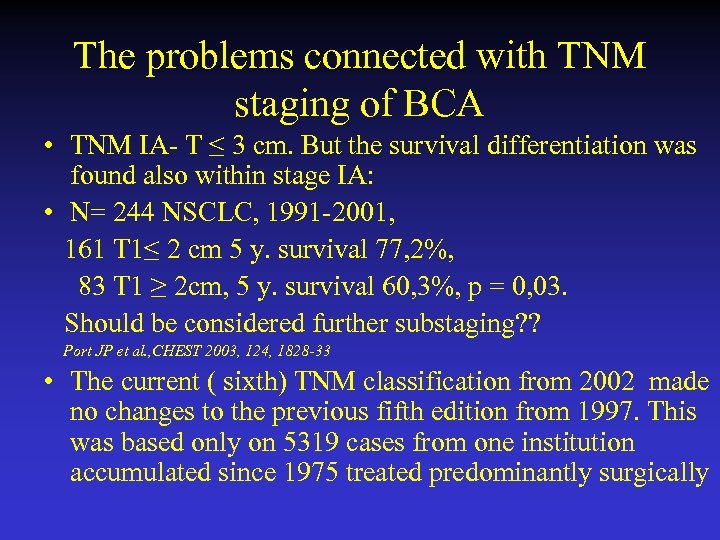
The problems connected with TNM staging of BCA • TNM IA- T ≤ 3 cm. But the survival differentiation was found also within stage IA: • N= 244 NSCLC, 1991 -2001, 161 T 1≤ 2 cm 5 y. survival 77, 2%, 83 T 1 ≥ 2 cm, 5 y. survival 60, 3%, p = 0, 03. Should be considered further substaging? ? Port JP et al. , CHEST 2003, 124, 1828 -33 • The current ( sixth) TNM classification from 2002 made no changes to the previous fifth edition from 1997. This was based only on 5319 cases from one institution accumulated since 1975 treated predominantly surgically
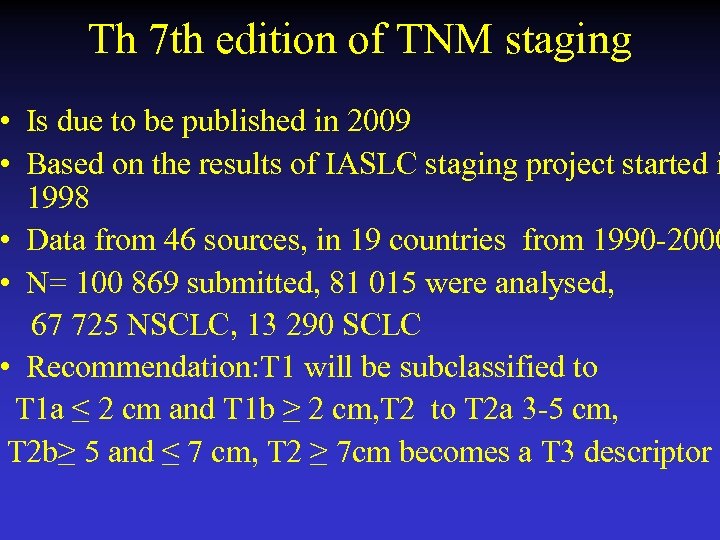
Th 7 th edition of TNM staging • Is due to be published in 2009 • Based on the results of IASLC staging project started i 1998 • Data from 46 sources, in 19 countries from 1990 -2000 • N= 100 869 submitted, 81 015 were analysed, 67 725 NSCLC, 13 290 SCLC • Recommendation: T 1 will be subclassified to T 1 a ≤ 2 cm and T 1 b ≥ 2 cm, T 2 to T 2 a 3 -5 cm, T 2 b≥ 5 and ≤ 7 cm, T 2 ≥ 7 cm becomes a T 3 descriptor

Th 7 th edition of TNM staging • Additional tumor in the same lobe will be T 3(was T 4), in other ipsilateral lobe T 4(was M 1), in contralateral lung M 1 a( This classification was applied to 657 pts with BAC – accurately reflect survival) Zell JA et al, J Thorac Oncol, 2007, 1078 -85) • Pleural nodule(was T 4), malignant pleural and pericardial eff. . (was T 4), contralateral tumor –will be M 1 a, distant metastases -M 1 b • Existing N descriptors were validated and no changes are proposed
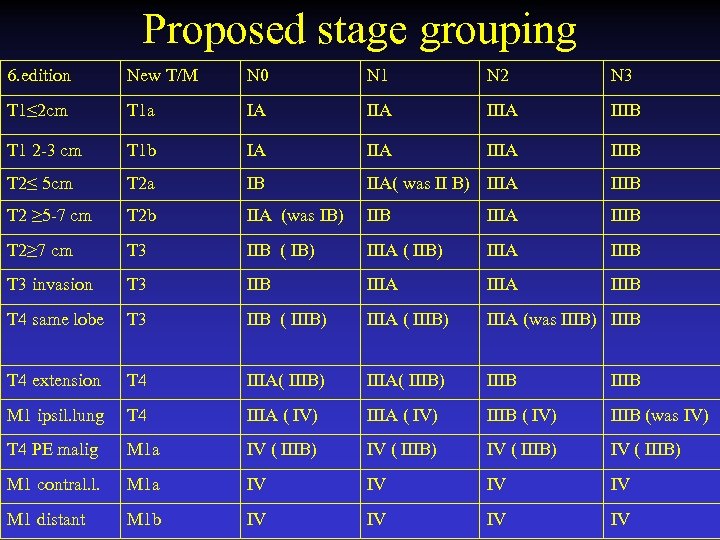
Proposed stage grouping 6. edition New T/M N 0 N 1 N 2 N 3 T 1≤ 2 cm T 1 a IA IIIA IIIB T 1 2 -3 cm T 1 b IA IIIA IIIB T 2≤ 5 cm T 2 a IB IIA( was II B) IIIA IIIB T 2 ≥ 5 -7 cm T 2 b IIA (was IB) IIB IIIA IIIB T 2≥ 7 cm T 3 IIB ( IB) IIIA ( IIB) IIIA IIIB T 3 invasion T 3 IIB IIIA IIIB T 4 same lobe T 3 IIB ( IIIB) IIIA (was IIIB) IIIB T 4 extension T 4 IIIA( IIIB) IIIB M 1 ipsil. lung T 4 IIIA ( IV) IIIB (was IV) T 4 PE malig M 1 a IV ( IIIB) M 1 contral. l. M 1 a IV IV M 1 distant M 1 b IV IV
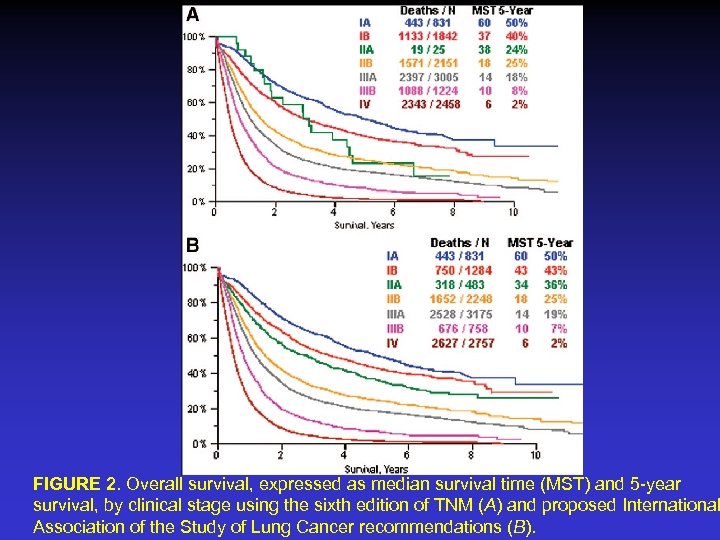
FIGURE 2. Overall survival, expressed as median survival time (MST) and 5 -year survival, by clinical stage using the sixth edition of TNM (A) and proposed International Association of the Study of Lung Cancer recommendations (B).
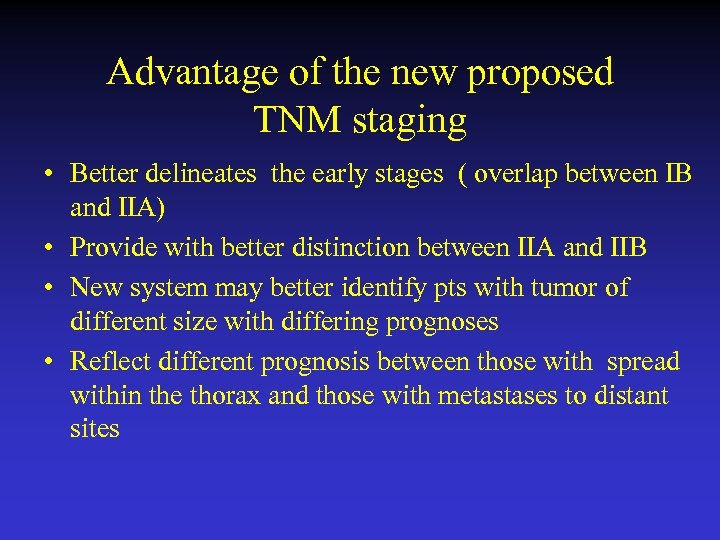
Advantage of the new proposed TNM staging • Better delineates the early stages ( overlap between IB and IIA) • Provide with better distinction between IIA and IIB • New system may better identify pts with tumor of different size with differing prognoses • Reflect different prognosis between those with spread within the thorax and those with metastases to distant sites

Solitary lesions - Coin lesion • asymptomatic pacient finding „ by chance“ or by screening ( 5 year survival in T 1 NO MO is 80 - 100 %) • X ray, CT signs of benign - rounded shape, sharp edge, calcification • benign - granulom - tuberculoma, cryptococosis, blastomykosis – absces, pneumonie, hamartoma – bronchogen cyst, , pl. infarctus, br. adenom – A -V aneurysma, reumatic nodule • over 50 year are 50 % malignant , 10 % meta !!!comparison with older X ray !!! To verify or removed ? ?

Other lung tumors • carcinoids – „bronchial adenoma“ today is one of the 9 subtypes of LC – „semimalignant“, production of vasoactive amins – APUD but today belong to LC subtype – bronchial obstruction – 1 / 3 hemoptysis – local removing acceptable, or standard surgery • atypic carcinoids more malignant • adenoid cystic carcinom - cylindrom- high 5 year survival • papilomas • mesenchym benign tu - hamartoma, chondroma, lipoma, leiomyoma

Other chest tumors - 2 Pleura - malignant mesothelioma - asbestos diffuse form - benign fibromesotelioma - demarcate Mediastinum - thymus, lymphoms, neurinoms, thyreoid gland Bronchogenic cysts, Meta of lung cancer

Differential diagnosis • • • metastatic disease other tumors – Hodgkin l. , mesothelioma, thymoma benign tumors pleural effusion nontumorous masses tuberculoma pneumonia – lung absces sarcoidosis aspergiloma pulmonary emboli

Screening ? ? ? • 3 USA and 1 Czechoslovak study chest Xray + sputum cytology no effect on mortality • biomarkers ? • molecular genetics ? • high risk population ? • low dose spiral CT ?
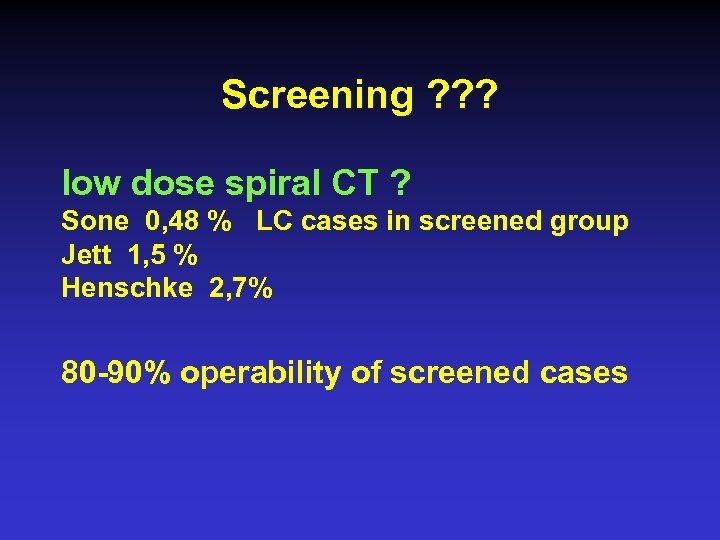
Screening ? ? ? low dose spiral CT ? Sone 0, 48 % LC cases in screened group Jett 1, 5 % Henschke 2, 7% 80 -90% operability of screened cases
Lung Cancer and Early Detection • No tests are recommended for screening the general population • A low-dose helical computerized tomography (CT or CAT) scan is currently being studied for this purpose
Treatment in the Czech Republic 1985 -2005 surgical treatment 10, 4% - 11, 4% in men 9, 4 % - 13, 2% in women radiotherapy 26% in 1985 - 23%in 2005 chemotherapy 20, 7% in 1985 – 34% in 2005 no treatment 47% in 1985 - 42% in 2005

Interventional bronchoscopy • currative intent • paliative intent

Interventional bronchoscopy Situation in the Czech Republic • Nd -YAG laser 10 x • stenting 8 x • brachytherapy 9 x • autofluorescence 4 x Life, 1 x SAFE • EBUS 4 x • photodynamic therapy - 0 • cryotherapy 2 x • argon-plasma coagulation - 2 • endobronchial drug and gene therapy - 0
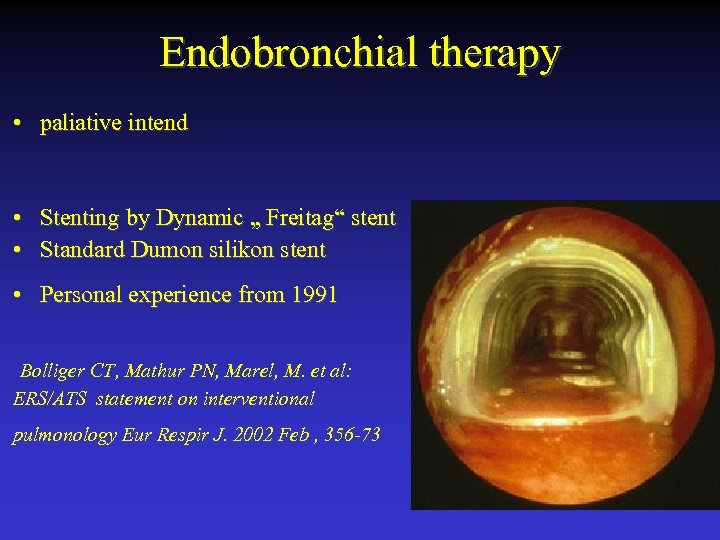
Endobronchial therapy • paliative intend • Stenting by Dynamic „ Freitag“ stent • Standard Dumon silikon stent • Personal experience from 1991 Bolliger CT, Mathur PN, Marel, M. et al: ERS/ATS statement on interventional pulmonology Eur Respir J. 2002 Feb , 356 -73
Contribution of BRS to diagnosis • BRS finding: direct Tu changes indirect tu changes normal findings BRS not done

BRS findings in 353 „our“ pts • The whole set: • • direct Tu changes at BRS 35% indirect Tu changes 26% normal findings 20% BRS not done 19%
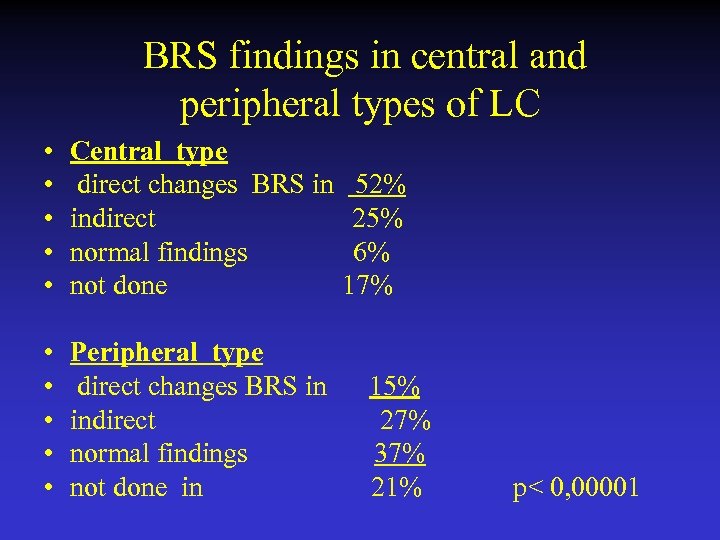
BRS findings in central and peripheral types of LC • • • Central type direct changes BRS in 52% indirect 25% normal findings 6% not done 17% • • • Peripheral type direct changes BRS in 15% indirect 27% normal findings 37% not done in 21% p< 0, 00001
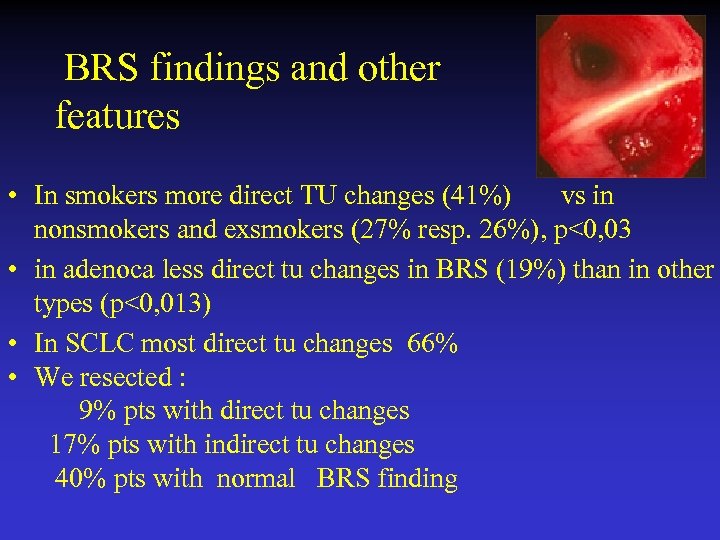
BRS findings and other features • In smokers more direct TU changes (41%) vs in nonsmokers and exsmokers (27% resp. 26%), p<0, 03 • in adenoca less direct tu changes in BRS (19%) than in other types (p<0, 013) • In SCLC most direct tu changes 66% • We resected : 9% pts with direct tu changes 17% pts with indirect tu changes 40% pts with normal BRS finding
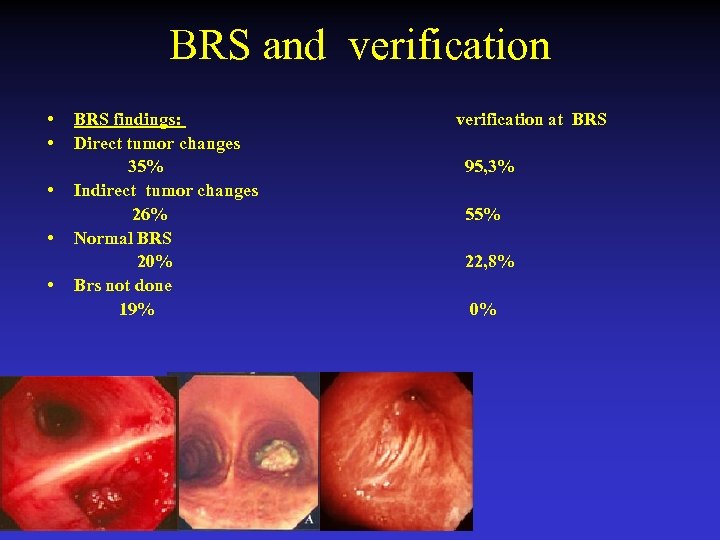
BRS and verification • • • BRS findings: Direct tumor changes 35% Indirect tumor changes 26% Normal BRS 20% Brs not done 19% verification at BRS 95, 3% 55% 22, 8% 0%
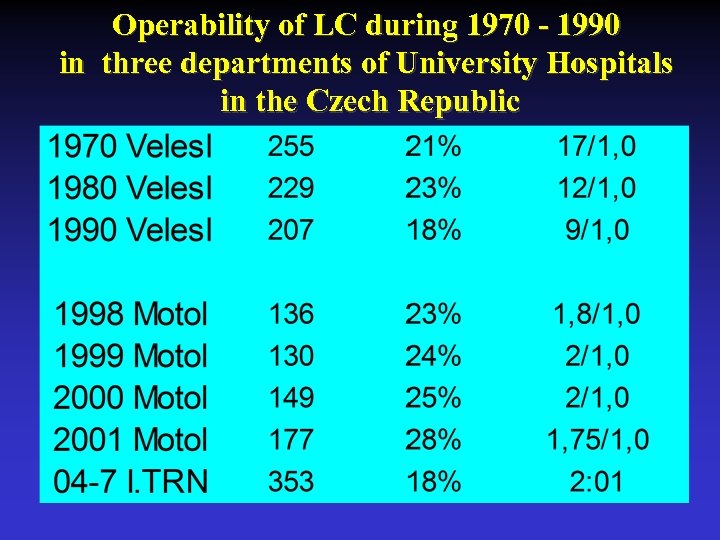
Operability of LC during 1970 - 1990 in three departments of University Hospitals in the Czech Republic
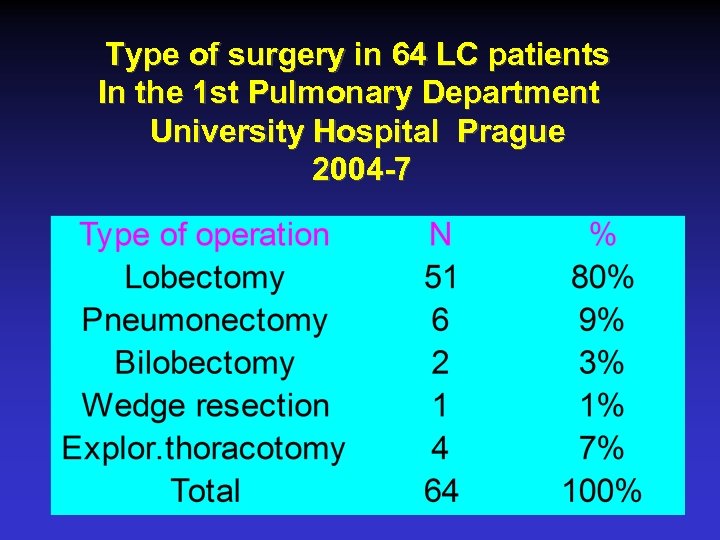
Type of surgery in 64 LC patients In the 1 st Pulmonary Department University Hospital Prague 2004 -7

Conclusions • The situation throughout the world is unsatisfactory • Non smoking society - is it a reality of Eastern Europe and world? • Earlier diagnosis • New: autofluorescence bronchoscopy, EBUS, TBNA, electromagnetic navigation active approach to the disease screening of LC ? ? in CR best possibility may be low dose spiral CT in the world biomarkers, genetic changes, low dose spiral CT • it is realistic to await a new „revolutionary drug„? ? ?

• Thank you for your attention
baa509b688d12b863843a1269a2f570a.ppt