3bae32380d5c668070ec384c7914edd3.ppt
- Количество слайдов: 17



Brief History of the Catalytic Converter. The catalytic converter was first invented by Eugene Houdry in the 1950’s. Tetra-ethyl lead present in gasoline "poisoned" the converter by forming a coating on the catalyst's surface, effectively disabling it. The catalytic converter was further developed by John J. Mooney and Carl D. Keith at the Engelhard Corporation, creating the first production of catalytic converter in 1973.

Uses of a Catalytic Converter A catalytic converter is a device used to reduce the toxicity of emissions from an internal combustion engine. Catalytic converters are most commonly used in motor vehicle exhaust systems. Catalytic converters are also used on generator sets, forklifts, mining equipment, trucks, buses, trains, and other engine-equipped machines. A catalytic converter provides an environment for a chemical reaction wherein toxic combustion by-products are converted to less-toxic substances.


Metal-core converter Ceramic-core converter The core is often a ceramic honeycomb in modern catalytic converters, but stainless steel foil honeycombs are used, too. The honey-comb surface increases the amount of surface area available to support the catalyst, and therefore is often called a "catalyst support". A washcoat is used to make converters more efficient, often as a mixture of silica and alumina. The washcoat, when added to the core, forms a rough, irregular surface, which has a far greater surface area than the flat core surfaces do, which then gives the converter core a larger surface area, and therefore more places for active precious metal sites.

The catalyst is added to the washcoat (in suspension) before being applied to the core. Platinum is the most active catalyst and is widely used. Palladium and rhodium are two other precious metals used. Platinum and rhodium are used as a reduction catalyst. Platinum and palladium are used as an oxidization catalyst. Cerium, iron, manganese and nickel are also used.


Two Way Catalytic Converter A two-way catalytic converter has two simultaneous tasks. Oxidation of carbon monoxide to carbon dioxide: 2 CO + O 2 → 2 CO 2 Oxidation of unburnt hydrocarbons (unburnt and partially-burnt fuel) to carbon dioxide and water: Cx. H(2 x+2) + [(3 x+1)/2] O 2 → x. CO 2 + (x+1) H 2 O (a combustion reaction) Unable to control NOx gases.

Three Way Catalytic Converter Reduction of nitrogen oxides to nitrogen and oxygen: 2 NOx → x. O 2 + N 2 Oxidation of carbon monoxide to carbon dioxide: 2 CO + O 2 → 2 CO 2 Oxidation of unburnt hydrocarbons (HC) to carbon dioxide and water: Cx. H(2 x+2) + [(3 x+1)/2]O 2 → x. CO 2 + (x+1)H 2 O Unwanted reactions can occur in the three-way catalyst, such as the formation of odiferous hydrogen sulfide and ammonia.
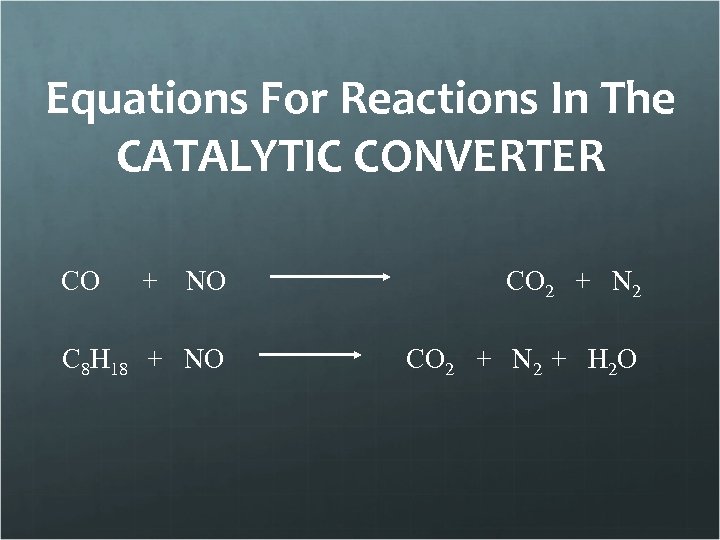
Equations For Reactions In The CATALYTIC CONVERTER CO + NO CO 2 + N 2 C 8 H 18 + NO CO 2 + N 2 + H 2 O
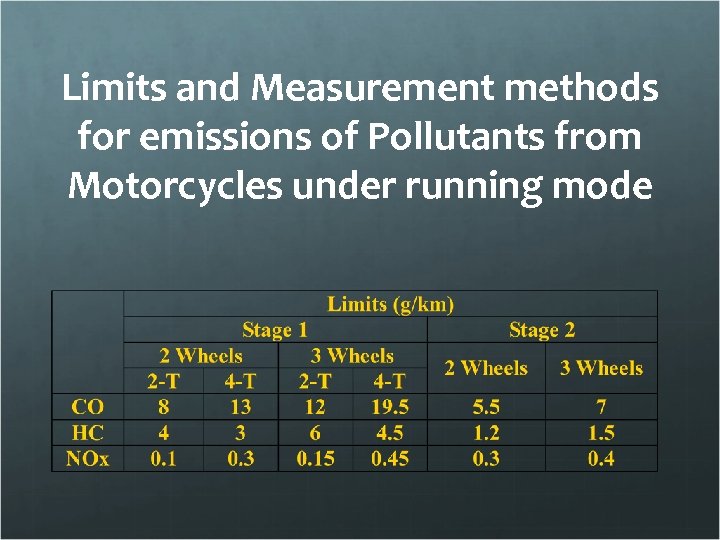
Limits and Measurement methods for emissions of Pollutants from Motorcycles under running mode

Damage to Catalytic Converters Catalyst poisoning occurs when the catalytic converter is exposed to exhaust containing substances that coat the working surfaces, encapsulating the catalyst so that it cannot contact and treat the exhaust. The most notable contaminant is lead. Any condition that causes abnormally high levels of unburned hydrocarbons — raw or partially-burnt fuel — to reach the converter will tend to significantly elevate its temperature, bringing the risk of a meltdown of the substrate and resultant catalytic deactivation and severe exhaust restriction.

Negative Aspects of Catalytic Converters. Some early converter designs created a great deal of restriction to the flow of exhaust, which negatively affected vehicle performance, drivability, and fuel economy. It had been stated that catalytic converters are known in a lot of cases to have an excessively long warm-up time period, in a great deal of cases ranging up to thirty-minutes.

The Environmental Impact of Catalytic Converters Reduces fuel economy of cars resulting in a greater use of fossil fuels. Although catalytic converters are effective at removing hydrocarbons and other harmful emissions, most of exhaust gas leaving the engine through a catalytic converter is carbon dioxide (CO 2), which is responsible for the green house effect. Catalytic converter production requires palladium and/or platinum; part of the world supply of these precious metals is produced near the Russian city of Norilsk, where the industry (among others) has caused Norilsk to be added to Time Magazine's list of most polluted places. It removes up to 90% of the harmful gases.

Thank You Have a Nice Day
3bae32380d5c668070ec384c7914edd3.ppt