c9f43c3133e34c71aa9c9493a66c4ed4.ppt
- Количество слайдов: 37

Breakthroughs in Lupus in 2015 Jennifer H. Anolik, MD, Ph. D Associate Professor of Medicine, Pathology, and Microbiology/Immunology Division of Allergy, Immunology & Rheumatology University of Rochester Medical Center Oct 2015 9 th Annual Lupus Education Day MEDICINE OF THE HIGHEST ORDER

Outline • Basic Review • Diagnosis • Pathogenesis • Treatment (leads to treatment)

Research in Lupus • The more that is known about clinical outcomes and immune abnormalities associated with lupus, the better equipped we are to fight the disease!

What we’re doing at the U of R: • NIH funded networks • Autoimmunity Center of Excellence for clinical trials and basic mechanisms of lupus and clinical trials • Accelerating Medicines Partnership • Clinical Cohorts: Lupus Clinical Trials Consortium • • • 20 centers Collaborative Longitudinal Lupus Registry Clinical Trials • • The AIR unit has an active program in clinical trials in SLE Investigation of new, targeted biological interventions in SLE

What are the different forms of lupus? Systemic Lupus Erythematosus Discoid or Cutaneous Lupus Drug-Induced Lupus Neonatal Lupus
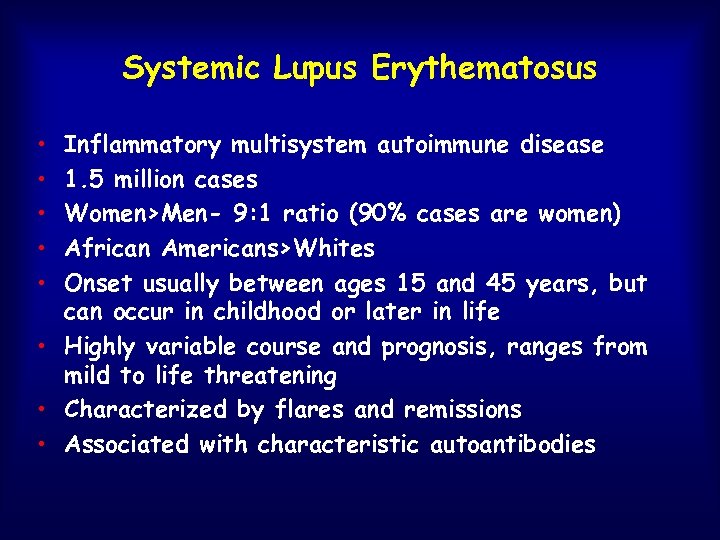
Systemic Lupus Erythematosus • • • Inflammatory multisystem autoimmune disease 1. 5 million cases Women>Men- 9: 1 ratio (90% cases are women) African Americans>Whites Onset usually between ages 15 and 45 years, but can occur in childhood or later in life • Highly variable course and prognosis, ranges from mild to life threatening • Characterized by flares and remissions • Associated with characteristic autoantibodies
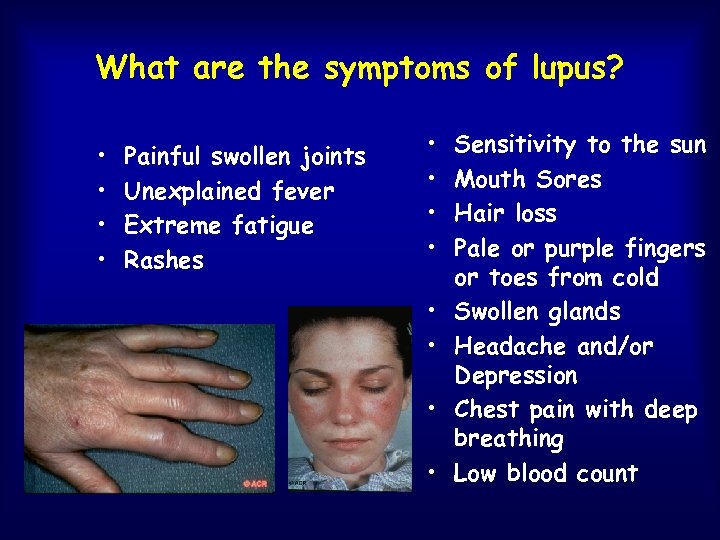
What are the symptoms of lupus? • • Painful swollen joints Unexplained fever Extreme fatigue Rashes • • Sensitivity to the sun Mouth Sores Hair loss Pale or purple fingers or toes from cold Swollen glands Headache and/or Depression Chest pain with deep breathing Low blood count
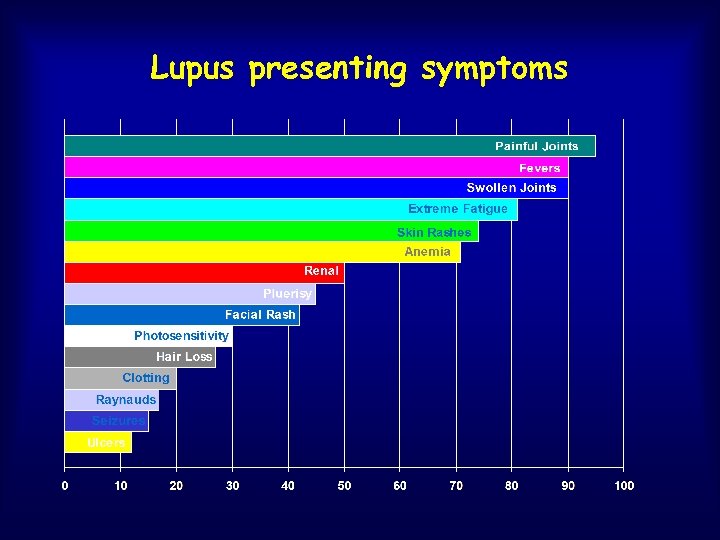
Lupus presenting symptoms

How do we diagnose lupus? Skin criteria 1. Malar rash 2. Discoid Rash 3. Photosensitivity 4. Oral Ulcers Systemic criteria 5. Arthritis 6. Serositis 7. Kidney 8. Neurologic Lab criteria 9. Anti-nuclear antibody 10. Immunologic 11. Hematologic *4 criteria simultaneously or serially for diagnosis New SLICC criteria

SLE Diagnosis: Autoantibodies • ANA • Seen in 99% of SLE • Not specific for SLE • Seen in many inflammatory, infectious, and neoplastic diseases • Seen in 5% to 15% of normal persons • Other more specific autoantibodies- anti. DNA, anti. Smith
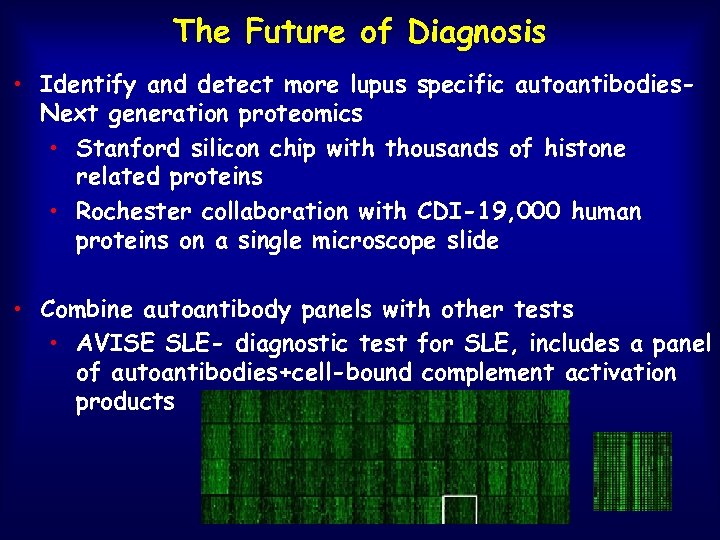
The Future of Diagnosis • Identify and detect more lupus specific autoantibodies. Next generation proteomics • Stanford silicon chip with thousands of histone related proteins • Rochester collaboration with CDI-19, 000 human proteins on a single microscope slide • Combine autoantibody panels with other tests • AVISE SLE- diagnostic test for SLE, includes a panel of autoantibodies+cell-bound complement activation products
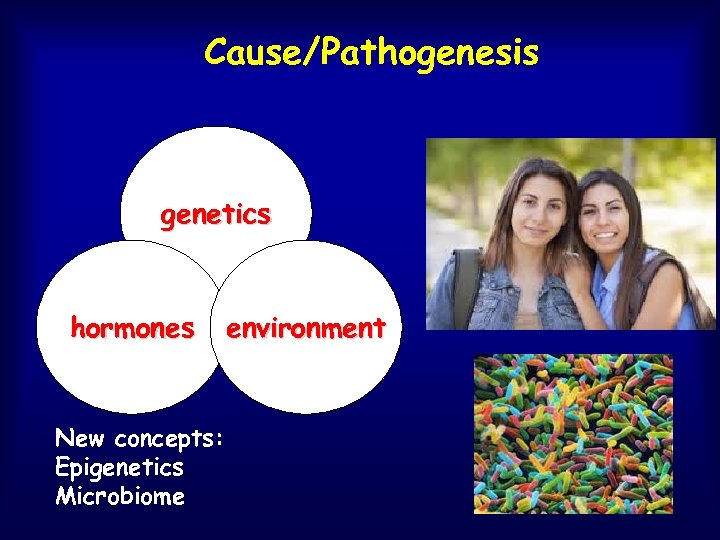
Cause/Pathogenesis genetics hormones New concepts: Epigenetics Microbiome environment
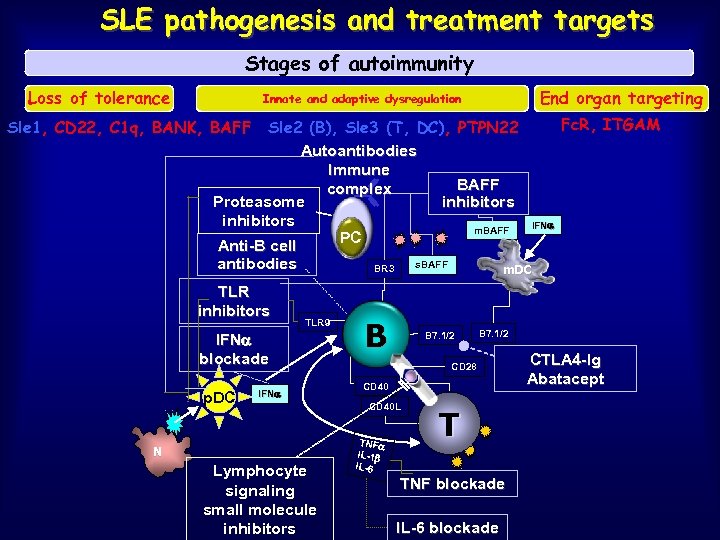
SLE pathogenesis and treatment targets Stages of autoimmunity Loss of tolerance End organ targeting Innate and adaptive dysregulation Fc. R, ITGAM Sle 2 (B), Sle 3 (T, DC), PTPN 22 Autoantibodies Immune BAFF complex Proteasome inhibitors IFN m. BAFF PC Anti-B cell s. BAFF antibodies BR 3 m. DC Sle 1, CD 22, C 1 q, BANK, BAFF TLR inhibitors TLR 9 IFN blockade p. DC IFN N Lymphocyte signaling small molecule inhibitors B B 7. 1/2 CD 28 CD 40 L TNF IL-1 IL-6 T TNF blockade IL-6 blockade CTLA 4 -Ig Abatacept
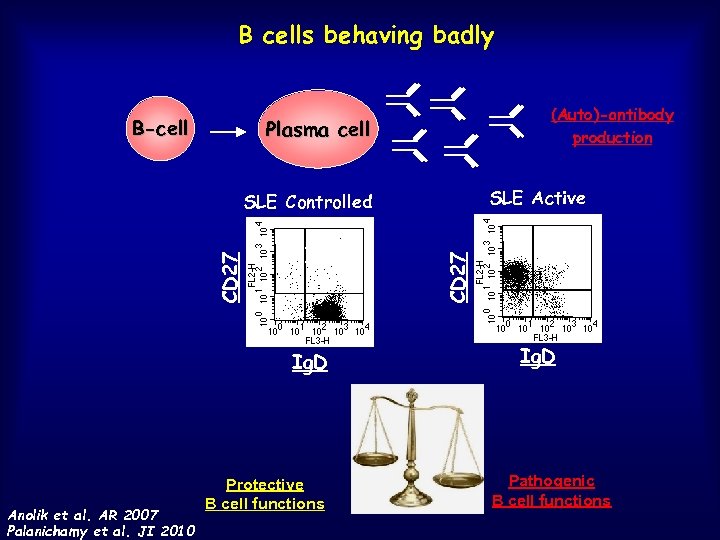
B cells behaving badly B-cell (Auto)-antibody production Plasma cell SLE Active 1 1 1 97 CD 27 SLE Controlled 73 9 9 9 R 6 Ig. D Anolik et al. AR 2007 Palanichamy et al. JI 2010 Protective B cell functions Ig. D Pathogenic B cell functions
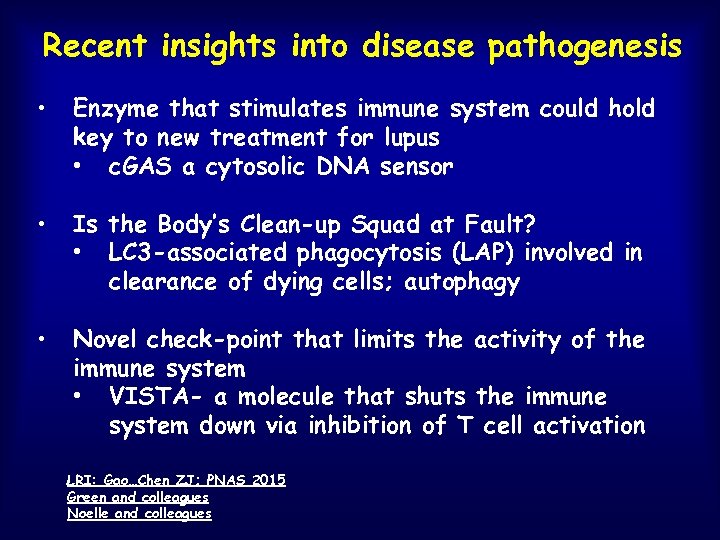
Recent insights into disease pathogenesis • Enzyme that stimulates immune system could hold key to new treatment for lupus • c. GAS a cytosolic DNA sensor • Is the Body’s Clean-up Squad at Fault? • LC 3 -associated phagocytosis (LAP) involved in clearance of dying cells; autophagy • Novel check-point that limits the activity of the immune system • VISTA- a molecule that shuts the immune system down via inhibition of T cell activation LRI: Gao…Chen ZJ; PNAS 2015 Green and colleagues Noelle and colleagues
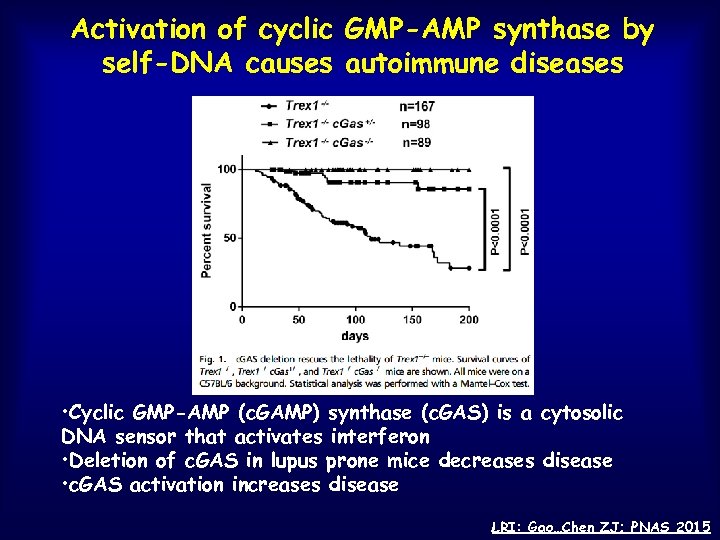
Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases • Cyclic GMP-AMP (c. GAMP) synthase (c. GAS) is a cytosolic DNA sensor that activates interferon • Deletion of c. GAS in lupus prone mice decreases disease • c. GAS activation increases disease LRI: Gao…Chen ZJ; PNAS 2015
The spectrum of lupus treatment Treating inflammation or autoimmunity • Anti-inflammatory agents • Antimalarials • Immunosupressive/cytotoxic agents Other • Prevention: management of cardiovascular risk, immunization • Anti-thrombotic therapy • Dialysis and kidney transplantation

The ‘traditional treatment armamentarium’ Benlysta

New Treatments for Lupus Until April 2011 it had been over 50 years since a new drug was approved for lupus! WHY? • Lupus is hard to study: • • • Clinical expression is heterogeneous Pathology is diverse Disease activity is intermittent Lack of agreed upon disease activity measures and endpoints Small patient populations- rare disease • Development costs: Estimated $1 billion to take a drug from the research stage to FDA approval • Lack of a clinical trial infrastructure WE NEED CLINICAL TRIALS TO KNOW WHAT WORKS AND TO GET FDA APPROVAL OF NEW DRUGS
HOW DO WE IDENTIFY NEW TARGETS? Accelerating Medicines Partnership Initiative (AMP) • New venture between lupus and RA scientists, NIH, biopharmaceutical companies and non-profit organizations • UR is one of the 9 sites • Goal is to transform the current model for developing new diagnostics and treatments by jointly identifying and validating promising biological targets of disease in TARGET TISSUE • The ultimate goal is to increase the number of new diagnostics and therapies for patients and reduce the time and cost of developing them
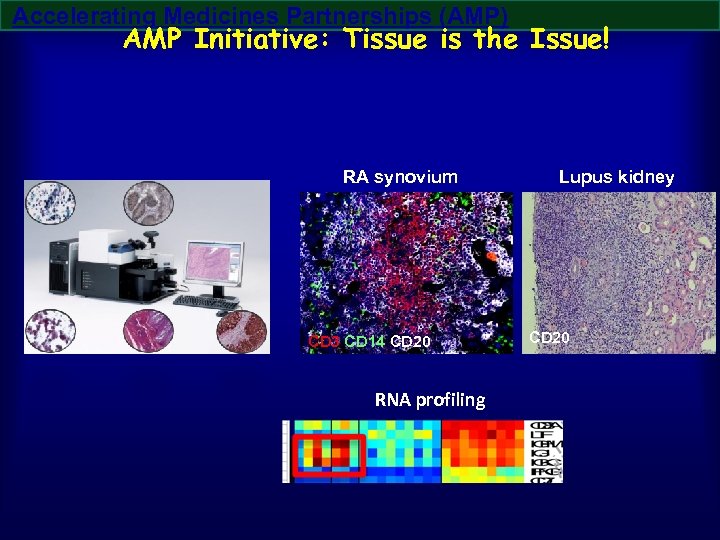
Accelerating Medicines Partnerships (AMP) AMP Initiative: Tissue is the Issue! RA synovium CD 3 CD 14 CD 20 RNA profiling Lupus kidney CD 20
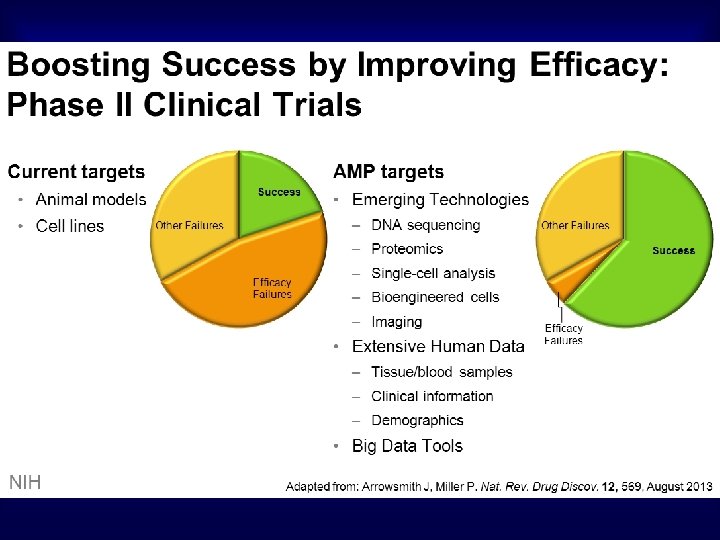

Repurposing drugs: LRx. L-STAT (Lupus Rx List-SLE Treatment Acceleration Trials) • New ALR-LRI collaboration • Finding drugs and other treatment strategies that may be ripe for repurposing in lupus • 155 candidate drugs have emerged for further study in small focused science-rich clinical trials • 1 st clinical trials of the STAT initiative will kickoff soon. Site selection underway. https: //www. linkedin. com/in/lrxlstat
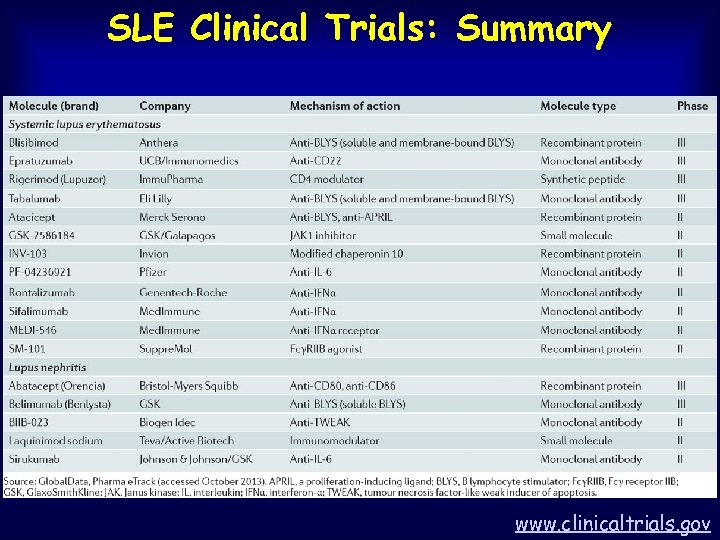
SLE Clinical Trials: Summary www. clinicaltrials. gov
Rituximab= anti-CD 20= B cell depletion • Initial promising studies • Two large trials of anti-CD 20 (rituximab) in SLE failed to meet their primary outcomes • Advances in the field on how to successfully do lupus clinical trials • Rituximab is still thought to be effective in lupus and indicated for a subset of refractory patients
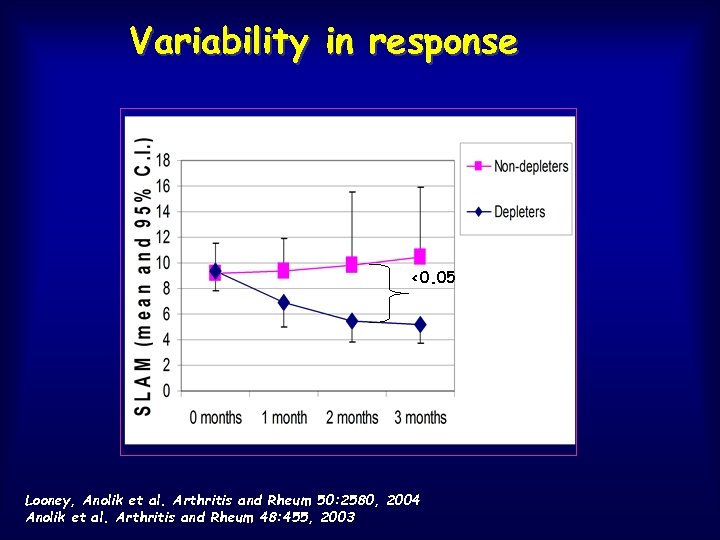
Variability in response CD 19+ (Lymphocytes/ul) 100 Non-depleters (6) 10 Depleters (11) <0. 05 1 0 3 6 Months Looney, Anolik et al. Arthritis and Rheum 50: 2580, 2004 Anolik et al. Arthritis and Rheum 48: 455, 2003 9 12
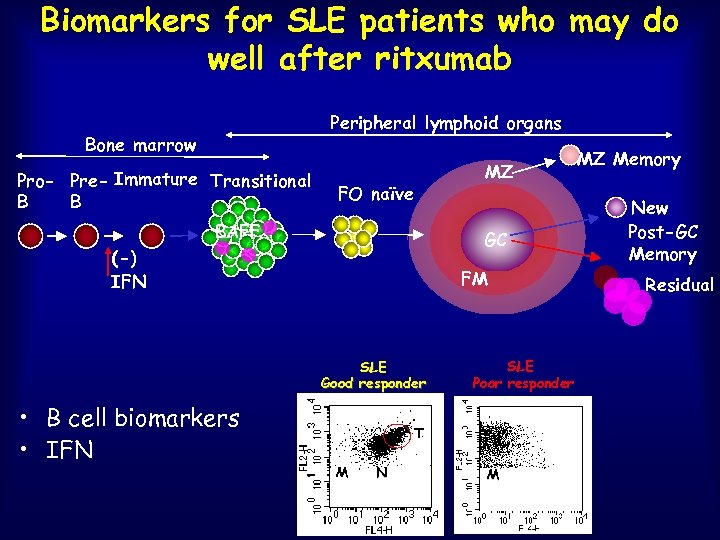
Biomarkers for SLE patients who may do well after ritxumab Peripheral lymphoid organs Bone marrow Pro- Pre- Immature Transitional B B FO naïve BAFF GC (-) IFN FM SLE Good responder • B cell biomarkers • IFN MZ SLE Poor responder T M N M MZ Memory New Post-GC Memory Residual
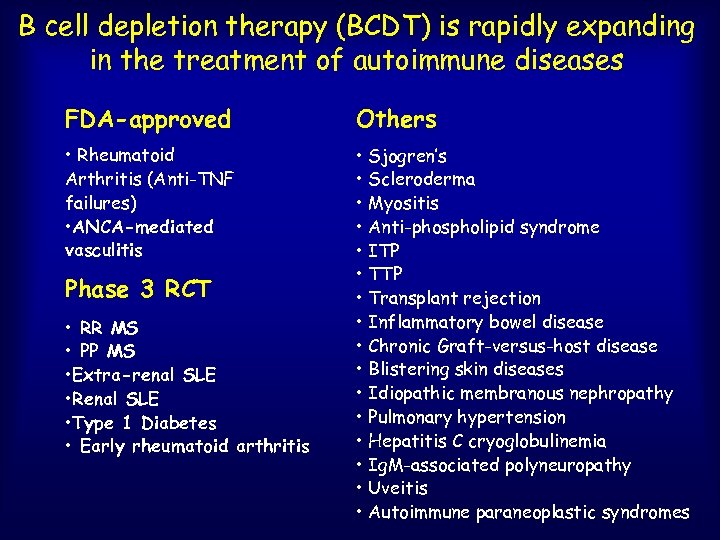
B cell depletion therapy (BCDT) is rapidly expanding in the treatment of autoimmune diseases FDA-approved Others • Rheumatoid Arthritis (Anti-TNF failures) • ANCA-mediated vasculitis • Sjogren’s • Scleroderma • Myositis • Anti-phospholipid syndrome • ITP • Transplant rejection • Inflammatory bowel disease • Chronic Graft-versus-host disease • Blistering skin diseases • Idiopathic membranous nephropathy • Pulmonary hypertension • Hepatitis C cryoglobulinemia • Ig. M-associated polyneuropathy • Uveitis • Autoimmune paraneoplastic syndromes Phase 3 RCT • RR MS • PP MS • Extra-renal SLE • Renal SLE • Type 1 Diabetes • Early rheumatoid arthritis

Rituximab/B cell biology paves the way for Belimumab (anti-BAFF)- Benlysta • Blocks a B cell survival factor, inducing B cell death • Recently approved (3/9/2011) for the treatment of SLE • 1 ST DRUG APPROVED FOR LUPUS IN OVER 50 YRS • 1 ST BIOLOGIC APPROVED FOR LUPUS http: //www. youtube. com/watch? v=i 24 UTv. OKK-8

B cell targeted 2015 What’s new? • Innovative ways to combine rituximab with benlysta • Other B cell targeted therapies: • • Anti-CD 22: phase III completed, did not meet endpoints Other anti-CD 20 s-largely halted Anti-CD 19 Proteasome inhibitors • Cytokine blockade • Benlysta for nephritis, black patients, pediatric, long-term safety, treatment holiday/restart • Different forms of BAFF blockade in Phase 3 - blisibimod
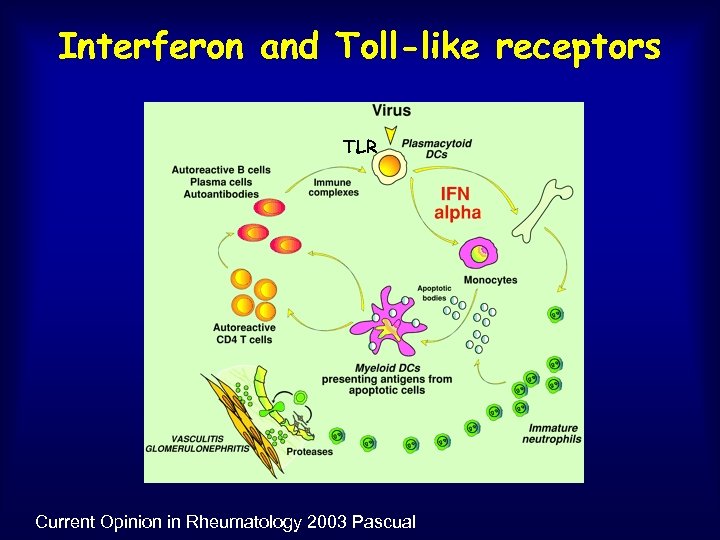
Interferon and Toll-like receptors TLR Current Opinion in Rheumatology 2003 Pascual
IFN and TLR blockade • Multiple studies on monoclonal antibodies against IFN alpha have been in various stages of developmentsomewhat disappointing • Press release that anifrolumab met primary endpoint of reduction in global disease activity score in moderate/severe SLE in Phase 2 - Astra. Zeneca starting Phase 3 • TLRs are key receptors of the innate immune system that can induce strong inflammatory responses- important in production of IFN. Interest in small molecules inhibitors of Toll-like Receptors (TLRs) 7, 8, and/or 9
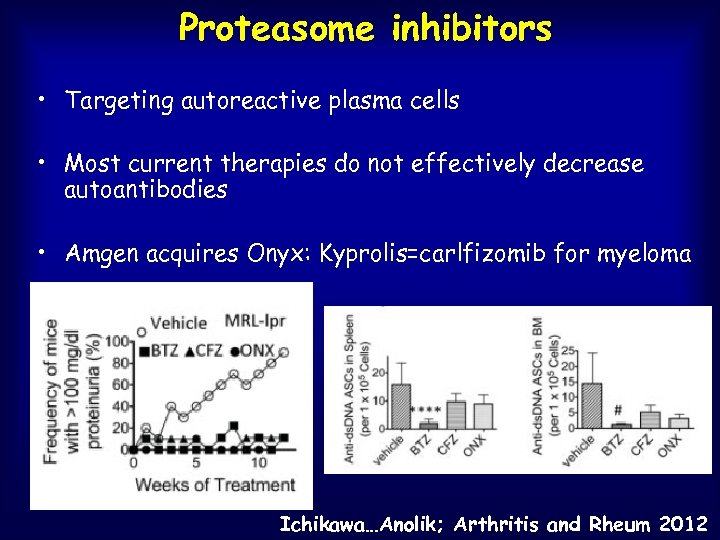
Proteasome inhibitors • Targeting autoreactive plasma cells • Most current therapies do not effectively decrease autoantibodies • Amgen acquires Onyx: Kyprolis=carlfizomib for myeloma Ichikawa…Anolik; Arthritis and Rheum 2012
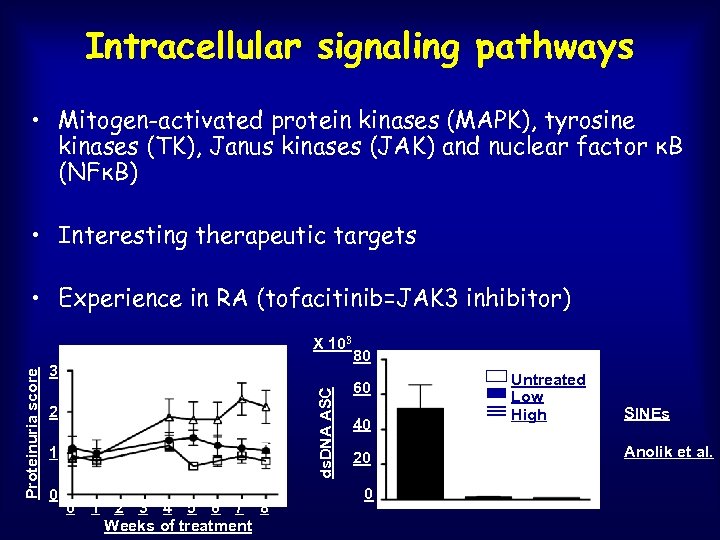
Intracellular signaling pathways • Mitogen-activated protein kinases (MAPK), tyrosine kinases (TK), Janus kinases (JAK) and nuclear factor κB (NFκB) • Interesting therapeutic targets • Experience in RA (tofacitinib=JAK 3 inhibitor) 3 2 1 ** ** 0 0 1 2 3 4 5 6 7 8 Weeks of treatment ds. DNA ASC Proteinuria score X 103 80 60 40 20 0 Untreated Low High SINEs Anolik et al.

Currently (or soon) enrolling trials at UR • Inhibition of intracellular signaling with small molecule oral agents • Inhibitor of ubiquination in Phase II placebo controlled, multi-center trial by Celgene • We are CURRENTLY enrolling • May have particular efficacy in skin disease • Cell based therapies • Mesenchymal stem cell transfer

Concluding points • We are learning how to “borrow” drugs used to treat other diseases • Some drugs may provide clues about how lupus develops • Despite barriers, novel mechanism-based therapies are in development for SLE • Therapy will attempt to target specific pathways in the body • Eventual treatments may involve combination therapies, i. e. , “cocktails” of targeted and semi-targeted therapies • Patients receive an “individualized” treatment

Learn More • • • www. lupusresearch. org/research_update. html Lupus. Trials. org www. clinicaltrials. gov The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the Office on Women’s Heath have developed a strategic plan for reducing health disparities. Lupus is included as an area of research focus. Further information on disparities in lupus and educational material at: http: //thelupusinitiative. org www. couldihavelupus. gov
c9f43c3133e34c71aa9c9493a66c4ed4.ppt