8498512f772d1e6d172785931801c0d9.ppt
- Количество слайдов: 13

BPAC Meeting Washington D. C. March 13 – 14, 2003 Development of a Supplemental WNV Taqman Assay and Other Critical Reagents in Support of the Procleix WNV Assay Bruce Phelps Blood Testing R&D Chiron Corporation

Objectives of the Chiron WNV Program • WNV Taqman Assay Development • Propagation of West Nile Virus • Development of Assay Standards • Reagents for Serological Assay Development

WNV Taqman Assay Development • A qualitative WNV Taqman assay is being developed at Chiron in support of the Procleix WNV assay development program. • The assay will be fully validated and may be used as a supplemental alternative NAT assay during Phase 2 trials to be initiated at the advent of the 2003 mosquito season.

WNV Taqman Assay Development • The capsid region of the WNV genome is targeted for amplification. • A 965 nucleotide RNA transcript from the capsid region is included as an Internal Control. • The Taqman assay detects 10 -50 copies of purified RNA transcript.
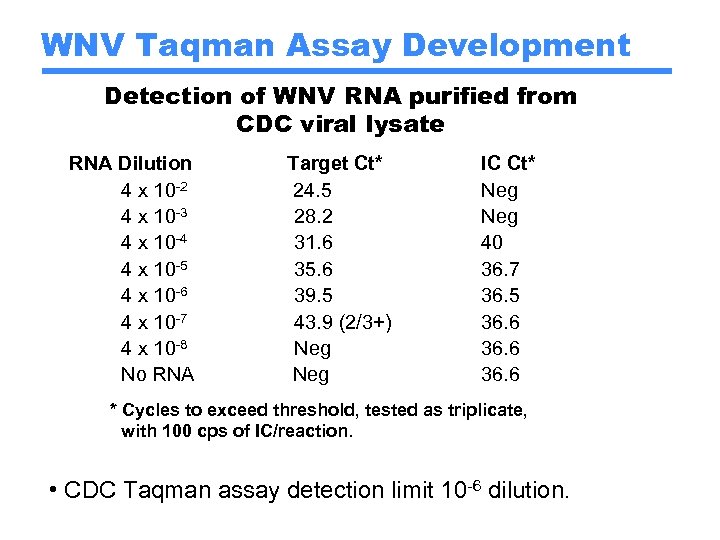
WNV Taqman Assay Development Detection of WNV RNA purified from CDC viral lysate RNA Dilution 4 x 10 -2 4 x 10 -3 4 x 10 -4 4 x 10 -5 4 x 10 -6 4 x 10 -7 4 x 10 -8 No RNA Target Ct* 24. 5 28. 2 31. 6 35. 6 39. 5 43. 9 (2/3+) Neg IC Ct* Neg 40 36. 7 36. 5 36. 6 * Cycles to exceed threshold, tested as triplicate, with 100 cps of IC/reaction. • CDC Taqman assay detection limit 10 -6 dilution.
WNV Taqman Assay Development • Two methods are being evaluated for target isolation: – Magnetic silica adsorption – Magnetic beads for oligonucleotide capture

Propagation of West Nile Virus • West Nile virus strain 385 -99, originally isolated from a snowy owl in the Bronx Zoo, New York, 1999, is being propagated in Vero cells in our BL 3 facility. • Viral titers of 107 PFU/m. L have been obtained from several viral cultures.

Propagation of West Nile Virus • A quantitative Taq. Man assay (developed in -house) is being used to estimate viral genomic equivalents/m. L using supernatants of viral infected cells. • Efficient WNV inactivation, using a validated HCV heat-inactivation protocol, has been confirmed by Vero cells infectivity studies.
Development of Assay Standards • Viral suspensions of heat-inactivated virus (total of 109 PFU of pre-inactivated virus) have been prepared. • Three RNA transcripts of different genomic regions have been prepared: – 5'NC/C/Pre M/M (~900 nt) – E/NS 1/NS 2 a (~1500 nt) – NS 5/3'NC (~1000 nt)

Development of Assay Standards • A genetically engineered non-infectious full -length West Nile Virus genome is being cloned in vectors suitable for RNA production. • Once fully characterized, this full length West Nile virus transcript could be used as a standard for nucleic acid test evaluation.

Reagents for Serological Assay Development • Cloning and yeast expression of West Nile Virus* recombinant proteins – Pr. M/E region – Envelope protein – Capsid protein – NS 1 region * New York Strain, 385 -99

Reagents for Serological Assay Development • These recombinant West Nile virus proteins will facilitate development of supplemental or diagnostic tests to detect Ig. M and/or Ig. G antibodies in the serum/blood of infected individuals. • These recombinant proteins can also be used to generate MAbs for antigen test development.

Acknowledgements We acknowledge Dr. Laura Kramer and Alan P. Dupuis of the Arbovirus Laboratories, Wadsworth Center, New York State Dept. of Health, for providing protocols and valuable assistance to culture and propagate West Nile virus.
8498512f772d1e6d172785931801c0d9.ppt