cfd4fda73b8569a88970c3d509ed9956.ppt
- Количество слайдов: 21

Boston Scientific Corporation: DES Bioabsorbable Technologies Keith Dawkins MD FRCP FACC FSCAI Global Chief Medical Officer Executive Vice President Boston Scientific Corporation

Conflicts of Interest Boston Scientific Corporation Employee Stockholder

DES Polymer Considerations Purpose of the Polymer Provides mechanically stable matrix for drug Modulates drug release into vessel wall Polymer has no function after drug release is complete All polymer coatings have potential to be damaged Damaged durable polymers are permanent

Potential Issues with Durable Polymer DES Safety Efficacy Late / very late stent thrombosis Higher risk in certain patient populations Potentially require long-term DAPT Chronic inflammation with neoatherosclerosis Constant irritant may lead to late restenosis Diminished efficacy in diabetic populations
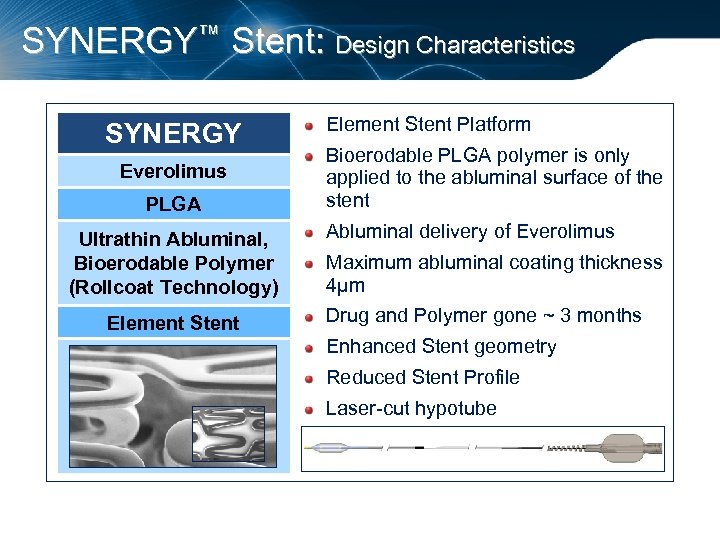
SYNERGY™ Stent: Design Characteristics SYNERGY Everolimus PLGA Ultrathin Abluminal, Bioerodable Polymer (Rollcoat Technology) Element Stent Platform Bioerodable PLGA polymer is only applied to the abluminal surface of the stent Abluminal delivery of Everolimus Maximum abluminal coating thickness 4μm Drug and Polymer gone ~ 3 months Enhanced Stent geometry Reduced Stent Profile Laser-cut hypotube
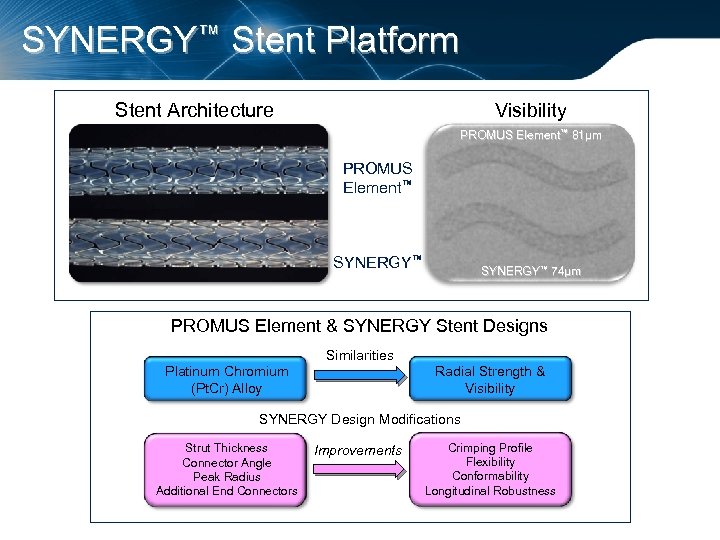
SYNERGY™ Stent Platform Stent Architecture Visibility PROMUS Element™ 81µm PROMUS Element™ SYNERGY™ 74µm PROMUS Element & SYNERGY Stent Designs Similarities Platinum Chromium (Pt. Cr) Alloy Radial Strength & Visibility SYNERGY Design Modifications Strut Thickness Connector Angle Peak Radius Additional End Connectors Improvements Crimping Profile Flexibility Conformability Longitudinal Robustness
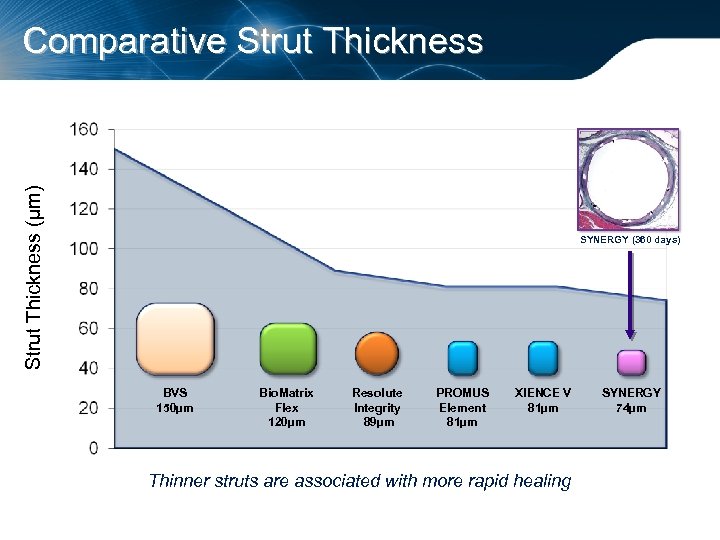
Strut Thickness (µm) Comparative Strut Thickness SYNERGY (360 days) BVS 150µm Bio. Matrix Flex 120µm Resolute Integrity 89µm PROMUS Element 81µm XIENCE V 81µm Thinner struts are associated with more rapid healing SYNERGY 74µm
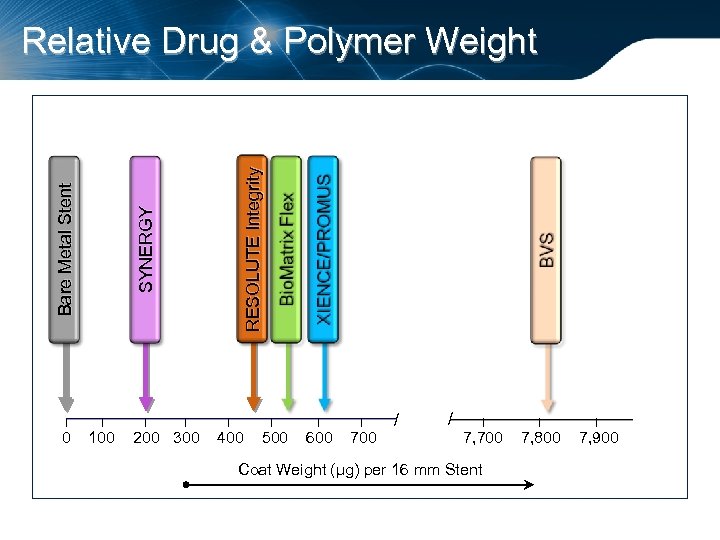
I 0 RESOLUTE Integrity SYNERGY Bare Metal Stent Relative Drug & Polymer Weight I 100 I I 200 300 I 400 // I 500 I 600 I 700 / / I 7, 700 Coat Weight (µg) per 16 mm Stent I 7, 800 I 7, 900
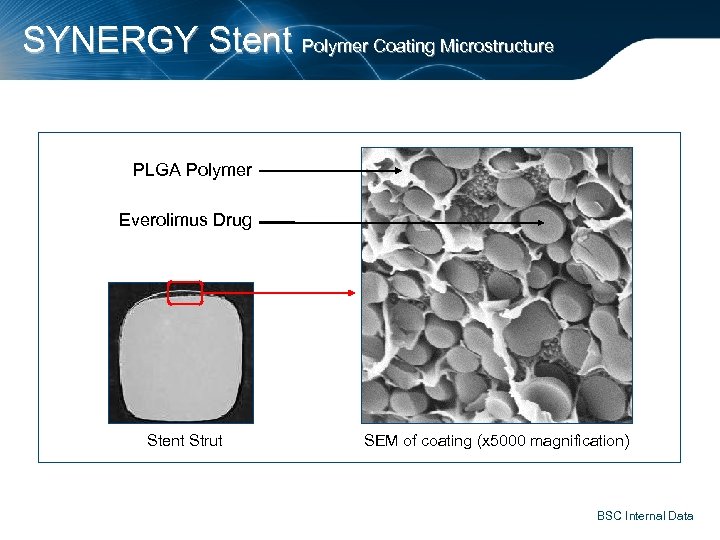
SYNERGY Stent Polymer Coating Microstructure PLGA Polymer Everolimus Drug Stent Strut SEM of coating (x 5000 magnification) BSC Internal Data
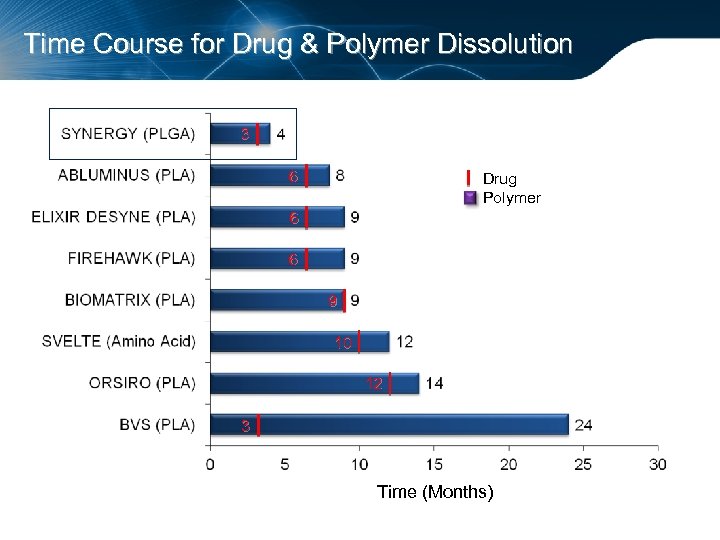
Time Course for Drug & Polymer Dissolution 3 6 Drug Polymer 6 6 9 10 12 3 Time (Months)
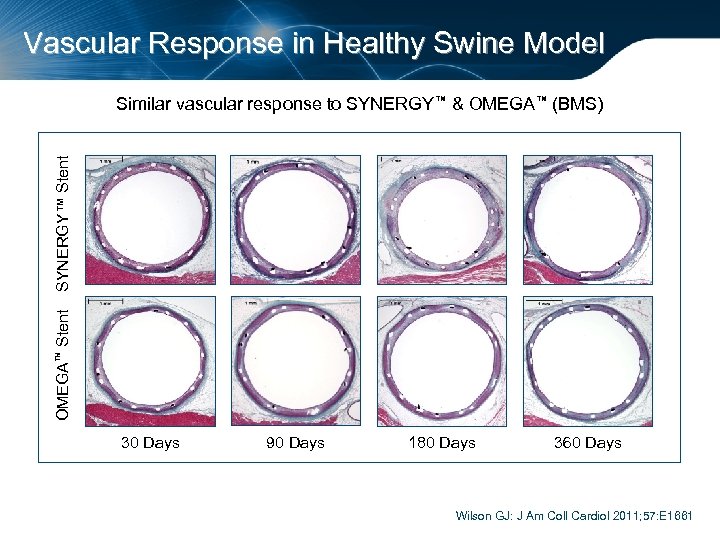
Vascular Response in Healthy Swine Model OMEGA™ Stent SYNERGY™ Stent Similar vascular response to SYNERGY™ & OMEGA™ (BMS) 30 Days 90 Days 180 Days 360 Days Wilson GJ: J Am Coll Cardiol 2011; 57: E 1661
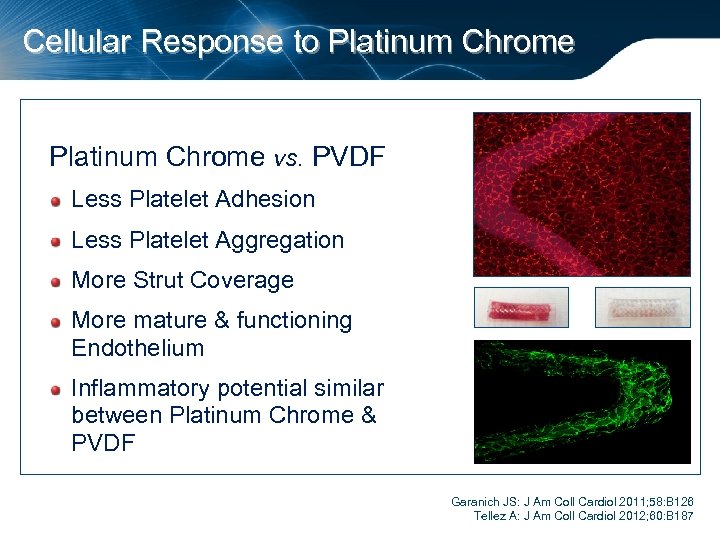
Cellular Response to Platinum Chrome vs. PVDF Less Platelet Adhesion Less Platelet Aggregation More Strut Coverage More mature & functioning Endothelium Inflammatory potential similar between Platinum Chrome & PVDF Garanich JS: J Am Coll Cardiol 2011; 58: B 126 Tellez A: J Am Coll Cardiol 2012; 60: B 187
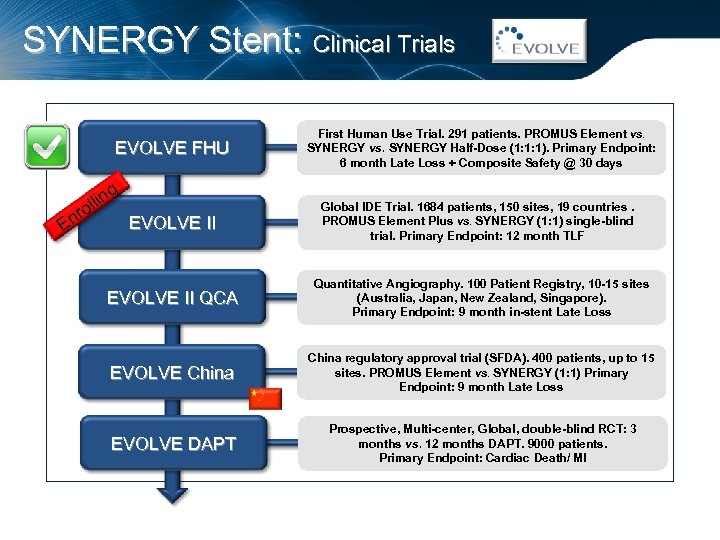
SYNERGY Stent: Clinical Trials EVOLVE FHU g llin o nr EVOLVE II E First Human Use Trial. 291 patients. PROMUS Element vs. SYNERGY vs. SYNERGY Half-Dose (1: 1: 1). Primary Endpoint: 6 month Late Loss + Composite Safety @ 30 days Global IDE Trial. 1684 patients, 150 sites, 19 countries. PROMUS Element Plus vs. SYNERGY (1: 1) single-blind trial. Primary Endpoint: 12 month TLF EVOLVE II QCA Quantitative Angiography. 100 Patient Registry, 10 -15 sites (Australia, Japan, New Zealand, Singapore). Primary Endpoint: 9 month in-stent Late Loss EVOLVE China regulatory approval trial (SFDA). 400 patients, up to 15 sites. PROMUS Element vs. SYNERGY (1: 1) Primary Endpoint: 9 month Late Loss EVOLVE DAPT Prospective, Multi-center, Global, double-blind RCT: 3 months vs. 12 months DAPT. 9000 patients. Primary Endpoint: Cardiac Death/ MI
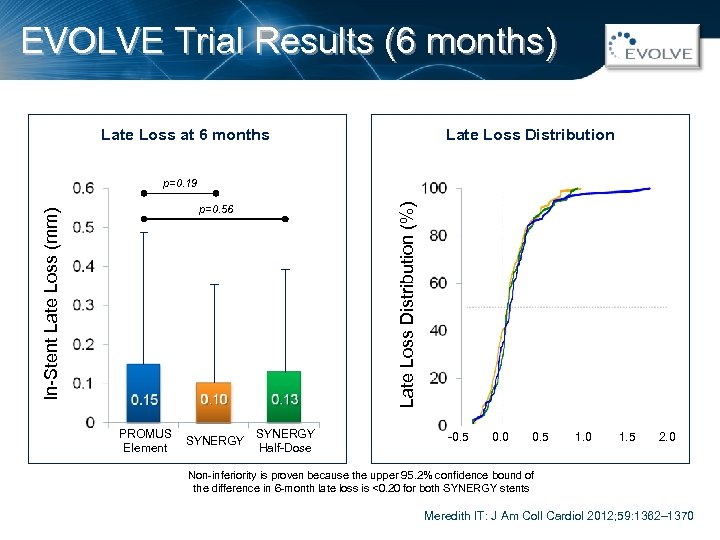
EVOLVE Trial Results (6 months) Late Loss Distribution Late Loss at 6 months Late Loss Distribution (%) p=0. 19 In-Stent Late Loss (mm) p=0. 56 PROMUS Element SYNERGY Half-Dose -0. 5 0. 0 0. 5 1. 0 1. 5 2. 0 Non-inferiority is proven because the upper 95. 2% confidence bound of the difference in 6 -month late loss is <0. 20 for both SYNERGY stents Meredith IT: J Am Coll Cardiol 2012; 59: 1362– 1370
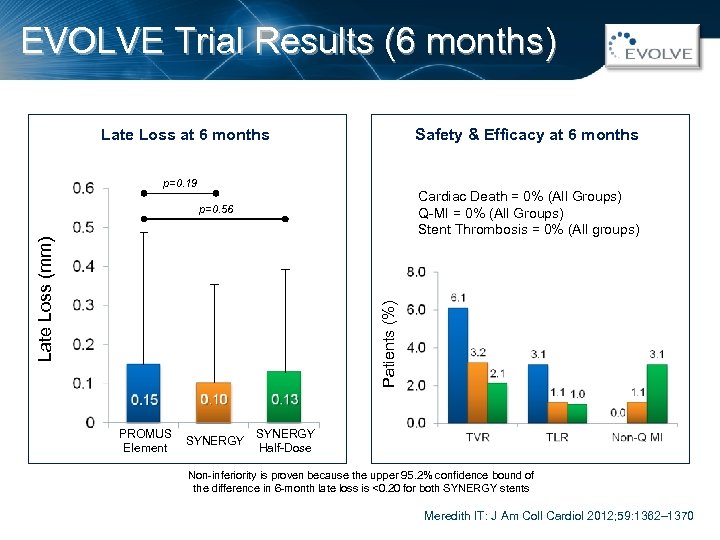
EVOLVE Trial Results (6 months) Safety & Efficacy at 6 months Late Loss at 6 months p=0. 19 Cardiac Death = 0% (All Groups) Q-MI = 0% (All Groups) Stent Thrombosis = 0% (All groups) Patients (%) Late Loss (mm) p=0. 56 PROMUS Element SYNERGY Half-Dose Non-inferiority is proven because the upper 95. 2% confidence bound of the difference in 6 -month late loss is <0. 20 for both SYNERGY stents Meredith IT: J Am Coll Cardiol 2012; 59: 1362– 1370
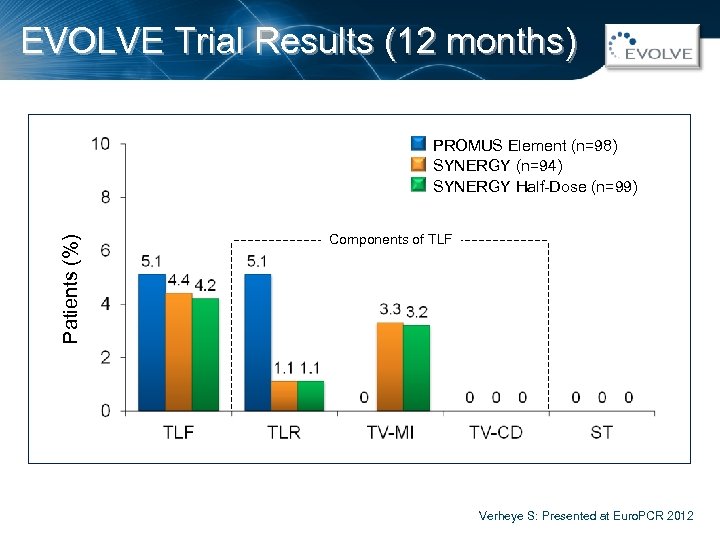
EVOLVE Trial Results (12 months) Patients (%) PROMUS Element (n=98) SYNERGY (n=94) SYNERGY Half-Dose (n=99) Components of TLF Verheye S: Presented at Euro. PCR 2012
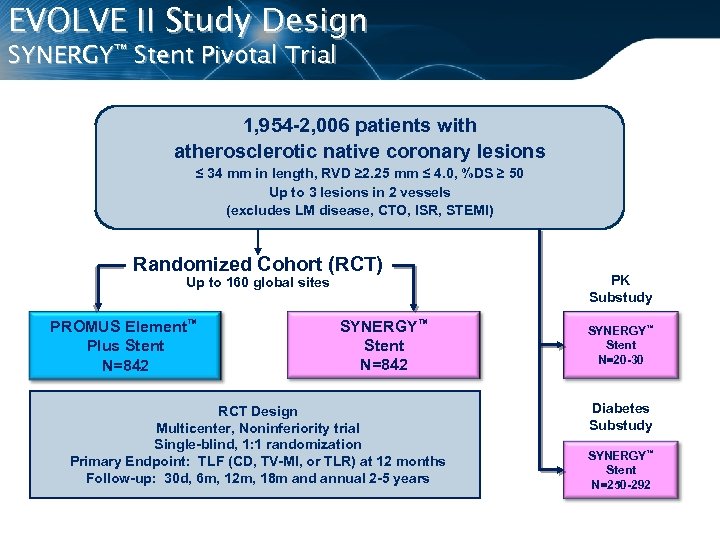
EVOLVE II Study Design SYNERGY™ Stent Pivotal Trial 1, 954 -2, 006 patients with atherosclerotic native coronary lesions ≤ 34 mm in length, RVD ≥ 2. 25 mm ≤ 4. 0, %DS ≥ 50 Up to 3 lesions in 2 vessels (excludes LM disease, CTO, ISR, STEMI) Randomized Cohort (RCT) Up to 160 global sites PROMUS Element™ Plus Stent N=842 SYNERGY™ Stent N=842 RCT Design Multicenter, Noninferiority trial Single-blind, 1: 1 randomization Primary Endpoint: TLF (CD, TV-MI, or TLR) at 12 months Follow-up: 30 d, 6 m, 12 m, 18 m and annual 2 -5 years PK Substudy SYNERGY™ Stent N=20 -30 Diabetes Substudy SYNERGY™ Stent N=250 -292
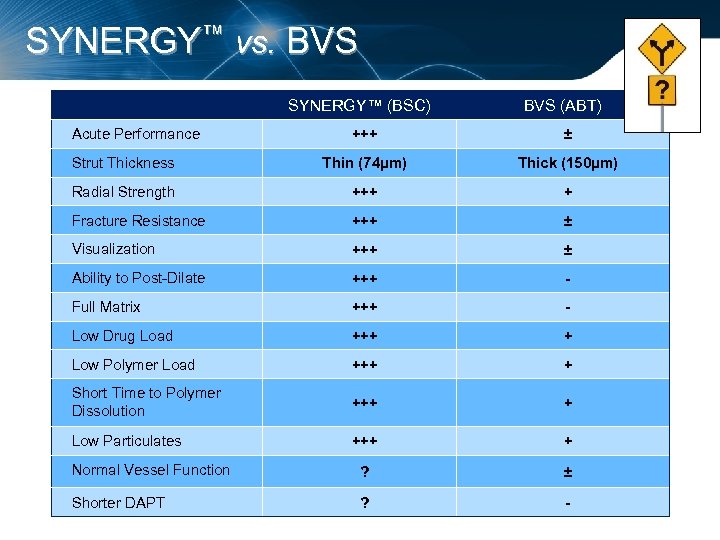
SYNERGY™ vs. BVS SYNERGY™ (BSC) Acute Performance BVS (ABT) +++ ± Strut Thickness Thin (74µm) Thick (150µm) Radial Strength +++ + Fracture Resistance +++ ± Visualization +++ ± Ability to Post-Dilate +++ - Full Matrix +++ - Low Drug Load +++ + Low Polymer Load +++ + Short Time to Polymer Dissolution +++ + Low Particulates +++ + Normal Vessel Function ? ± Shorter DAPT ? -
The Burden of Stent Thrombosis. . . Thrombus Cost DAPT Hemorrhage
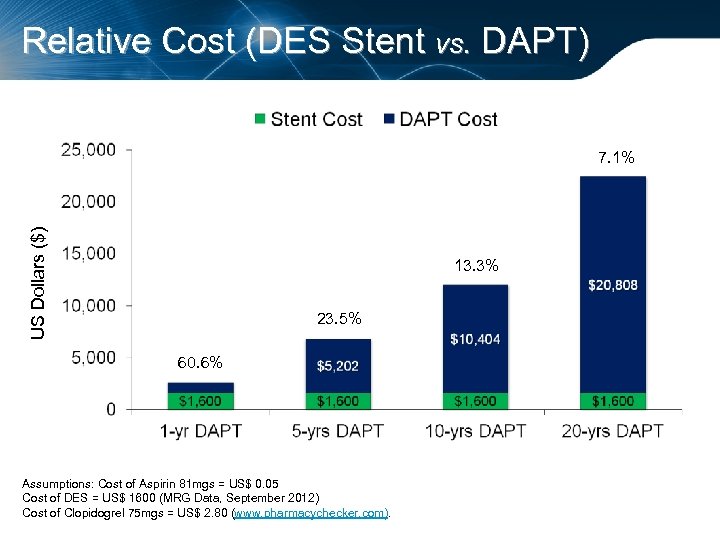
Relative Cost (DES Stent vs. DAPT) US Dollars ($) 7. 1% 13. 3% 23. 5% 60. 6% Assumptions: Cost of Aspirin 81 mgs = US$ 0. 05 Cost of DES = US$ 1600 (MRG Data, September 2012) Cost of Clopidogrel 75 mgs = US$ 2. 80 (www. pharmacychecker. com).

Conclusions Long term durable polymer exposure is potentially undesirable The SYNERGY™ Stent is a next generation bioabsorbable polymer technology with unique properties: Polymer gone shortly after drug elution is complete at 3 months Parallel, synchronous drug release and polymer absorption Ultra-thin abluminal coating and lower polymer load than previous technologies Presence of drug in arterial tissue throughout entire course of polymer degradation to promote optimal healing 12 -month safety and efficacy data from the EVOLVE Trial support positive clinical performance Bioabsorbable polymer DES may improve late outcomes (reduce late/very late ST), minimize DAPT dependency, and enhance healing vs. durable polymer DES. Further confirmatory trials are ongoing
cfd4fda73b8569a88970c3d509ed9956.ppt