bimetallic_2015_Bukhtiyarov_IIC.pptx
- Количество слайдов: 51

BORESKOV INSTITUTE OF CATALYSIS Siberian Branch, Russian Academy of Sciences Dedicated to Prof. D. W. Goodman teacher, colleague, friend Bimetallic Pd-based Supported Catalysts for Selective Oxidation and Hydrogenation: improving the selectivity via tuning the structure and composition of active centers Valerii I. Bukhtiyarov Pr. Akademika Lavrentieva, 5 Novosibirsk, 630090 Russia Tel. : +7 -(383)-330 -67 -71 Fax: +7 -(383)-330 -8356 E-mail: vib@catalysis. ru
OUTLINE Bimetallic catalysts for organic synthesis: motivation & overview Pd-Au alloy catalysts Structure of surface sites Vinyl acetate synthesis Selective oxidation of glucose to gluconic acid Pd-Zn Alloy Catalysts Preparation via molecular complexes Selective hydrogenation of acetylene Summary
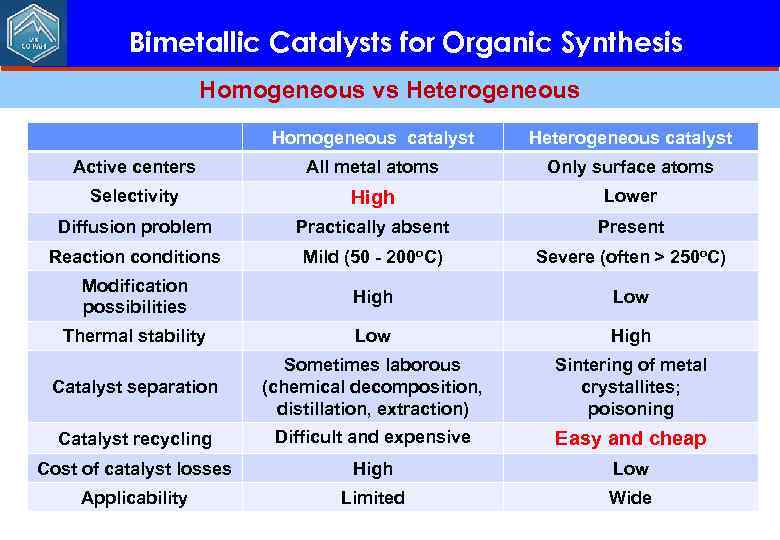
Bimetallic Catalysts for Organic Synthesis Homogeneous vs Heterogeneous Homogeneous catalyst Heterogeneous catalyst Active centers All metal atoms Only surface atoms Selectivity High Lower Diffusion problem Practically absent Present Reaction conditions Mild (50 - 200 o. C) Severe (often > 250 o. C) Modification possibilities High Low Thermal stability Low High Catalyst separation Sometimes laborous (chemical decomposition, distillation, extraction) Sintering of metal crystallites; poisoning Catalyst recycling Difficult and expensive Easy and cheap Cost of catalyst losses High Low Applicability Limited Wide
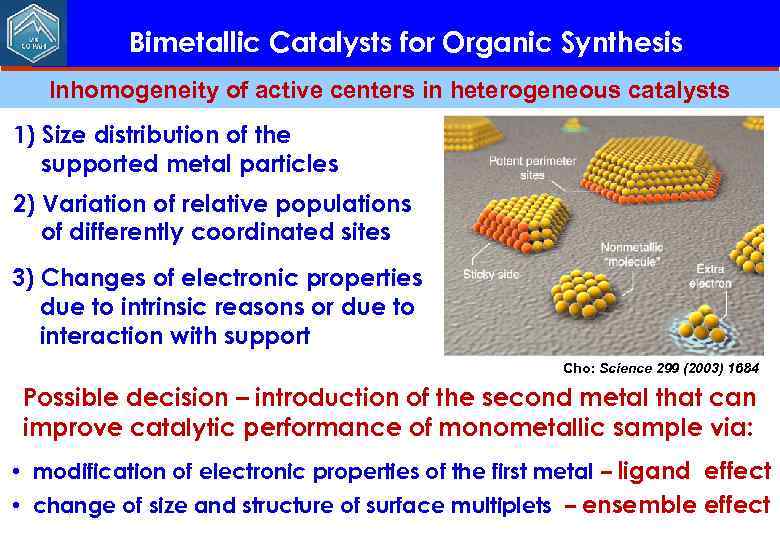
Bimetallic Catalysts for Organic Synthesis Inhomogeneity of active centers in heterogeneous catalysts 1) Size distribution of the supported metal particles 2) Variation of relative populations of differently coordinated sites 3) Changes of electronic properties due to intrinsic reasons or due to interaction with support Cho: Science 299 (2003) 1684 Possible decision – introduction of the second metal that can improve catalytic performance of monometallic sample via: • modification of electronic properties of the first metal – ligand effect • change of size and structure of surface multiplets – ensemble effect
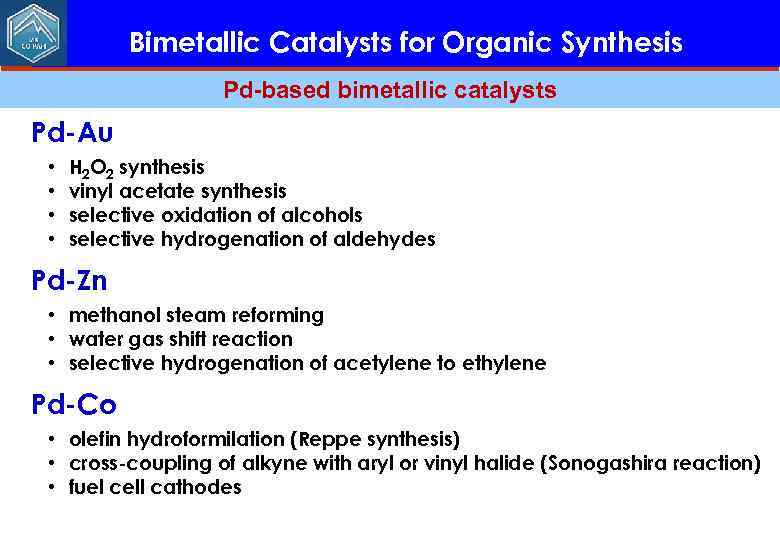
Bimetallic Catalysts for Organic Synthesis Pd-based bimetallic catalysts Pd-Au • • H 2 O 2 synthesis vinyl acetate synthesis selective oxidation of alcohols selective hydrogenation of aldehydes Pd-Zn • methanol steam reforming • water gas shift reaction • selective hydrogenation of acetylene to ethylene Pd-Co • olefin hydroformilation (Reppe synthesis) • cross-coupling of alkyne with aryl or vinyl halide (Sonogashira reaction) • fuel cell cathodes
OUTLINE Bimetallic catalysts for organic synthesis: motivation & overview Pd-Au alloy catalysts Structure of surface sites Vinyl acetate synthesis Selective oxidation of glucose to gluconic acid Pd-Zn Alloy Catalysts Preparation via molecular complexes Selective hydrogenation of acetylene Summary
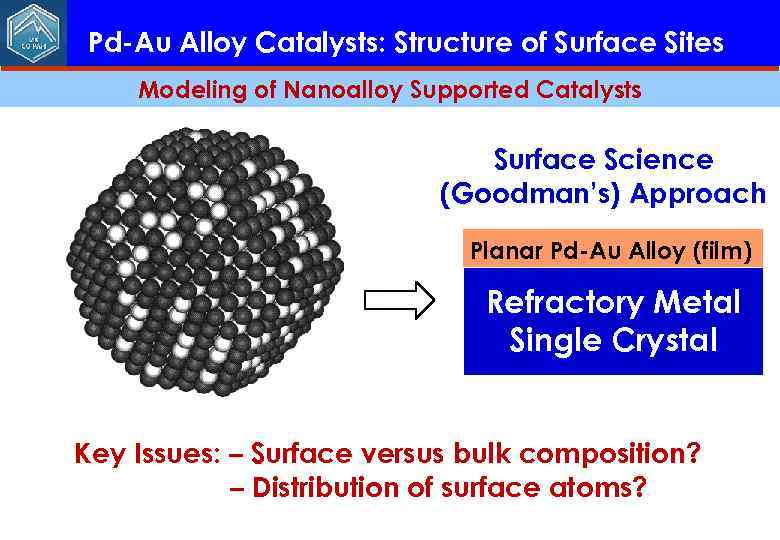
Pd-Au Alloy Catalysts: Structure of Surface Sites Modeling of Nanoalloy Supported Catalysts Surface Science (Goodman’s) Approach Planar Pd-Au Alloy (film) Refractory Metal Single Crystal Key Issues: – Surface versus bulk composition? – Distribution of surface atoms?
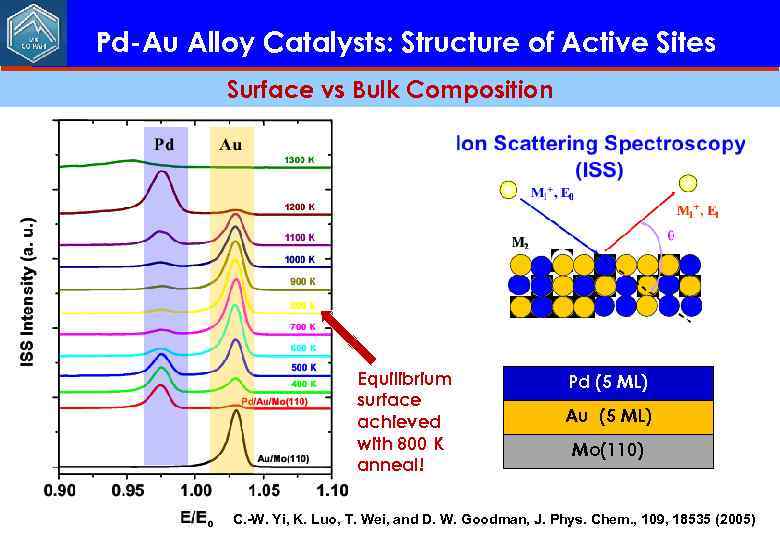
Pd-Au Alloy Catalysts: Structure of Active Sites Surface vs Bulk Composition Equilibrium surface achieved with 800 K anneal! Pd (5 ML) Au (5 ML) Mo(110) C. -W. Yi, K. Luo, T. Wei, and D. W. Goodman, J. Phys. Chem. , 109, 18535 (2005)
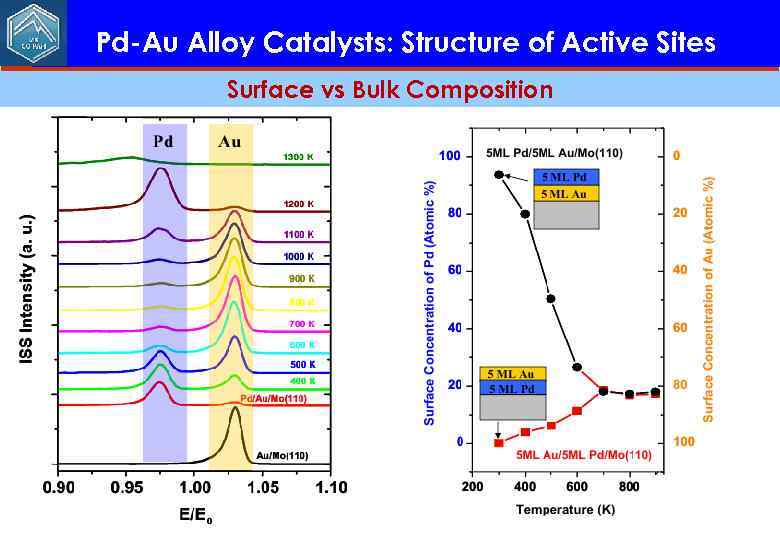
Pd-Au Alloy Catalysts: Structure of Active Sites Surface vs Bulk Composition
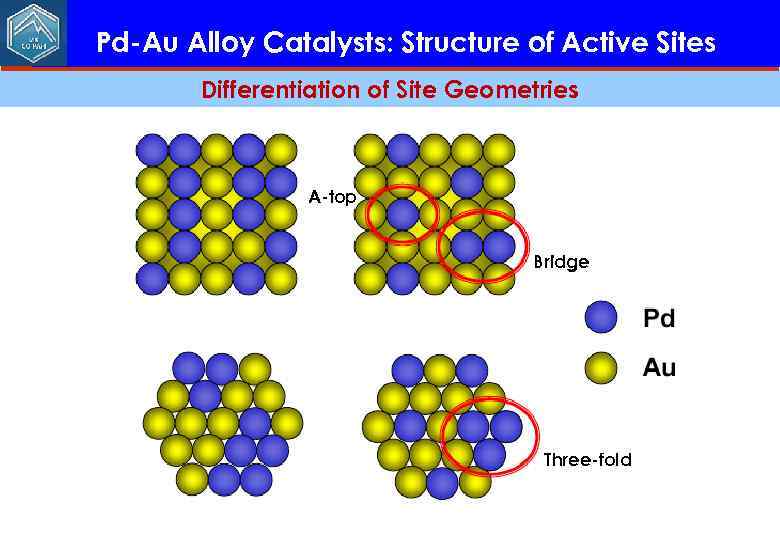
Pd-Au Alloy Catalysts: Structure of Active Sites Differentiation of Site Geometries A-top Bridge Three-fold
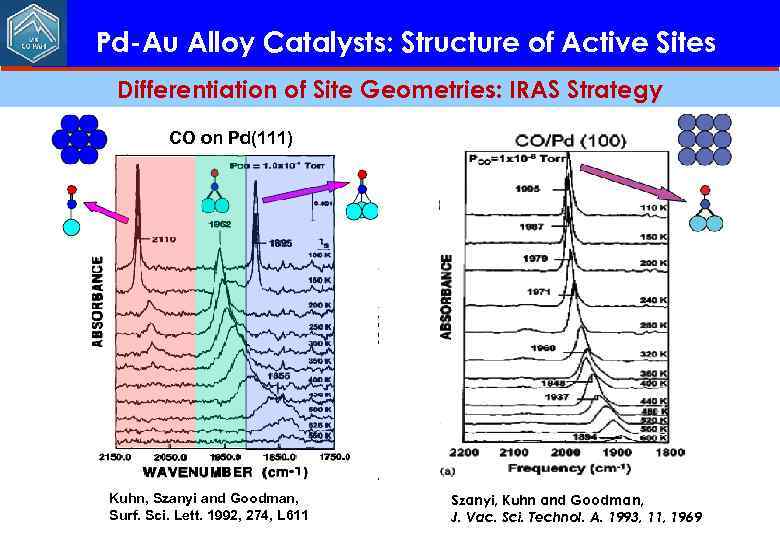
Pd-Au Alloy Catalysts: Structure of Active Sites Differentiation of Site Geometries: IRAS Strategy CO on Pd(111) Kuhn, Szanyi and Goodman, Surf. Sci. Lett. 1992, 274, L 611 Szanyi, Kuhn and Goodman, J. Vac. Sci. Technol. A. 1993, 11, 1969
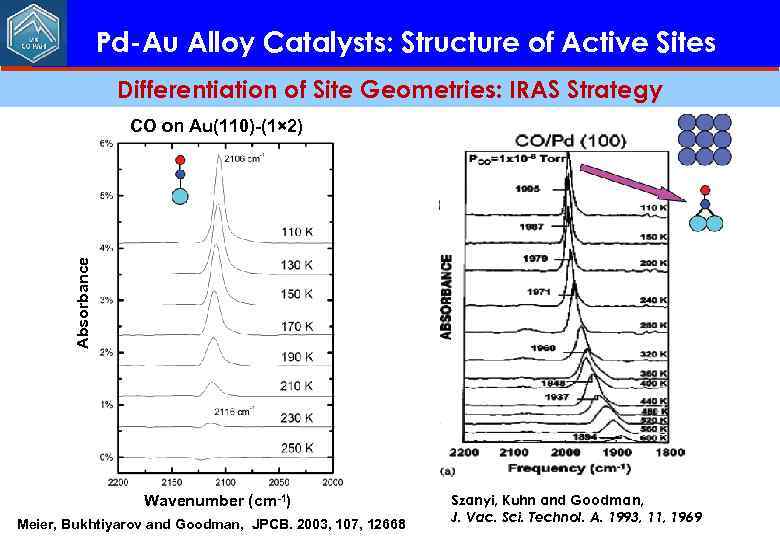
Pd-Au Alloy Catalysts: Structure of Active Sites Differentiation of Site Geometries: IRAS Strategy Absorbance CO on Au(110)-(1× 2) Wavenumber (cm-1) Meier, Bukhtiyarov and Goodman, JPCB. 2003, 107, 12668 Szanyi, Kuhn and Goodman, J. Vac. Sci. Technol. A. 1993, 11, 1969
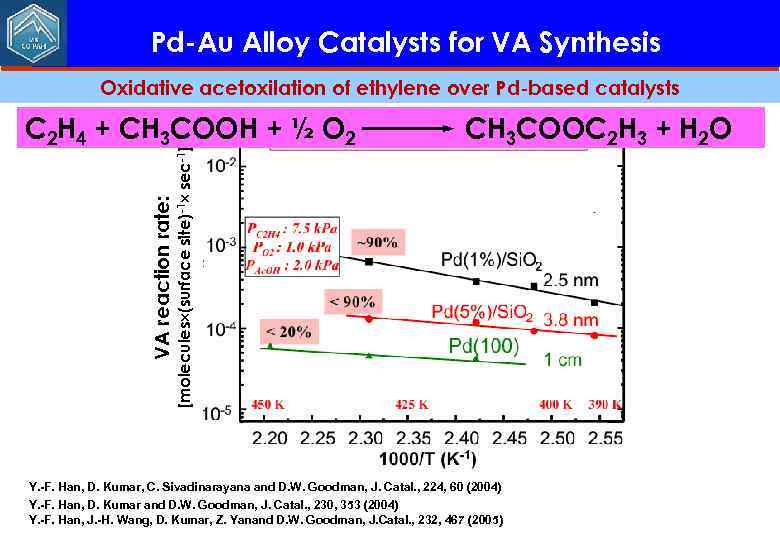
Pd-Au Alloy Catalysts for VA Synthesis Oxidative acetoxilation of ethylene over Pd-based catalysts CH 3 COOC 2 H 3 + H 2 O VA reaction rate: [molecules (surface site)-1 sec-1] C 2 H 4 + CH 3 COOH + ½ O 2 Y. -F. Han, D. Kumar, C. Sivadinarayana and D. W. Goodman, J. Catal. , 224, 60 (2004) Y. -F. Han, D. Kumar and D. W. Goodman, J. Catal. , 230, 353 (2004) Y. -F. Han, J. -H. Wang, D. Kumar, Z. Yanand D. W. Goodman, J. Catal. , 232, 467 (2005)
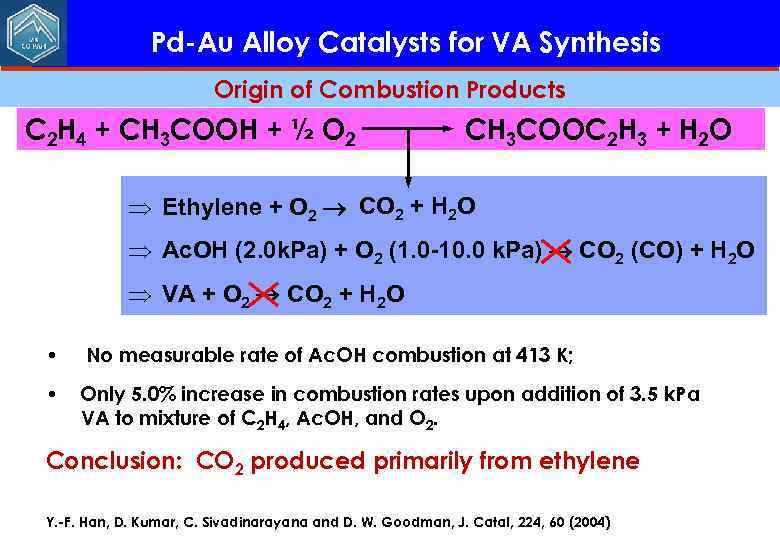
Pd-Au Alloy Catalysts for VA Synthesis Origin of Combustion Products C 2 H 4 + CH 3 COOH + ½ O 2 CH 3 COOC 2 H 3 + H 2 O Þ Ethylene + O 2 CO 2 + H 2 O Þ Ac. OH (2. 0 k. Pa) + O 2 (1. 0 -10. 0 k. Pa) CO 2 (CO) + H 2 O Þ VA + O 2 CO 2 + H 2 O • No measurable rate of Ac. OH combustion at 413 K; • Only 5. 0% increase in combustion rates upon addition of 3. 5 k. Pa VA to mixture of C 2 H 4, Ac. OH, and O 2. Conclusion: CO 2 produced primarily from ethylene Y. -F. Han, D. Kumar, C. Sivadinarayana and D. W. Goodman, J. Catal, 224, 60 (2004)
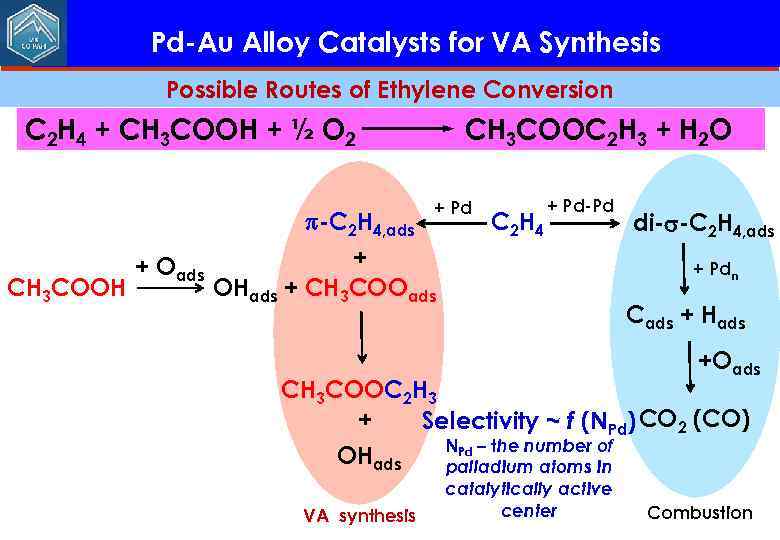
Pd-Au Alloy Catalysts for VA Synthesis Possible Routes of Ethylene Conversion C 2 H 4 + CH 3 COOH + ½ O 2 -C 2 H 4, ads CH 3 COOH + Oads CH 3 COOC 2 H 3 + H 2 O + Pd C 2 H 4 + Pd-Pd + OHads + CH 3 COOads di- -C 2 H 4, ads + Pdn Cads + Hads +Oads CH 3 COOC 2 H 3 + Selectivity ~ f (NPd) CO 2 (CO) NPd – the number of OHads palladium atoms in VA synthesis catalytically active center Combustion
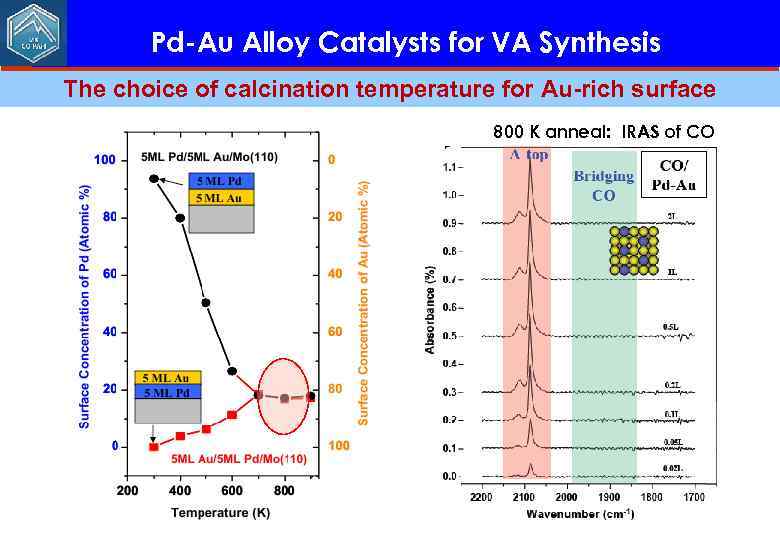
Pd-Au Alloy Catalysts for VA Synthesis The choice of calcination temperature for Au-rich surface 800 K anneal: IRAS of CO
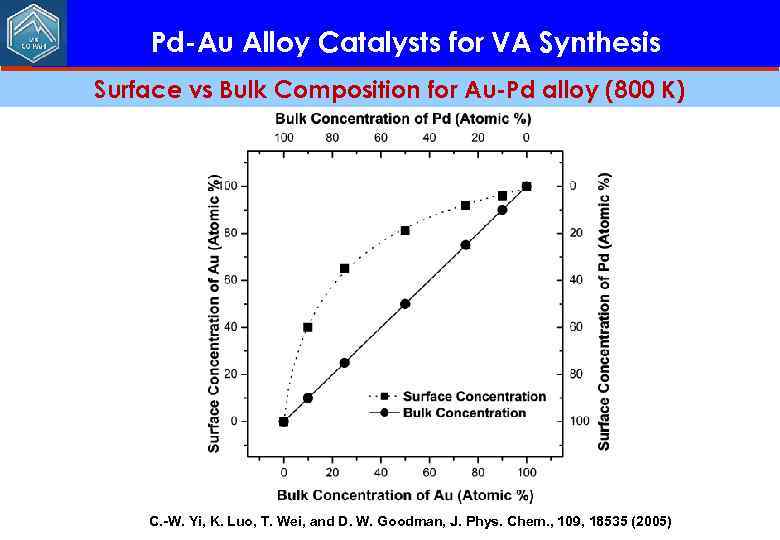
Pd-Au Alloy Catalysts for VA Synthesis Surface vs Bulk Composition for Au-Pd alloy (800 K) C. -W. Yi, K. Luo, T. Wei, and D. W. Goodman, J. Phys. Chem. , 109, 18535 (2005)
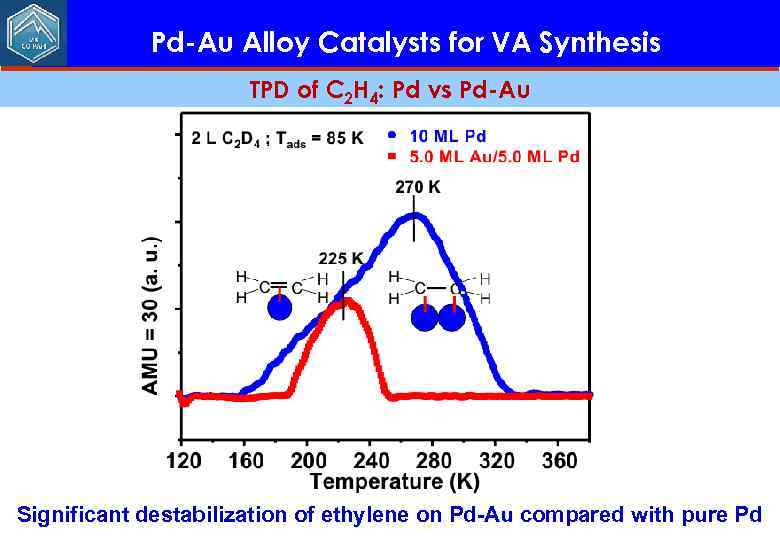
Pd-Au Alloy Catalysts for VA Synthesis TPD of C 2 H 4: Pd vs Pd-Au Significant destabilization of ethylene on Pd-Au compared with pure Pd
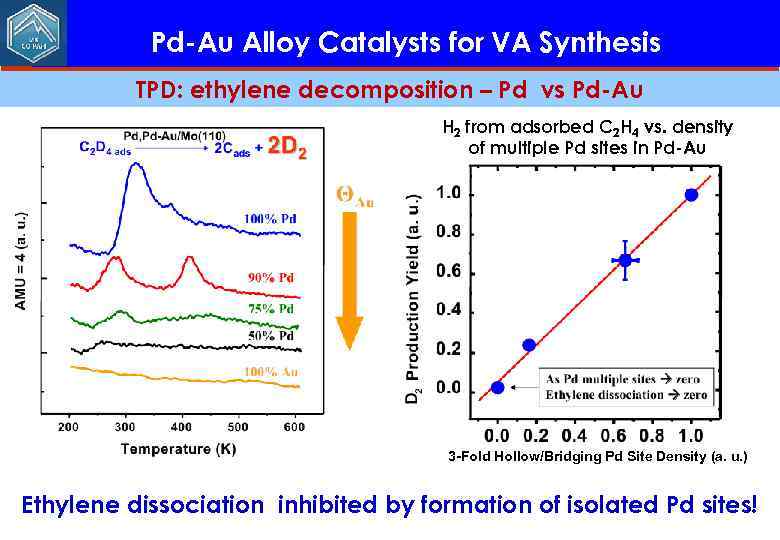
Pd-Au Alloy Catalysts for VA Synthesis TPD: ethylene decomposition – Pd vs Pd-Au H 2 from adsorbed C 2 H 4 vs. density of multiple Pd sites in Pd-Au 3 -Fold Hollow/Bridging Pd Site Density (a. u. ) Ethylene dissociation inhibited by formation of isolated Pd sites!
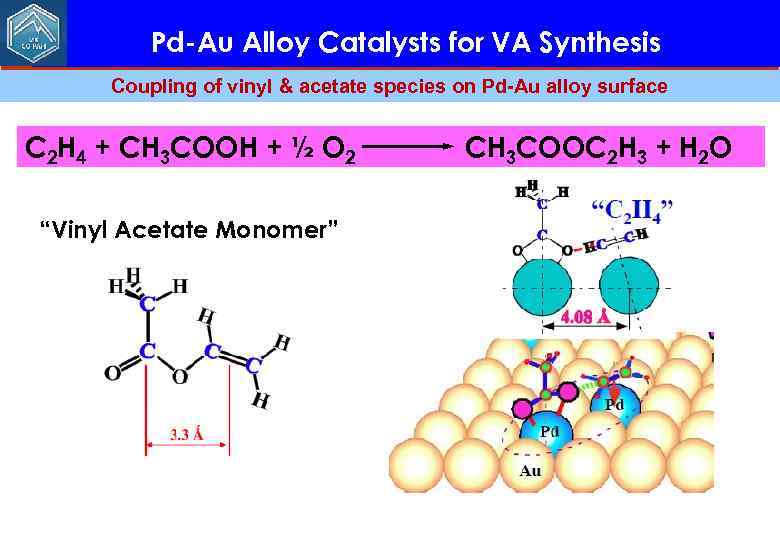
Pd-Au Alloy Catalysts for VA Synthesis Сoupling of vinyl & acetate species on Pd-Au alloy surface C 2 H 4 + CH 3 COOH + ½ O 2 “Vinyl Acetate Monomer” CH 3 COOC 2 H 3 + H 2 O
![Pd-Au Alloy Catalysts for VA Synthesis VA reaction rate: [molecules (surface site)-1 sec-1] Oxidative Pd-Au Alloy Catalysts for VA Synthesis VA reaction rate: [molecules (surface site)-1 sec-1] Oxidative](https://present5.com/presentation/-104014181_425067588/image-21.jpg)
Pd-Au Alloy Catalysts for VA Synthesis VA reaction rate: [molecules (surface site)-1 sec-1] Oxidative acetoxilation of ethylene over Pd-based catalysts
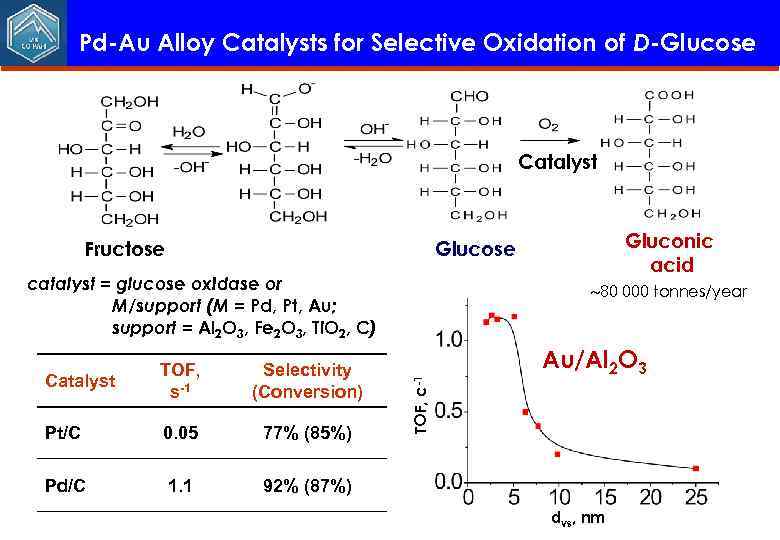
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Catalyst Fructose Gluconic acid Glucose catalyst = glucose oxidase or M/support (М = Pd, Pt, Au; support = Al 2 O 3, Fe 2 O 3, Ti. O 2, C) Selectivity (Conversion) Pt/С 0. 05 77% (85%) Pd/С 1. 1 Au/Al 2 O 3 92% (87%) TOF, c-1 Catalyst TOF, s-1 80 000 tonnes/year dvs, nm
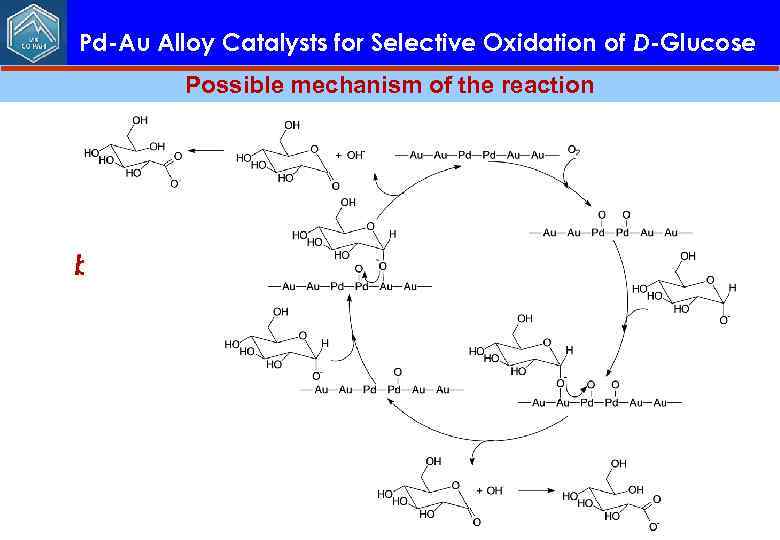
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Possible mechanism of the reaction In our opinion, the next important step would be tuning the composition and nanostructure of Au. Pd particles by changing a Pd: Au ratio in order to achieve optimal catalytic performance
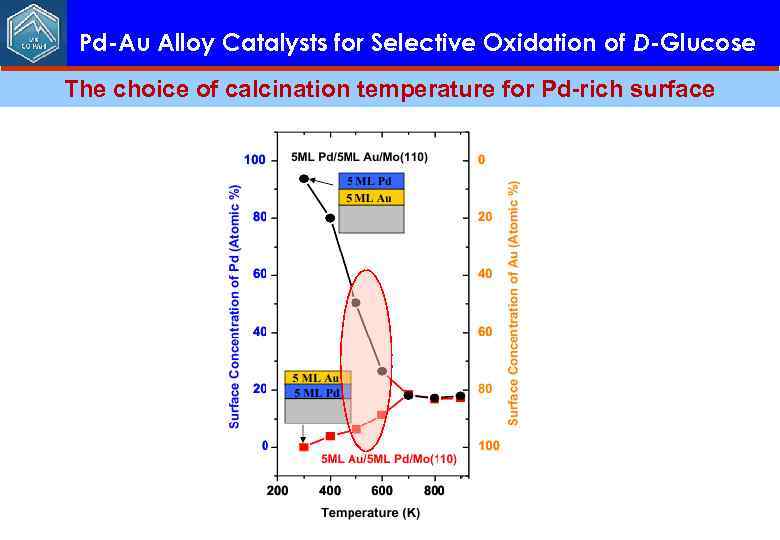
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose The choice of calcination temperature for Pd-rich surface
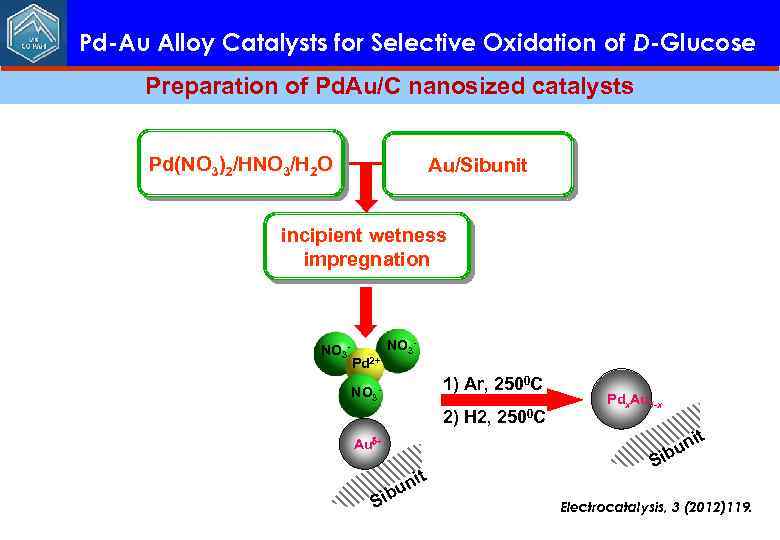
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Preparation of Pd. Au/C nanosized catalysts Pd(NO 3)2/HNO 3/H 2 O Au/Sibunit incipient wetness impregnation NO 3 - Pd 2+ NO 3 - 1) Ar, 2500 C NO 3 - 2) H 2, 2500 C Pdx. Au 1 -x it un ib Au + ibu S nit S Electrocatalysis, 3 (2012)119.
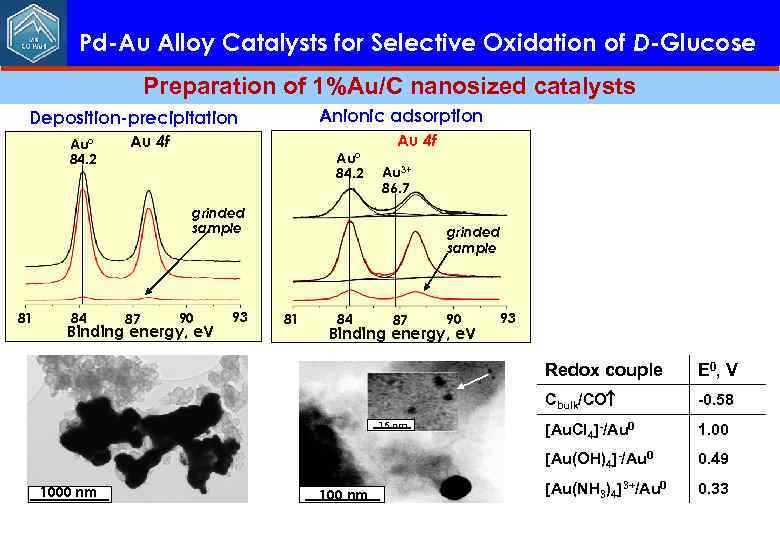
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Preparation of 1%Au/C nanosized catalysts Anionic adsorption Deposition-precipitation Au° 84. 2 Au 4 f Au 3+ 86. 7 grinded sample 81 84 87 90 Binding energy, e. V 93 Redox couple Cbulk/CO 1000 nm 100 nm -0. 58 [Au. Cl 4]-/Au 0 1. 00 [Au(OH)4]-/Au 0 15 nm E 0, V 0. 49 [Au(NH 3)4]3+/Au 0 0. 33
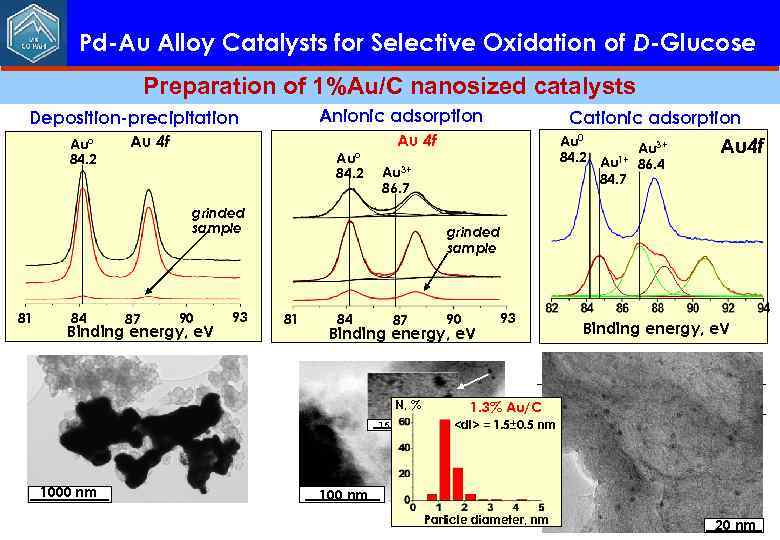
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Preparation of 1%Au/C nanosized catalysts Anionic adsorption Deposition-precipitation Au° 84. 2 Au 4 f 84 87 90 Binding energy, e. V 93 Au 0 Au 3+ 84. 2 Au 1+ 86. 4 84. 7 Au 3+ 86. 7 grinded sample 81 Cationic adsorption Au 4 f grinded sample 81 84 87 90 Binding energy, e. V 93 Binding energy, e. V Redox couple N, % 15 nm 1. 3% Au/C E 0, V Cbulk/CO -0. 58 <dl> = 1. 5± 0. 5 nm [Au. Cl ]-/Au 0 4 1. 00 [Au(OH)4]-/Au 0 1000 nm 100 nm 0. 49 [Au(NH 3)4]3+/Au 0 0. 33 Particle diameter, nm 20 nm
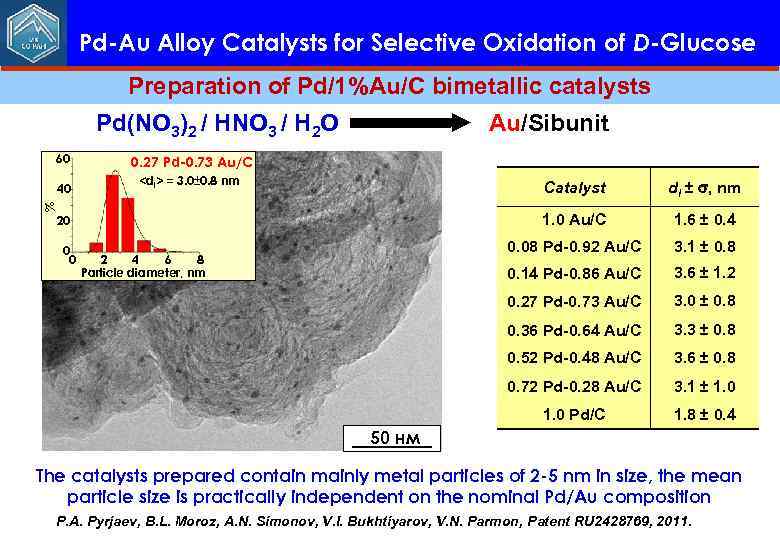
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Preparation of Pd/1%Au/C bimetallic catalysts Pd(NO 3)2 / HNO 3 / H 2 O 60 0. 27 Pd-0. 73 Au/C 40 Au/Sibunit <dl> = 3. 0± 0. 8 nm 0. 14 Pd-0. 86 Au/C 3. 6 ± 1. 2 3. 0 ± 0. 8 0. 36 Pd-0. 64 Au/C 3. 3 ± 0. 8 0. 52 Pd-0. 48 Au/C 3. 6 ± 0. 8 0. 72 Pd-0. 28 Au/C 3. 1 ± 1. 0 Pd/C 2 4 6 8 Particle diameter, nm 3. 1 ± 0. 8 0. 27 Pd-0. 73 Au/C 0 0 1. 6 ± 0. 4 0. 08 Pd-0. 92 Au/C 20 dl ± , nm 1. 0 Au/C % Catalyst 1. 8 ± 0. 4 50 нм The catalysts prepared contain mainly metal particles of 2 -5 nm in size, the mean particle size is practically independent on the nominal Pd/Au composition P. A. Pyrjaev, B. L. Moroz, A. N. Simonov, V. I. Bukhtiyarov, V. N. Parmon, Patent RU 2428769, 2011.
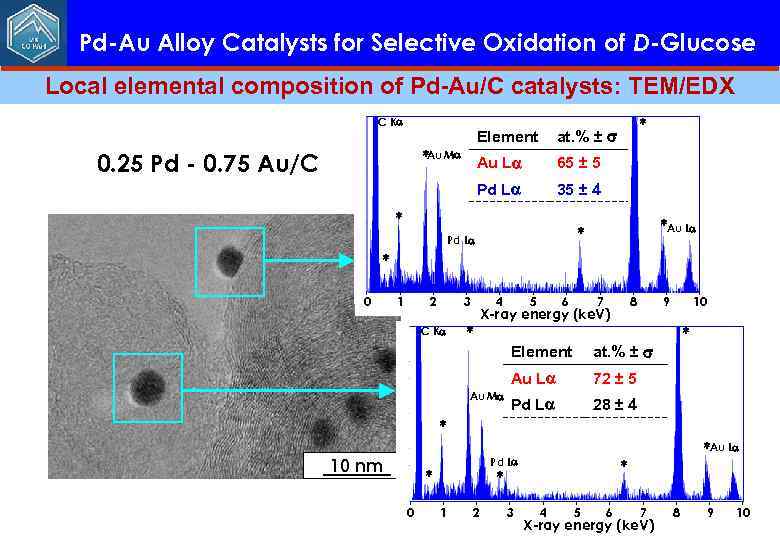
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Local elemental composition of Pd-Au/C catalysts: TEM/EDX C K *Au M Au L 0. 25 Pd - 0. 75 Au/C * at. % ± Element 65 ± 5 Pd L 35 ± 4 * *Au L * Pd L * 2 1 3 C K 0 * 4 5 6 7 8 X-ray energy (ke. V) Element 72 ± 5 Pd L * at. % ± Au L Au M 10 9 28 ± 4 * 10 nm Pd L * 0 * * 1 2 3 *Au L 4 5 6 7 X-ray energy (ke. V) 8 9 10
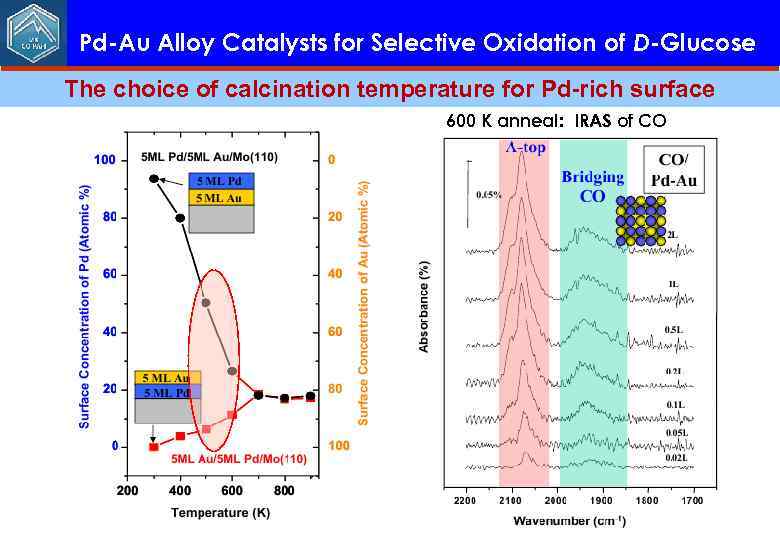
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose The choice of calcination temperature for Pd-rich surface 600 K anneal: IRAS of CO
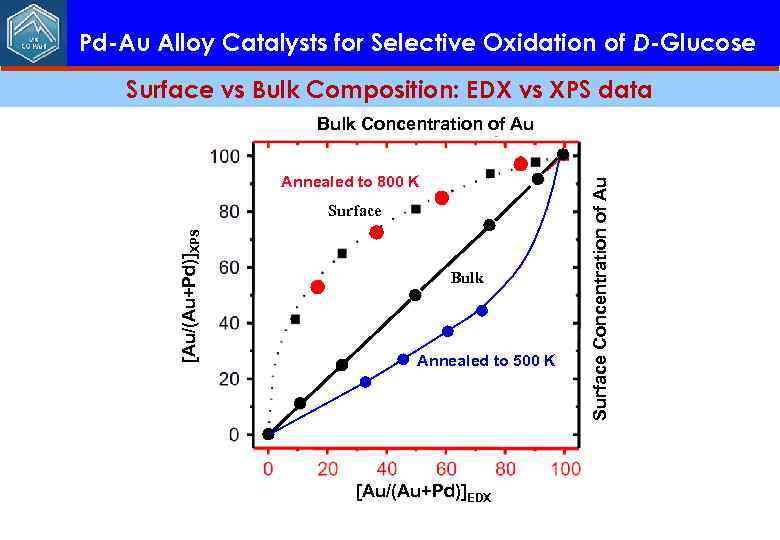
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Surface vs Bulk Composition: EDX vs XPS data Annealed to 800 K [Au/(Au+Pd)]XPS Surface Bulk Annealed to 500 K [Au/(Au+Pd)]EDX Surface Concentration of Au Bulk Concentration of Au
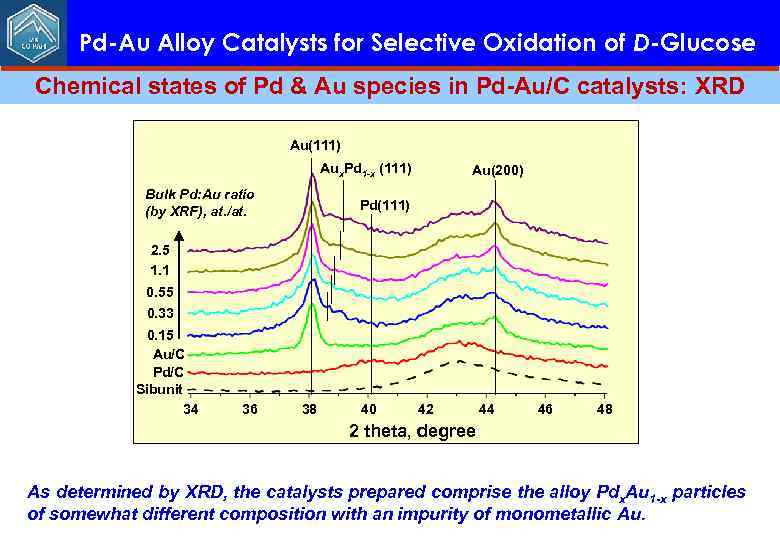
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Chemical states of Pd & Au species in Pd-Au/C catalysts: XRD Au(111) Aux. Pd 1 -x (111) Bulk Pd: Au ratio (by XRF), at. /at. Au(200) Pd(111) 2. 5 1. 1 0. 55 0. 33 0. 15 Au/C Pd/C Sibunit 34 36 38 40 42 44 46 48 2 theta, degree As determined by XRD, the catalysts prepared comprise the alloy Pdx. Au 1 -x particles of somewhat different composition with an impurity of monometallic Au.
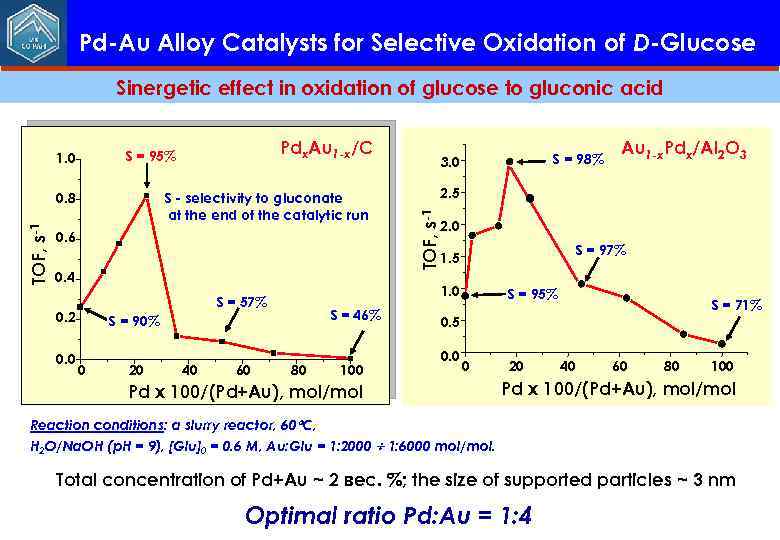
Pd-Au Alloy Catalysts for Selective Oxidation of D-Glucose Sinergetic effect in oxidation of glucose to gluconic acid 0. 6 0. 4 0. 0 S = 46% S = 90% 0 20 40 60 80 100 Au 1 -x. Pdx/Al 2 O 3 2. 5 2. 0 S = 97% 1. 5 1. 0 S = 57% 0. 2 S = 98% 3. 0 TOF, s-1 S - selectivity to gluconate at the end of the catalytic run 0. 8 TOF, s-1 Pdx. Au 1 -x/C S = 95% 1. 0 S = 95% S = 71% 0. 5 0. 0 0 Pd x 100/(Pd+Au), mol/mol 20 40 60 80 100 Pd x 100/(Pd+Au), mol/mol Reaction conditions: a slurry reactor, 60 C, Н 2 O/Na. OH (p. H = 9), [Glu]0 = 0. 6 M, Au: Glu = 1: 2000 1: 6000 mol/mol. Total concentration of Pd+Au ~ 2 вес. %; the size of supported particles ~ 3 nm Optimal ratio Pd: Au = 1: 4
OUTLINE Bimetallic catalysts for organic synthesis: motivation & overview Pd-Au alloy catalysts Vinyl acetate synthesis Structure of active sites Selective oxidation of glucose to gluconic acid Pd-Zn Alloy Catalysts Preparation via molecular complexes Selective hydrogenation of acetylene Summary
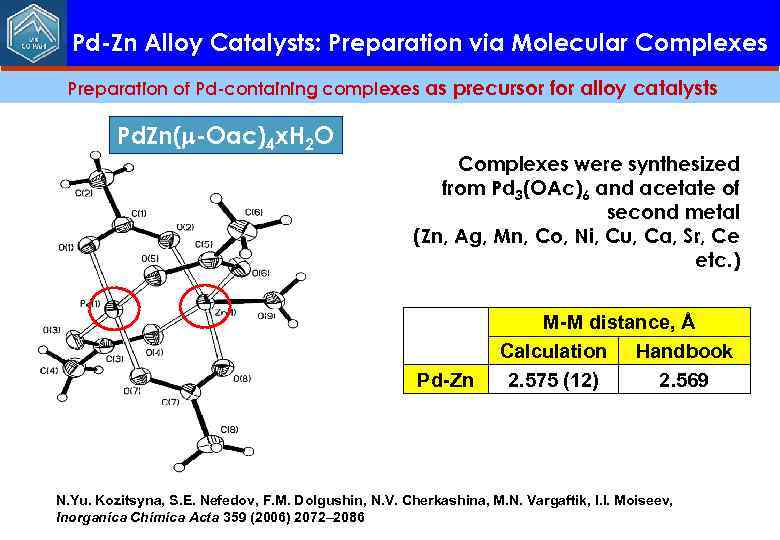
Pd-Zn Alloy Catalysts: Preparation via Molecular Complexes Preparation of Pd-containing complexes as precursor for alloy catalysts Pd. Zn(m-Oac)4 x. H 2 O Complexes were synthesized from Pd 3(OAc)6 and acetate of second metal (Zn, Ag, Mn, Co, Ni, Cu, Ca, Sr, Ce etc. ) Pd-Zn М-М distance, Å Calculation Handbook 2. 575 (12) 2. 569 N. Yu. Kozitsyna, S. E. Nefedov, F. M. Dolgushin, N. V. Cherkashina, M. N. Vargaftik, I. I. Moiseev, Inorganica Chimica Acta 359 (2006) 2072– 2086 35
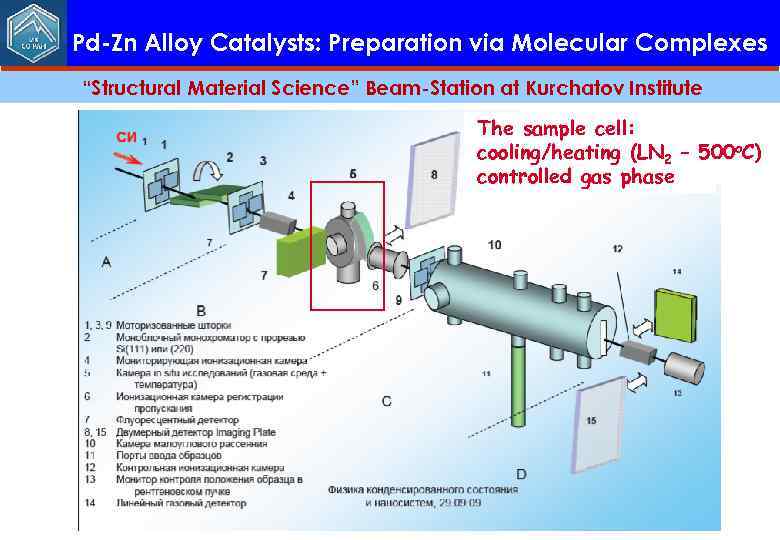
Pd-Zn Alloy Catalysts: Preparation via Molecular Complexes “Structural Material Science” Beam-Station at Kurchatov Institute The sample cell: cooling/heating (LN 2 – 500 o. C) controlled gas phase
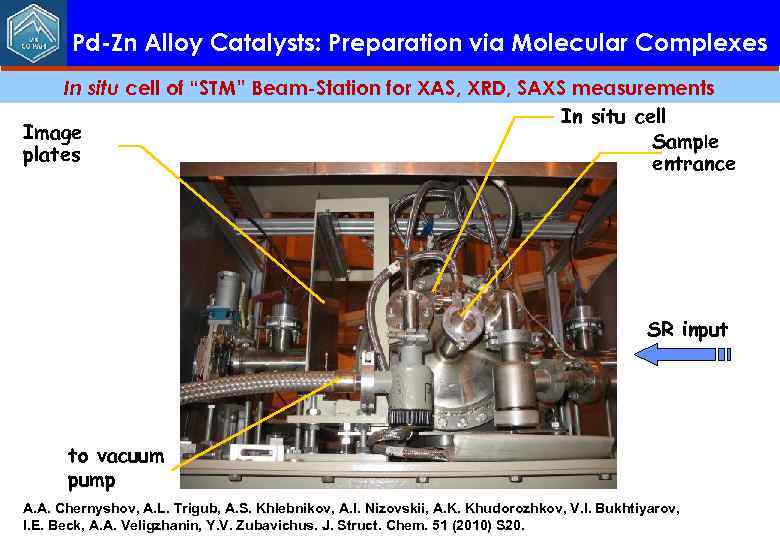
Pd-Zn Alloy Catalysts: Preparation via Molecular Complexes In situ cell of “STM” Beam-Station for XAS, XRD, SAXS measurements In situ cell Image Sample plates entrance SR input to vacuum pump A. A. Chernyshov, A. L. Trigub, A. S. Khlebnikov, A. I. Nizovskii, A. K. Khudorozhkov, V. I. Bukhtiyarov, I. E. Beck, A. A. Veligzhanin, Y. V. Zubavichus. J. Struct. Chem. 51 (2010) S 20.
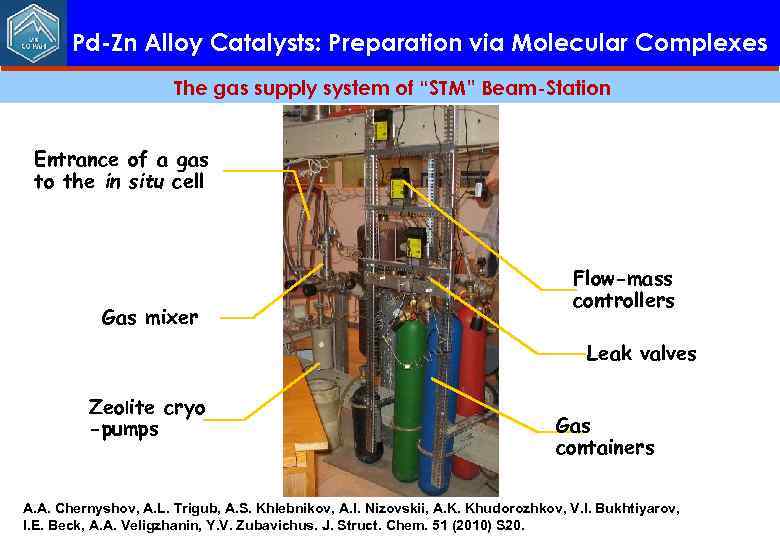
Pd-Zn Alloy Catalysts: Preparation via Molecular Complexes The gas supply system of “STM” Beam-Station Entrance of a gas to the in situ cell Gas mixer Flow-mass controllers Leak valves Zeolite cryo -pumps Gas containers A. A. Chernyshov, A. L. Trigub, A. S. Khlebnikov, A. I. Nizovskii, A. K. Khudorozhkov, V. I. Bukhtiyarov, I. E. Beck, A. A. Veligzhanin, Y. V. Zubavichus. J. Struct. Chem. 51 (2010) S 20.
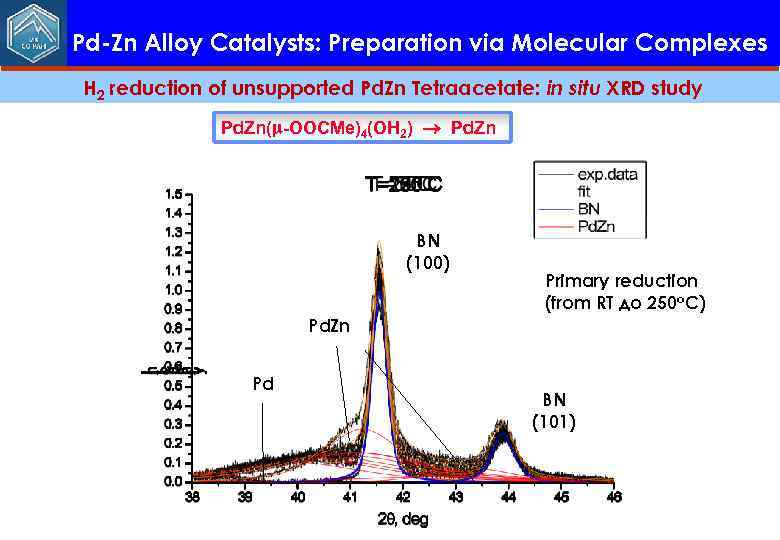
Pd-Zn Alloy Catalysts: Preparation via Molecular Complexes H 2 reduction of unsupported Pd. Zn Tetraacetate: in situ XRD study Pd. Zn(m-OOCMe)4(OH 2) Pd. Zn BN (100) Primary reduction (from RT до 250 о. С) Pd. Zn Pd BN (101)
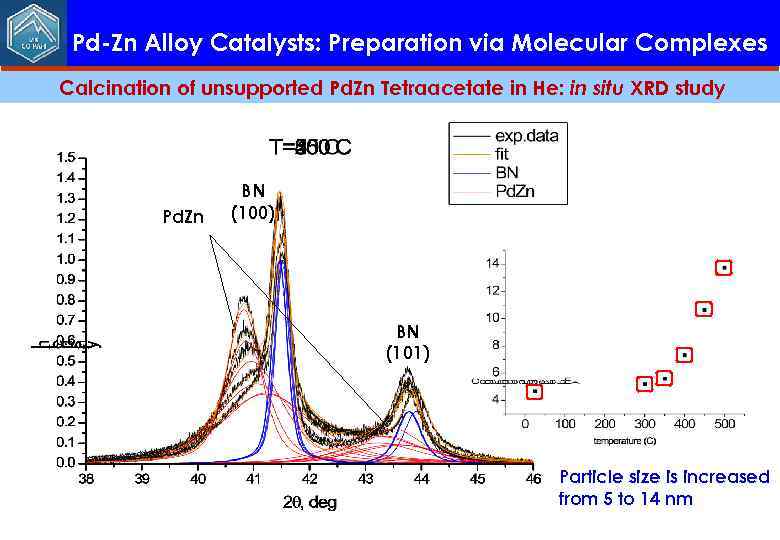
Pd-Zn Alloy Catalysts: Preparation via Molecular Complexes Calcination of unsupported Pd. Zn Tetraacetate in He: in situ XRD study Pd. Zn BN (100) BN (101) Particle size is increased from 5 to 14 nm
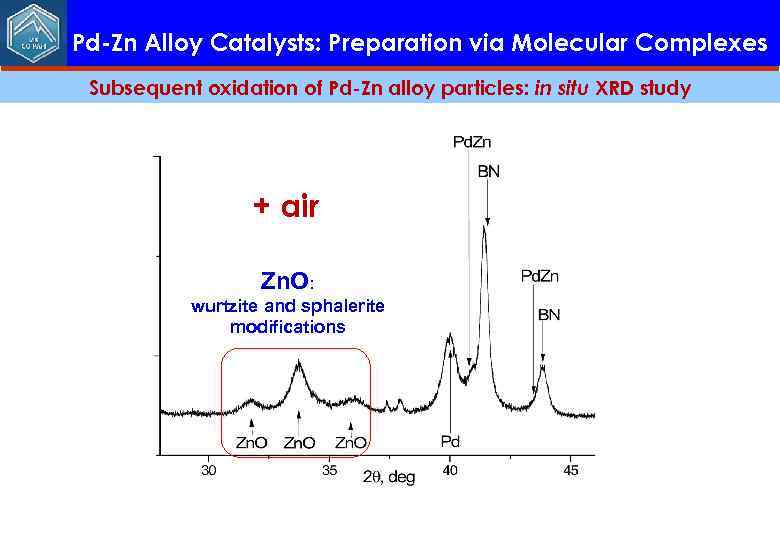
Pd-Zn Alloy Catalysts: Preparation via Molecular Complexes Subsequent oxidation of Pd-Zn alloy particles: in situ XRD study + air Zn. O: wurtzite and sphalerite modifications
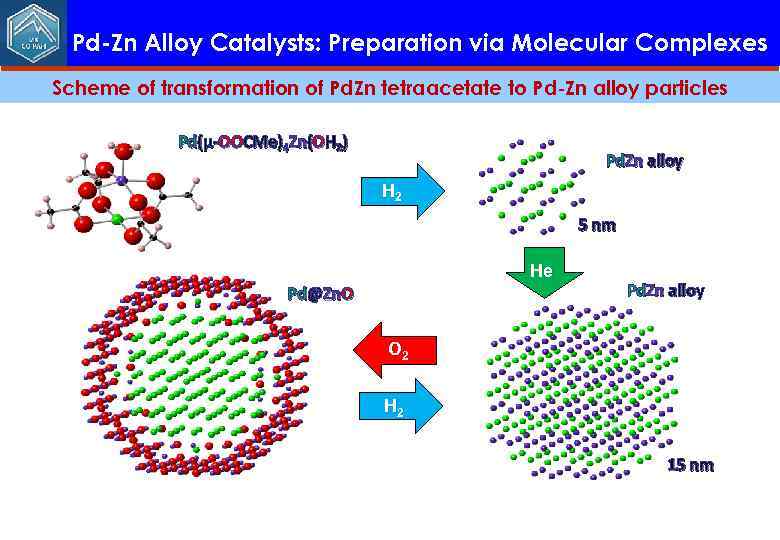
Pd-Zn Alloy Catalysts: Preparation via Molecular Complexes Scheme of transformation of Pd. Zn tetraacetate to Pd-Zn alloy particles Pd(μ-OOCMe)4 Zn(OH 2) Pd. Zn alloy H 2 5 nm He Pd@Zn. O Pd. Zn alloy O 2 H 2 15 nm
Pd-Zn Alloy Catalysts for Selective Hydrogenation of C 2 H 2 HC CH H 2 Cat. H 2 C CH 2 Х H 2 Cat. H 3 C-CH 3 Selective catalytic hydrogenation – the most effective way to remove acetylene and its homologs from olefin feeds to low concentration ( 3 -5 ppm) Industry applies supported Pd catalysts, but it exhibits low selectivity at acetylene high conversion. Increase in selectivity is provided : Ø by decrease of palladium particle size; Ø by modification of electronic properties of Pd via introduction of second metal (Ag, etc. )
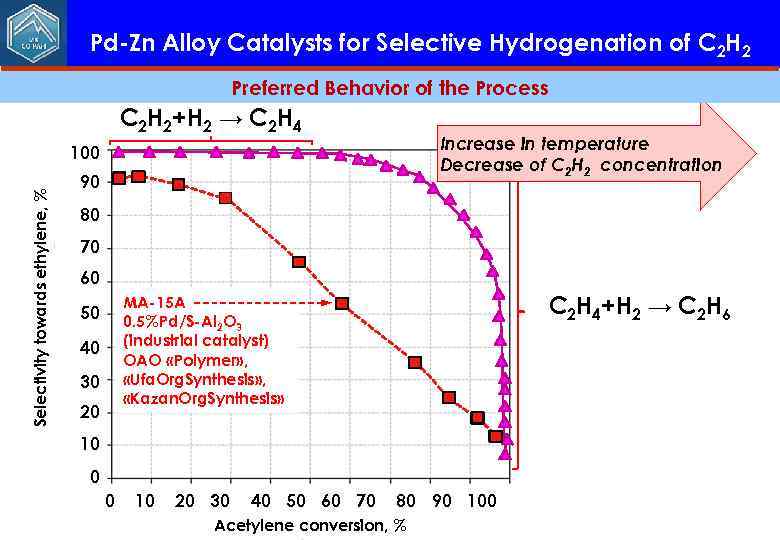
Pd-Zn Alloy Catalysts for Selective Hydrogenation of C 2 H 2 Preferred Behavior of the Process C 2 H 2+H 2 → C 2 H 4 Increase in temperature Decrease of С 2 Н 2 concentration Selectivity towards ethylene, % 100 90 80 70 60 C 2 H 4+H 2 → C 2 H 6 MA-15 А 0. 5%Pd/S-Al 2 O 3 (industrial catalyst) ОАО «Polymer» , «Ufa. Org. Synthesis» , «Kazan. Org. Synthesis» 50 40 30 20 10 0 0 10 20 30 40 50 60 70 80 90 100 Acetylene conversion, %
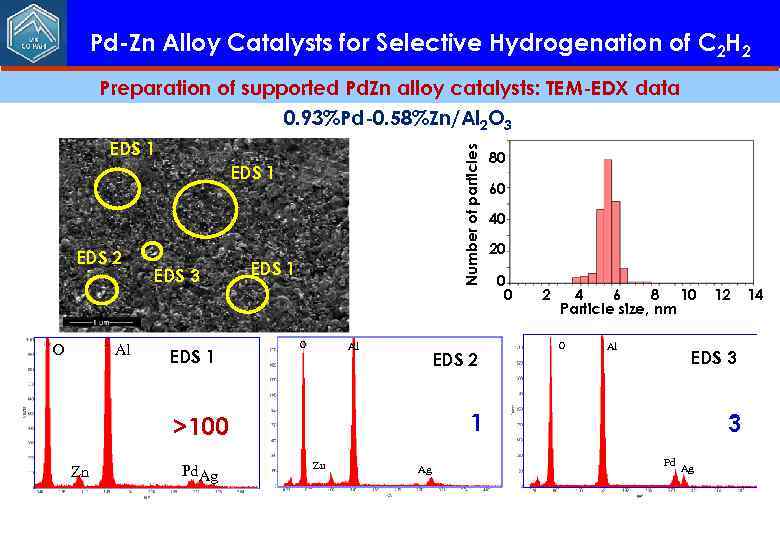
Pd-Zn Alloy Catalysts for Selective Hydrogenation of C 2 H 2 Preparation of supported Pd. Zn alloy catalysts: TEM-EDX data 0. 93%Pd-0. 58%Zn/Al 2 O 3 Number of particles EDS 1 EDS 2 O Al EDS 3 EDS 1 O Al EDS 2 Pd. Ag 60 40 20 0 0 2 4 6 8 10 Particle size, nm O Al Zn Ag 12 EDS 3 1 >100 Zn 80 3 Pd Ag 14
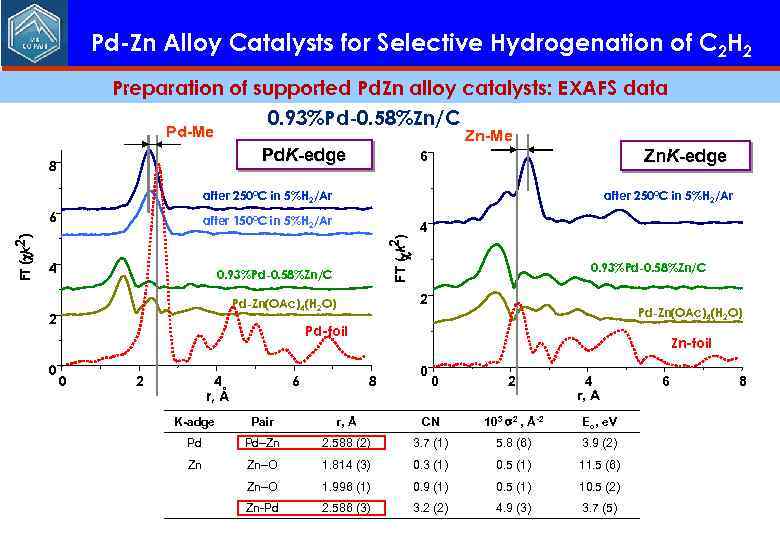
Pd-Zn Alloy Catalysts for Selective Hydrogenation of C 2 H 2 Preparation of supported Pd. Zn alloy catalysts: EXAFS data 0. 93%Pd-0. 58%Zn/C Pd-Me Pd. K-edge 8 Zn-Me Zn. K-edge 6 after 250°C in 5%H 2/Ar after 150°C in 5%H 2/Ar 4 0. 93%Pd-0. 58%Zn/C 2 Pd-Zn(OAc)4(H 2 O) 2 0 FT ( k 2) c FT (ck 2) 6 Pd-Zn(OAc)4(H 2 O) Pd-foil 0 2 4 r, Å 6 Zn-foil 8 0 0 2 4 r, A K-adge Pair r, Å CN 103 2 , Å-2 Eo, e. V Pd Pd–Zn 2. 588 (2) 3. 7 (1) 5. 8 (6) 3. 9 (2) Zn Zn–O 1. 814 (3) 0. 3 (1) 0. 5 (1) 11. 5 (6) Zn–O 1. 996 (1) 0. 9 (1) 0. 5 (1) 10. 5 (2) Zn-Pd 2. 586 (3) 3. 2 (2) 4. 9 (3) 3. 7 (5) 6 8
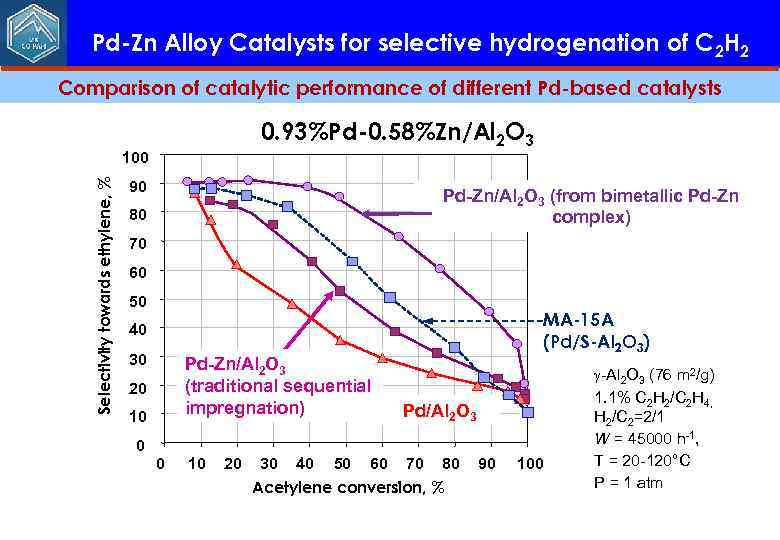
Pd-Zn Alloy Catalysts for selective hydrogenation of C 2 H 2 Comparison of catalytic performance of different Pd-based catalysts 0. 93%Pd-0. 58%Zn/Al 2 O 3 Selectivity towards ethylene, % 100 90 Pd-Zn/Al 2 O 3 (from bimetallic Pd-Zn complex) 80 70 60 50 МА-15 А (Pd/S-Al 2 O 3) 40 30 Pd-Zn/Al 2 O 3 (traditional sequential impregnation) 20 10 0 0 10 20 Pd/Al 2 O 3 30 40 50 60 70 80 Acetylene conversion, % 90 100 g-Al 2 O 3 (76 m 2/g) 1. 1% C 2 H 2/C 2 H 4, H 2/C 2=2/1 W = 45000 h-1, T = 20 -120°C P = 1 atm
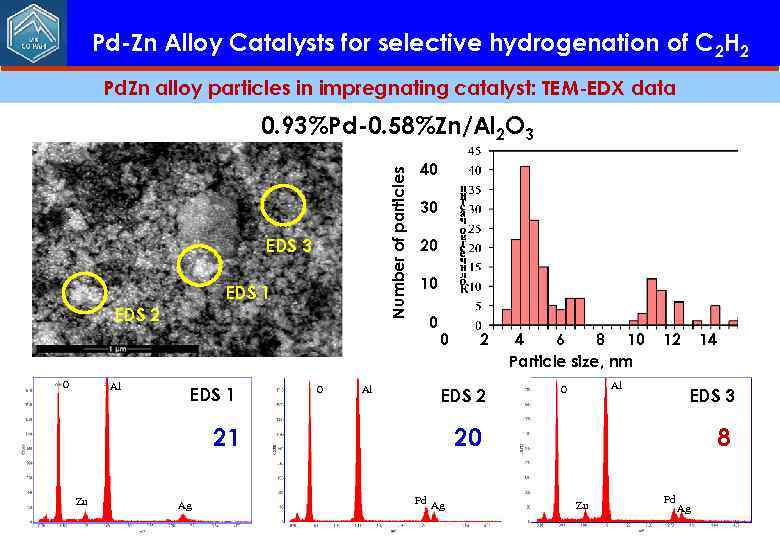
Pd-Zn Alloy Catalysts for selective hydrogenation of C 2 H 2 Pd. Zn alloy particles in impregnating catalyst: TEM-EDX data Number of particles 0. 93%Pd-0. 58%Zn/Al 2 O 3 EDS 1 EDS 2 O Al EDS 1 O Al 40 30 20 10 0 0 EDS 2 21 Zn Ag 2 4 6 8 10 Particle size, nm 12 Al O 14 EDS 3 20 Pd Ag 8 Zn Pd Ag
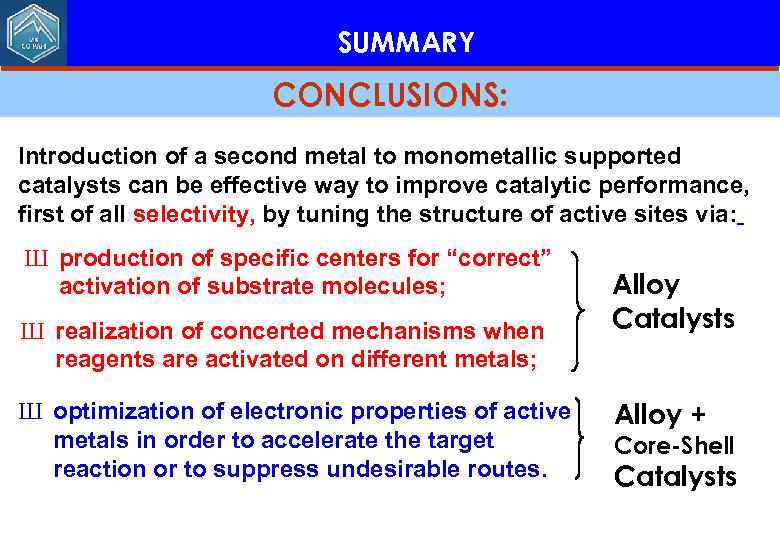
SUMMARY CONCLUSIONS: Introduction of a second metal to monometallic supported catalysts can be effective way to improve catalytic performance, first of all selectivity, by tuning the structure of active sites via: Ш production of specific centers for “correct” activation of substrate molecules; Ш realization of concerted mechanisms when reagents are activated on different metals; Ш optimization of electronic properties of active metals in order to accelerate the target reaction or to suppress undesirable routes. Alloy Catalysts Alloy + Core-Shell Catalysts

ACKNOWLEDGEMENTS: D. W. Goodman B. L. Moroz, P. A. Pyrjaev, I. V. Delidovich, O. P. Taran, I. P. Prosvirin, E. Yu. Gerasimov I. I. Moiseev, M. N. Vargaftik, N. Yu. Kositsyna, Ya. V. Zubavichus, M. V. Tsodikov A. Yu. Stakheev, N. S. Telegina, G. N. Baeva, O. P. Tkachenko

bimetallic_2015_Bukhtiyarov_IIC.pptx