e7c0a4186d09d7296b13a0d3d7c1cb9e.ppt
- Количество слайдов: 46

Bonding: General Concepts Chapter 8
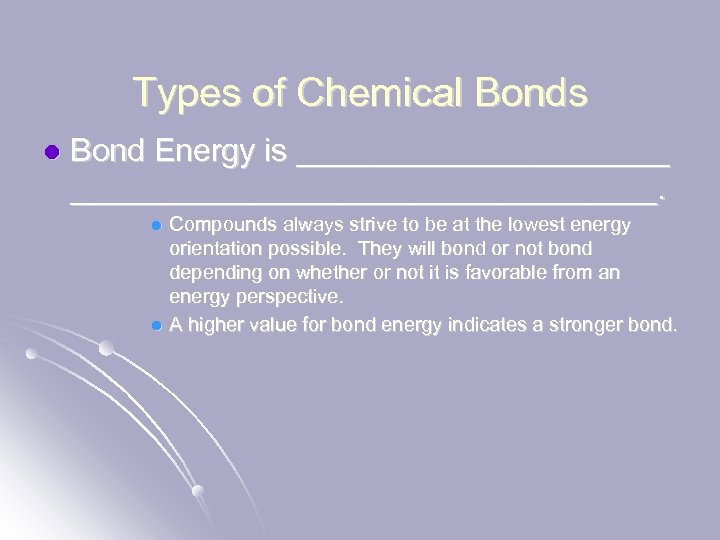
Types of Chemical Bonds l Bond Energy is ___________________________. Compounds always strive to be at the lowest energy orientation possible. They will bond or not bond depending on whether or not it is favorable from an energy perspective. l A higher value for bond energy indicates a stronger bond. l
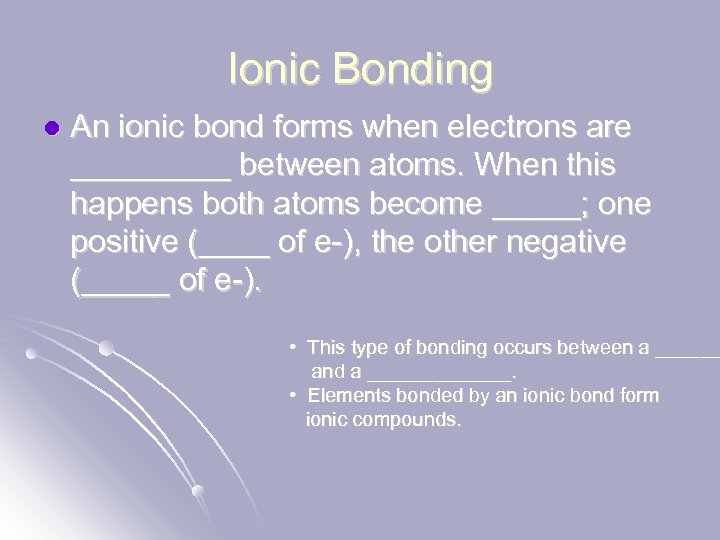
Ionic Bonding l An ionic bond forms when electrons are _____ between atoms. When this happens both atoms become _____; one positive (____ of e-), the other negative (_____ of e-). • This type of bonding occurs between a ______ and a _______. • Elements bonded by an ionic bond form ionic compounds.
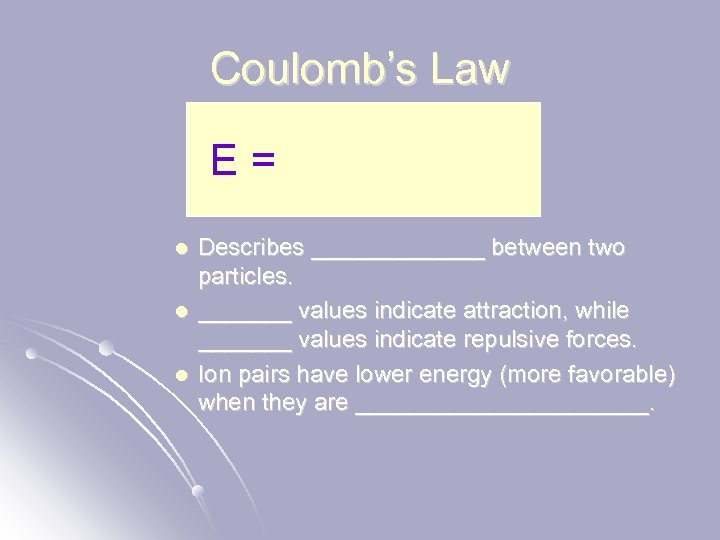
Coulomb’s Law E = l l l Describes _______ between two particles. _______ values indicate attraction, while _______ values indicate repulsive forces. Ion pairs have lower energy (more favorable) when they are ___________.

Bond Length l Bond length is the distance between two ions that maximizes the favorable attractions and minimizes the amount of repulsive forces. l Favorable attractive forces: l Unfavorable attractive forces (repulsive forces):
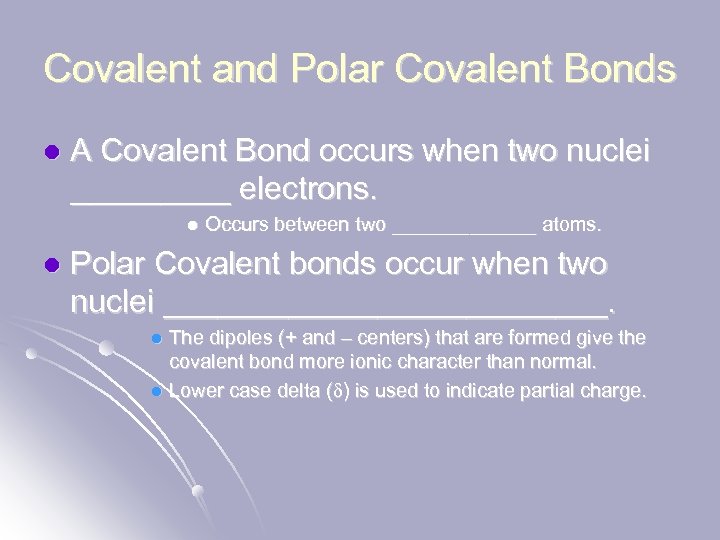
Covalent and Polar Covalent Bonds l A Covalent Bond occurs when two nuclei _____ electrons. l l Occurs between two _______ atoms. Polar Covalent bonds occur when two nuclei _____________. The dipoles (+ and – centers) that are formed give the covalent bond more ionic character than normal. l Lower case delta (d) is used to indicate partial charge. l

Electronegativity Follows the trend for electron affinity (synonymous), which means that it increases as we move ____ a group and _____ a period on the periodic table. l Electronegativity is __________________________. l

Electronegativity affects bonds l l If bonded atoms have a large difference in electronegativity (2. 0 or greater), they are considered to have an _____ bond because ___________________. If bonded atoms have a moderate difference in electronegativity (0. 5 -1. 6), they are considered to have a _______-_____ bond because ___________________. If bonded atoms have a negligible difference in electronegativity (below 0. 5), they will be considered to have a ______ bond because ___________________. If bonded atoms have a difference in electronegativity between 1. 6 and 2. 0, their identities have to be considered. If a metal is involved, it will be deemed ionic. If 2 non-metals are bonded, it will be considered polar-covalent.
Bond Polarity and Dipole Moments l Dipoles are formed in _____ bonds. l If the dipoles (a form of force) are aligned to point in exactly equal and opposite directions, they will _________. This makes the molecule non-polar. l The bond remains polar. l Molecules must be symmetrical for the cancellation to occur. Shapes that cancel are (draw them): l 1. 2. 3.
Assignment l Arrange the bonds in each of the following set in order of increasing polarity l C-F, O-F, Be-F l O-Cl, S-Br, C-P l C-S, B-F, N-O l Using only the periodic table as a guide, select A) the most electronegative element in group 6 A B) The least electronegative element out of Al, Si, P C) The element in the group K, C, Zn, F that is most likely to form an ionic compound with Ba.

l Give three ions that are isoelectronic with argon. Place these ions in order of increasing size. l What two requirements must be satisfied for a molecule to be polar? l Rank the following bonds in order of increasing ionic character: l N-O, Ca-O, C-F, Br-Br, K-F

Section 5: Formation of Binary Ionic Compounds l Lattice energy: the energy required to combine elements to form an ionic compound. (amount released when bond forms) Lattice energy is negative. l Elements must start as a gas l The more exothermic (negative), the more likely the substance is to form spontaneously. l
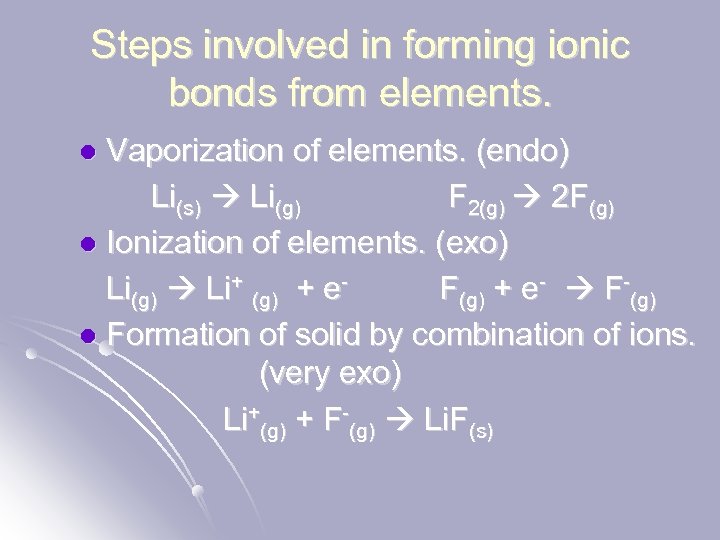
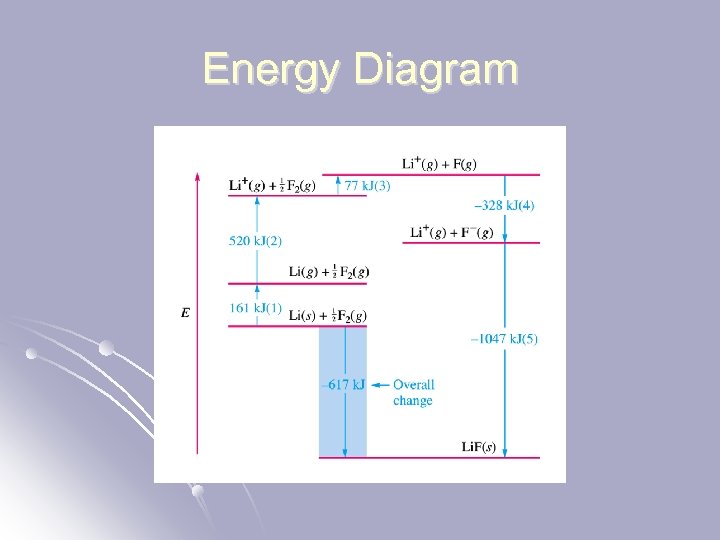
Steps involved in forming ionic bonds from elements. Vaporization of elements. (endo) Li(s) Li(g) F 2(g) 2 F(g) l Ionization of elements. (exo) Li(g) Li+ (g) + e. F(g) + e- F-(g) l Formation of solid by combination of ions. (very exo) Li+(g) + F-(g) Li. F(s) l
Energy Diagram

Partial Ionic Character of Covalent Bonds Any Compound That Conducts Electricity When Melted Is IONIC
Models of Chemical Bonds Models do not equal reality…they are merely something to help us visualize a concept near the truth. l Models are often wrong because they over-simplify. l

Covalent Bond Energy Single bond = one pair shared electrons l Double bond = two pair shared electrons l Triple bond = three pair shared electrons l As more pairs of electrons are shared, the bond length shortens. (more orbitals have to overlap to allow the sharing to happen) l Single bonds usually contain the least amount of energy, while triple bonds usually contain the most…as bond length shortens, bond energy increases. l Page 374 Tables 8. 4 and 8. 5
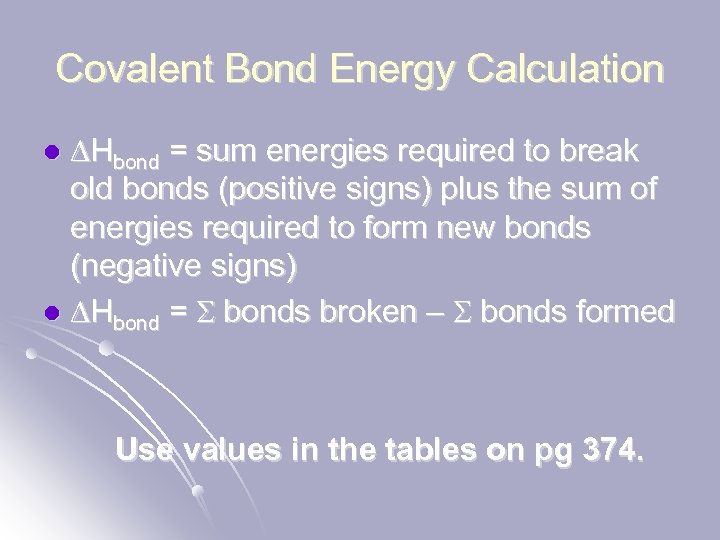
Covalent Bond Energy Calculation DHbond = sum energies required to break old bonds (positive signs) plus the sum of energies required to form new bonds (negative signs) l DHbond = S bonds broken – S bonds formed l Use values in the tables on pg 374.
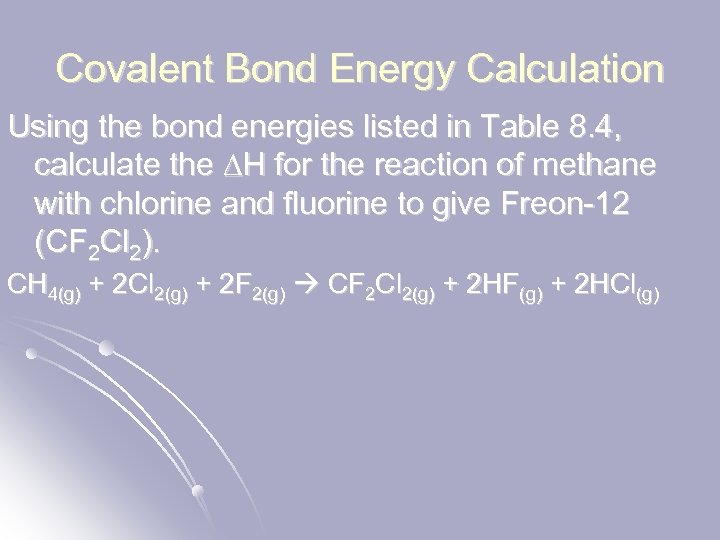
Covalent Bond Energy Calculation Using the bond energies listed in Table 8. 4, calculate the DH for the reaction of methane with chlorine and fluorine to give Freon-12 (CF 2 Cl 2). CH 4(g) + 2 Cl 2(g) + 2 F 2(g) CF 2 Cl 2(g) + 2 HF(g) + 2 HCl(g)
The VSEPR Model Valence Shell Electron Pair Repulsion l A model of molecular structure based on the idea that ideal structures minimizes electron pair repulsions. l Draw and evaluate Lewis Structures l
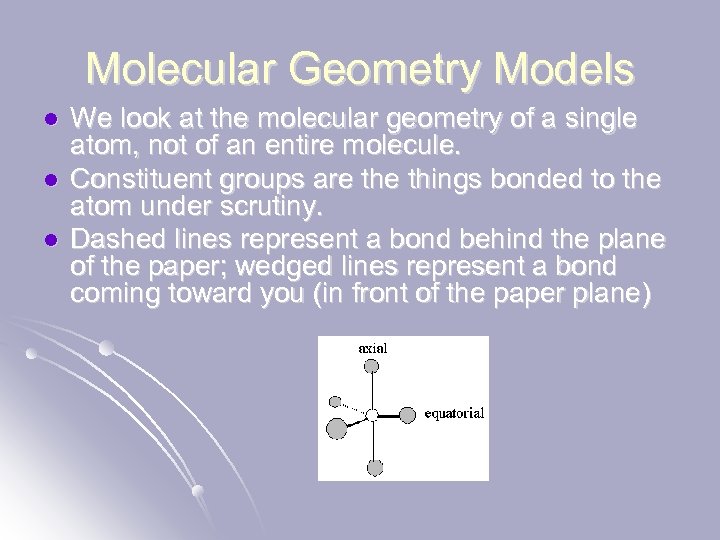
Molecular Geometry Models l l l We look at the molecular geometry of a single atom, not of an entire molecule. Constituent groups are things bonded to the atom under scrutiny. Dashed lines represent a bond behind the plane of the paper; wedged lines represent a bond coming toward you (in front of the paper plane)
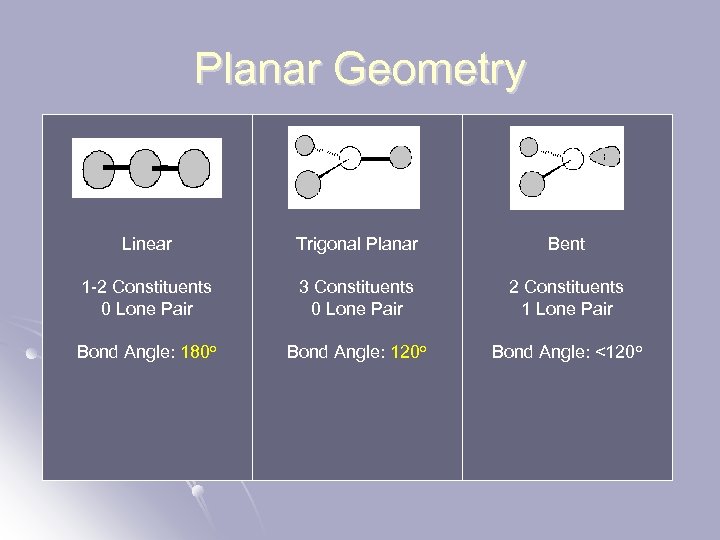
Planar Geometry Linear Trigonal Planar Bent 1 -2 Constituents 0 Lone Pair 3 Constituents 0 Lone Pair 2 Constituents 1 Lone Pair Bond Angle: 180 o Bond Angle: 120 o Bond Angle: <120 o
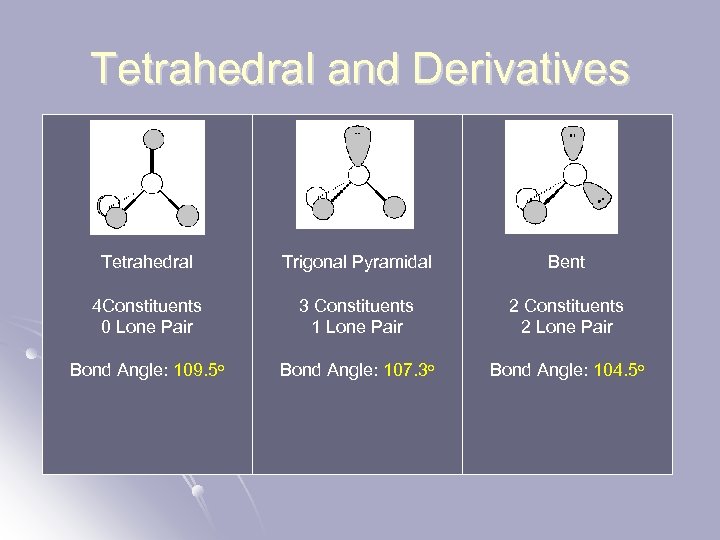
Tetrahedral and Derivatives Tetrahedral Trigonal Pyramidal Bent 4 Constituents 0 Lone Pair 3 Constituents 1 Lone Pair 2 Constituents 2 Lone Pair Bond Angle: 109. 5 o Bond Angle: 107. 3 o Bond Angle: 104. 5 o
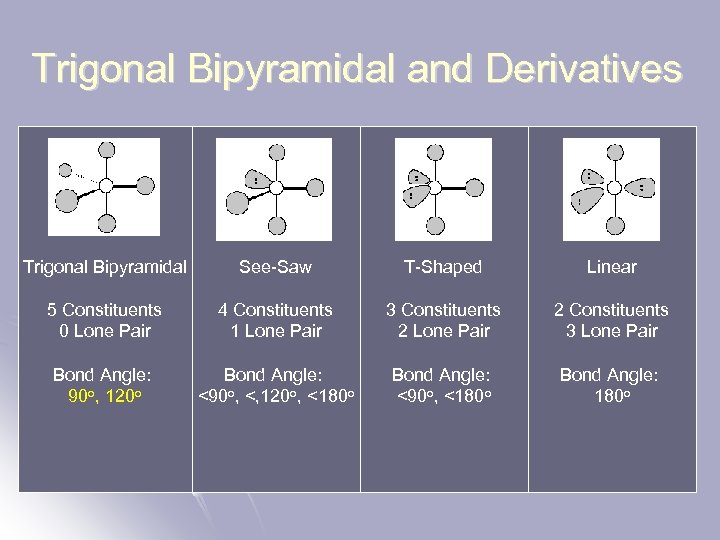
Trigonal Bipyramidal and Derivatives Trigonal Bipyramidal See-Saw T-Shaped Linear 5 Constituents 0 Lone Pair 4 Constituents 1 Lone Pair 3 Constituents 2 Lone Pair 2 Constituents 3 Lone Pair Bond Angle: 90 o, 120 o Bond Angle: <90 o, <, 120 o, <180 o Bond Angle: <90 o, <180 o Bond Angle: 180 o
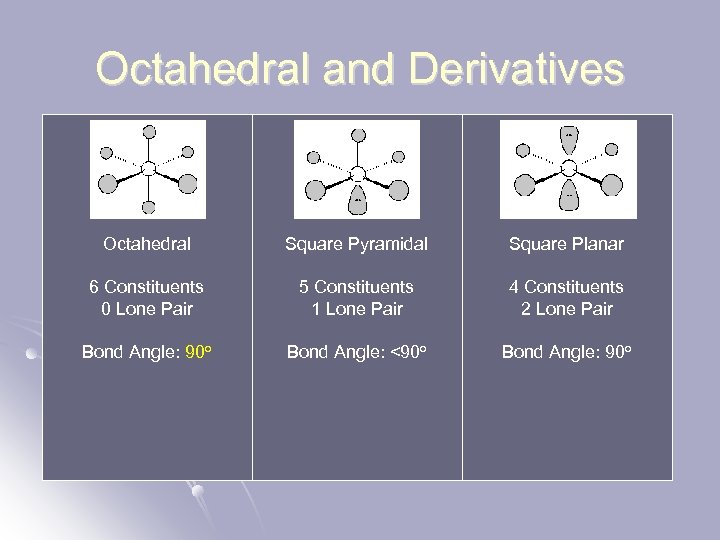
Octahedral and Derivatives Octahedral Square Pyramidal Square Planar 6 Constituents 0 Lone Pair 5 Constituents 1 Lone Pair 4 Constituents 2 Lone Pair Bond Angle: 90 o Bond Angle: <90 o Bond Angle: 90 o
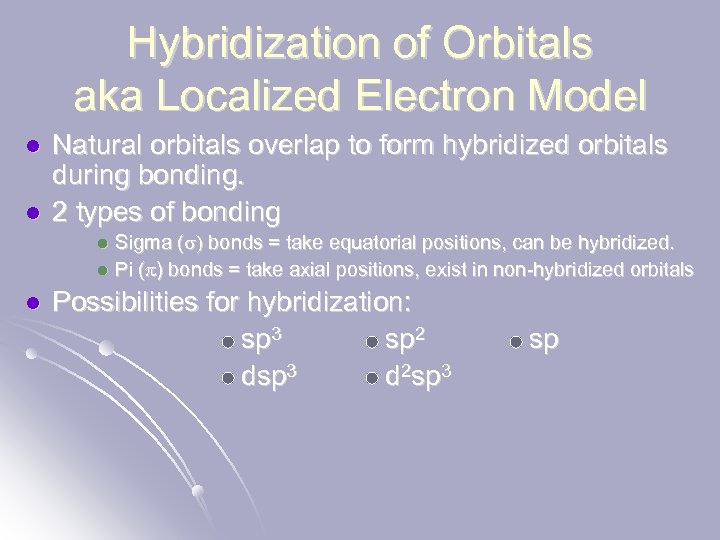
Hybridization of Orbitals aka Localized Electron Model l l Natural orbitals overlap to form hybridized orbitals during bonding. 2 types of bonding Sigma (s) bonds = take equatorial positions, can be hybridized. l Pi (p) bonds = take axial positions, exist in non-hybridized orbitals l l Possibilities for hybridization: sp 3 sp 2 dsp 3 d 2 sp 3 sp
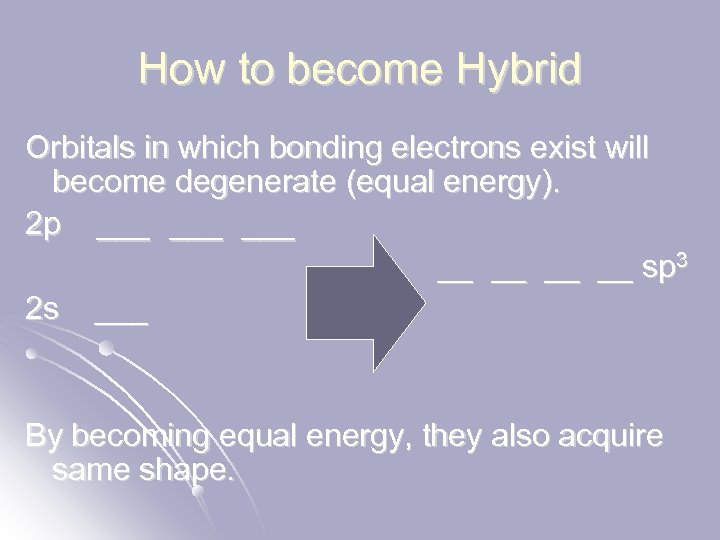
How to become Hybrid Orbitals in which bonding electrons exist will become degenerate (equal energy). 2 p ___ ___ __ __ sp 3 2 s ___ By becoming equal energy, they also acquire same shape.
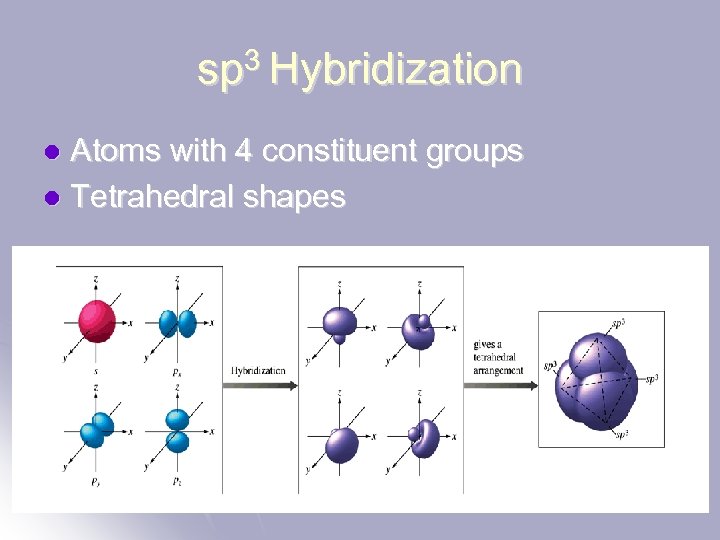
sp 3 Hybridization Atoms with 4 constituent groups l Tetrahedral shapes l
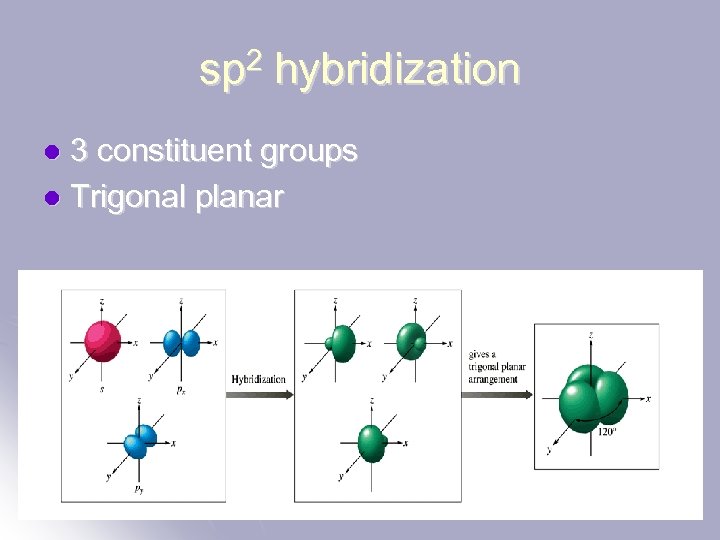
sp 2 hybridization 3 constituent groups l Trigonal planar l
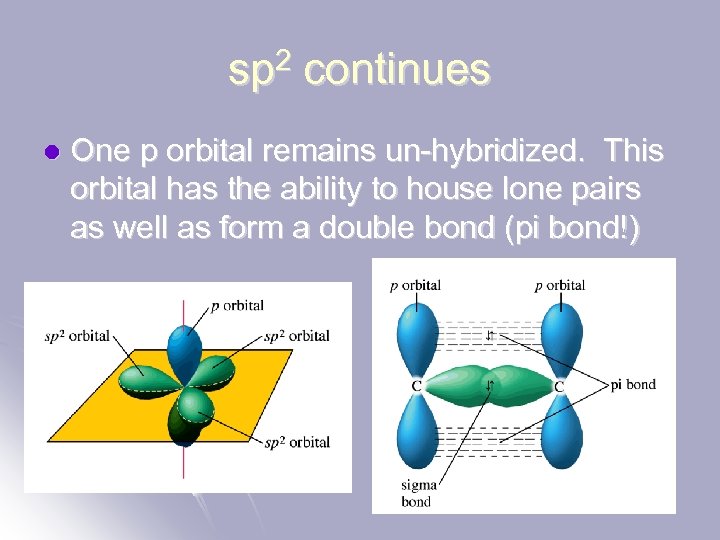
sp 2 continues l One p orbital remains un-hybridized. This orbital has the ability to house lone pairs as well as form a double bond (pi bond!)
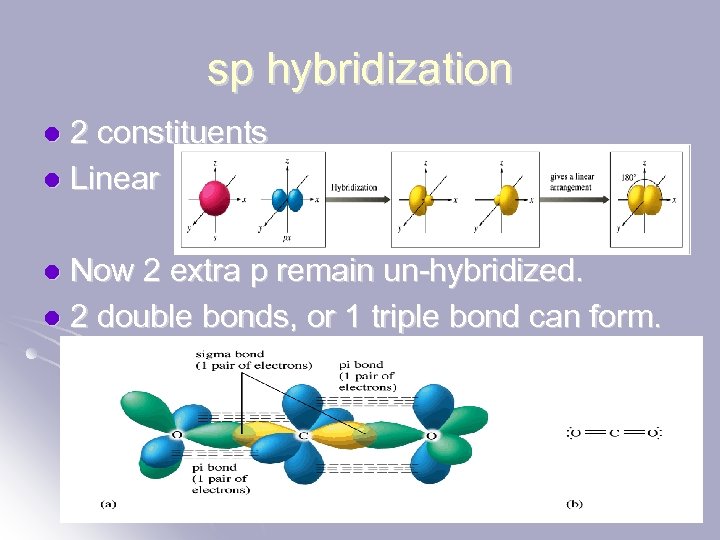
sp hybridization 2 constituents l Linear l Now 2 extra p remain un-hybridized. l 2 double bonds, or 1 triple bond can form. l
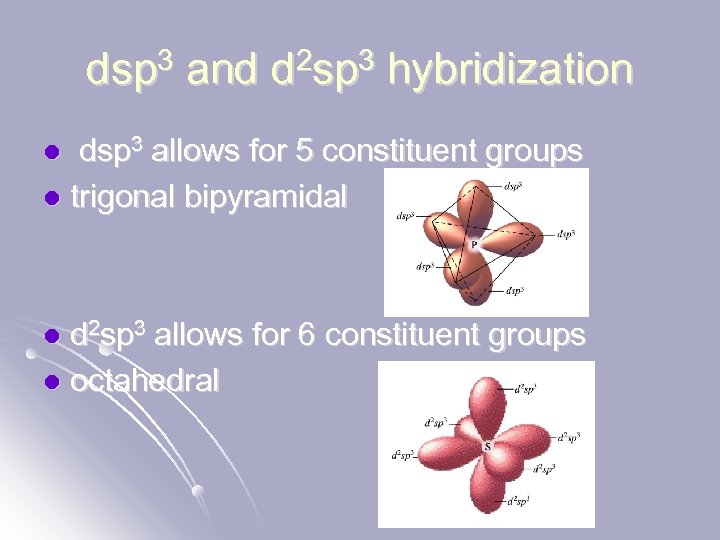
dsp 3 and d 2 sp 3 hybridization dsp 3 allows for 5 constituent groups l trigonal bipyramidal l d 2 sp 3 allows for 6 constituent groups l octahedral l
Molecular Orbital Theory This is an alternative to the idea of hybridized orbitals. l Essentially, when two atoms come together their orbitals merge and form two completely new orbitals. l l Bonding orbital (low energy, electrons fill first) l Anti-bonding orbital (high energy, electrons fill last)
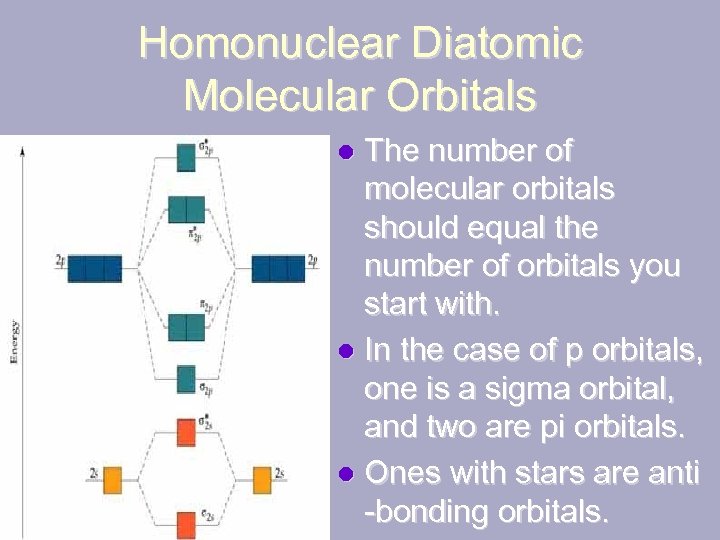
Homonuclear Diatomic Molecular Orbitals The number of molecular orbitals should equal the number of orbitals you start with. l In the case of p orbitals, one is a sigma orbital, and two are pi orbitals. l Ones with stars are anti -bonding orbitals. l

Determining Bond Order l You can use molecular orbitals to determine bond order to a much more specific degree than you can with a Lewis Dot Diagram.
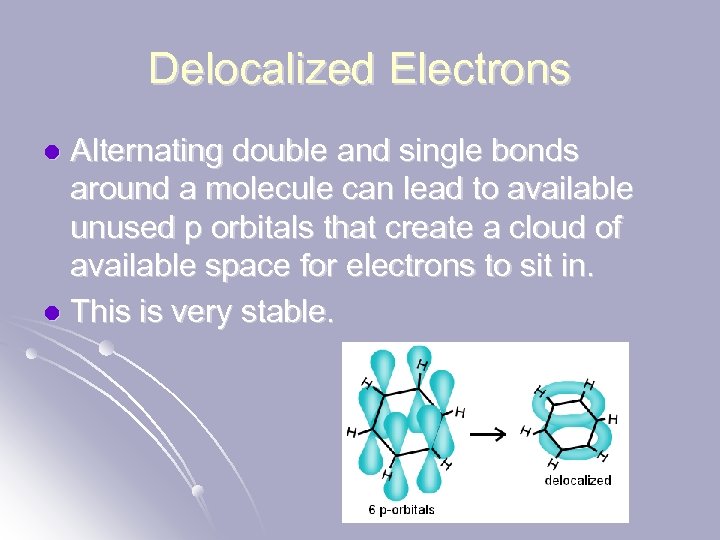
Delocalized Electrons Alternating double and single bonds around a molecule can lead to available unused p orbitals that create a cloud of available space for electrons to sit in. l This is very stable. l

Complex Ions and Coordination Compounds Highly colored compounds l Paramagnetic (unpaired electrons) l Made of a Transition Metal and Ligands l

How do they do that? l Transition metals have two sets of valence electrons. l Primary valence electrons affect oxidation state (charge in ions) and allow for trading of electrons (ionic bonds). l Secondary valence electrons affect coordination number (number of ligands) and allow for ligands to share electrons with the transition metal.

Ligands l Ligands are molecules or ions that have a lone pair of electrons available to coordinate with the transition metal. l Rule of Thumb: The coordination number of a metal can generally be assumed as twice its charge. l Ex: Co+3 can have a coordination number of 6.

Naming Complex Ions Name cations first l Change ending of ligands to –o l Use prefixes to tell how many. l List oxidation state of metal using Roman Numerals l List ligands in alphabetical order l If complex ion is an anion, end metal name with –ate. l
![Examples [Al(OH)4]l [Co(NH 3)4 ]2+ l Amminetetraaquachromium(II) sulfate l Potassium hexacyanoferrate(III) l Examples [Al(OH)4]l [Co(NH 3)4 ]2+ l Amminetetraaquachromium(II) sulfate l Potassium hexacyanoferrate(III) l](https://present5.com/presentation/e7c0a4186d09d7296b13a0d3d7c1cb9e/image-41.jpg)
Examples [Al(OH)4]l [Co(NH 3)4 ]2+ l Amminetetraaquachromium(II) sulfate l Potassium hexacyanoferrate(III) l
Isomers l A set of compounds with the same chemical formula that exhibit decidedly different properties. Structural Isomer = the same atoms are present in each molecule, but they are bonded to different things. l Stereoisomer = bonds are between same atoms, but have a different spatial relationship with each other. l
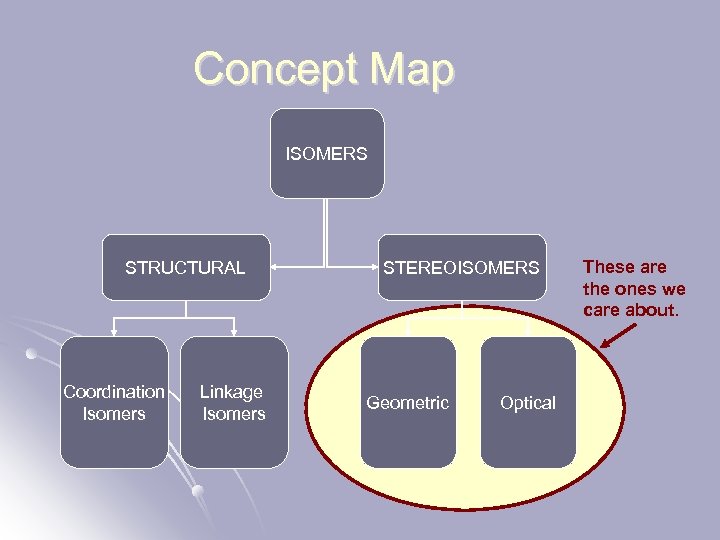
Concept Map ISOMERS STRUCTURAL Coordination Isomers Linkage Isomers STEREOISOMERS Geometric Optical These are the ones we care about.
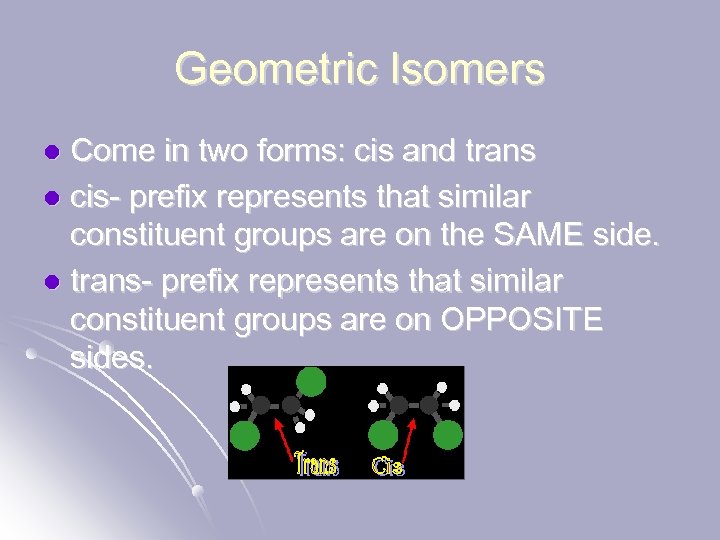
Geometric Isomers Come in two forms: cis and trans l cis- prefix represents that similar constituent groups are on the SAME side. l trans- prefix represents that similar constituent groups are on OPPOSITE sides. l
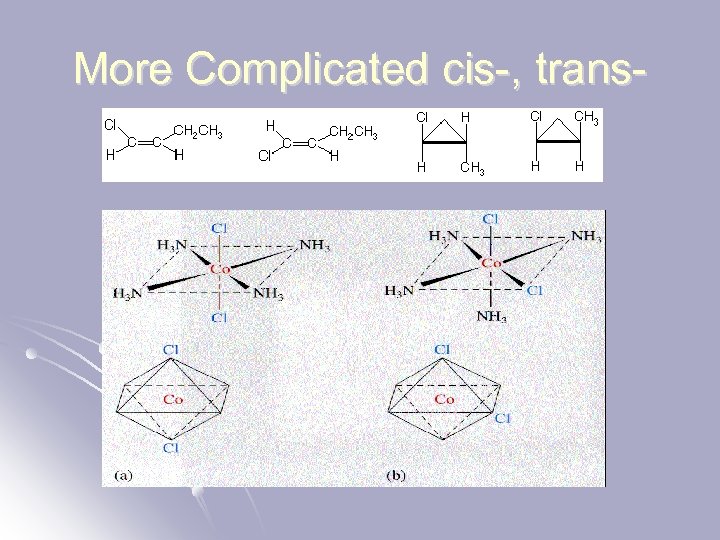
More Complicated cis-, trans-
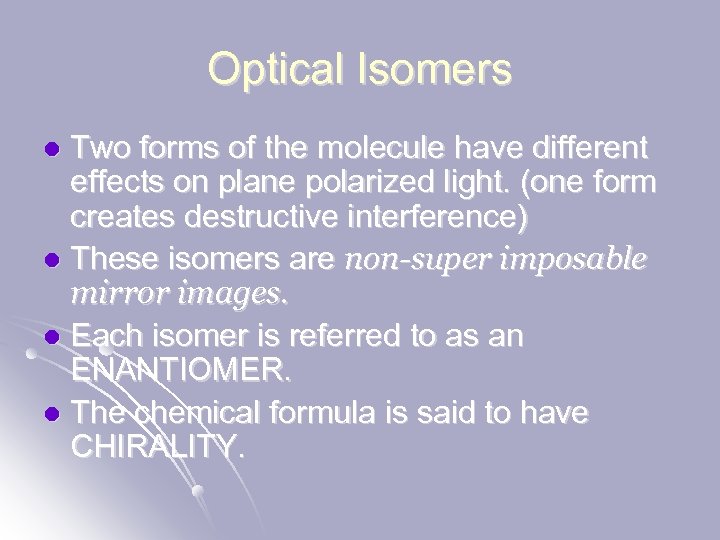
Optical Isomers Two forms of the molecule have different effects on plane polarized light. (one form creates destructive interference) l These isomers are non-super imposable mirror images. l Each isomer is referred to as an ENANTIOMER. l The chemical formula is said to have CHIRALITY. l
e7c0a4186d09d7296b13a0d3d7c1cb9e.ppt