afd832d6773c9adb511046d4dcd5d10a.ppt
- Количество слайдов: 52
© Boardworks Ltd 2003
Teacher’s Notes A slide contains teacher’s notes wherever this icon is displayed To access these notes go to ‘Notes Page View’ (Power. Point 97) or ‘Normal View’ (Power. Point 2000). Notes Page View Normal View Flash Files A flash file has been embedded into the Power. Point slide wherever this icon is displayed – These files are not editable. © Boardworks Ltd 2003

Observations • • • An important part of scientific work can be looking for patterns but to spot patterns we must first make careful observations. Some metals are softer and can be cut more easily than others Some metals look different to others. They might be different colours, shiny, dull, etc. © Boardworks Ltd 2003

Observing reactions with oxygen • E. g. Most metals react with oxygen (tarnish) – but they may do so in very different ways. Some react faster than others and some may not react at all. • The next slide records the observations made by Shaida as she investigated burning some metals. Some metals burn better than others © Boardworks Ltd 2003

Reaction with oxygen - observations Below are some observations. Magnesium The ribbon burned with a dazzling white flame giving grey-white smoke and ash. Copper The copper turnings went through reds and oranges and then slowly got a permanent coating of black. Iron The iron filings glowed red and sparkled leaving a brown-black looking solid. They are all forming metal oxides. © Boardworks Ltd 2003
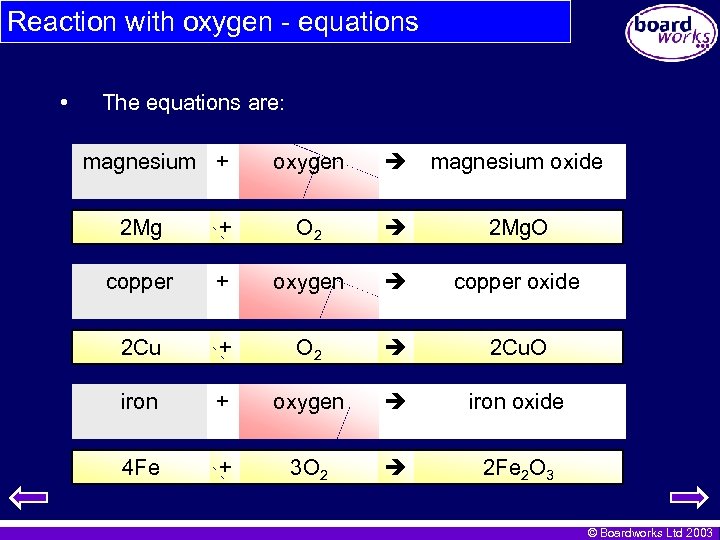
Reaction with oxygen - equations • The equations are: magnesium + oxygen magnesium oxide 2 Mg + O 2 2 Mg. O copper + oxygen copper oxide 2 Cu + O 2 2 Cu. O iron + oxygen iron oxide 4 Fe + 3 O 2 2 Fe 2 O 3 © Boardworks Ltd 2003

Activity Put these three metals in order of reactivity with oxygen - magnesium, copper, iron. 1. magnesium 2. iron 3. copper © Boardworks Ltd 2003

Observing reactions with water • • • Some metals (like lead and copper) react only slowly with water. The word plumbing comes from plumbum (Latin for lead) because the ancient Romans used lead for their water pipes. However, even lead does slowly dissolve and it is very poisonous. Because of this plumbers don’t use lead any more. (Should we re-name them coppers!) The next slide records some observations made by James as he investigated the reactions of metals with water. Modern pipes – not lead but copper or plastic © Boardworks Ltd 2003

Reaction with water - observations Below are some observations. Lithium Bubbles of hydrogen gas are given off quite quickly. The neutral water becomes alkaline. Sodium The sodium melts and skims over the surface producing a stream of small bubbles. Sometimes a yellow-orange flame appeared. Potassium immediately produced a lilac flame as it skimmed around making a fizzing noise. All the metals are reacting to form metal hydroxides and hydrogen gas. © Boardworks Ltd 2003

Reaction with water A rice sized grain of metal to be used and safety specs worn. The hydrogen produced from potassium always ignites in water. © Boardworks Ltd 2003
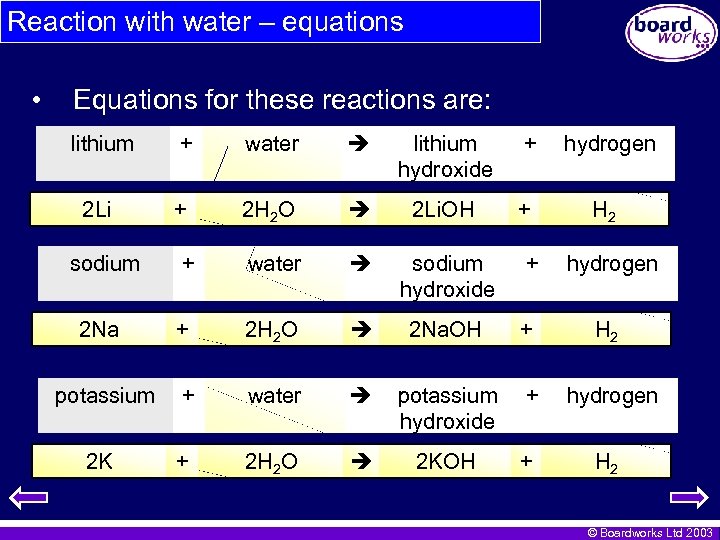
Reaction with water – equations • Equations for these reactions are: lithium + water lithium hydroxide + hydrogen 2 Li + 2 H 2 O 2 Li. OH + H 2 sodium + water sodium hydroxide + hydrogen 2 Na + 2 H 2 O 2 Na. OH + H 2 potassium + water potassium hydroxide + hydrogen 2 K + 2 H 2 O 2 KOH + H 2 © Boardworks Ltd 2003

Reaction with water Here are some more observations. Iron Copper Silver Gold No rapid reaction but gradual conversion of the iron to rust No reaction This is, of course, relevant to the use of copper for plumbing and of silver and of gold for jewellery. © Boardworks Ltd 2003

Activity • • Put the following metals in order in terms of their reaction with water. Iron, sodium, potassium, silver, copper, lithium, gold 1. potassium 2. sodium 3. lithium 4. iron 5 Copper, silver, gold © Boardworks Ltd 2003

Observing reactions with acid • • The very first chemists were called alchemists. They spent much of their time trying to find methods of changing cheap metals into gold. Some were very good at making metals look gold. But gold is so un-reactive that it wont dissolve even in really strong acids. Other metals do. This became known as “the acid test” because it stopped tricksters making false claims that something was gold. We still use the phrase “the acid test” to mean something that will show up fakes. Most metals dissolve in strong acid – but gold doesn’t © Boardworks Ltd 2003
Acids and metals © Boardworks Ltd 2003
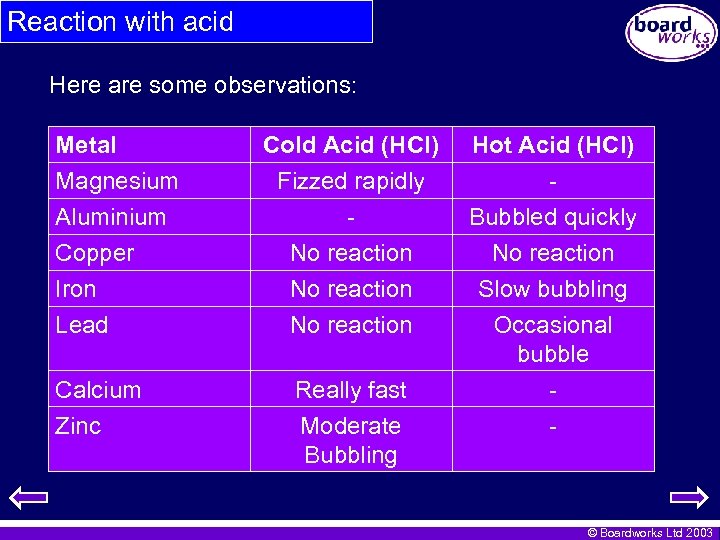
Reaction with acid Here are some observations: Metal Magnesium Aluminium Copper Iron Lead Calcium Zinc Cold Acid (HCl) Fizzed rapidly No reaction Really fast Moderate Bubbling Hot Acid (HCl) Bubbled quickly No reaction Slow bubbling Occasional bubble - © Boardworks Ltd 2003

Activity Match up the metals listed with the correct photo. Copper, magnesium, iron, zinc copper Magnesium iron zinc All the reactions involve the formation of a salt and hydrogen gas. © Boardworks Ltd 2003
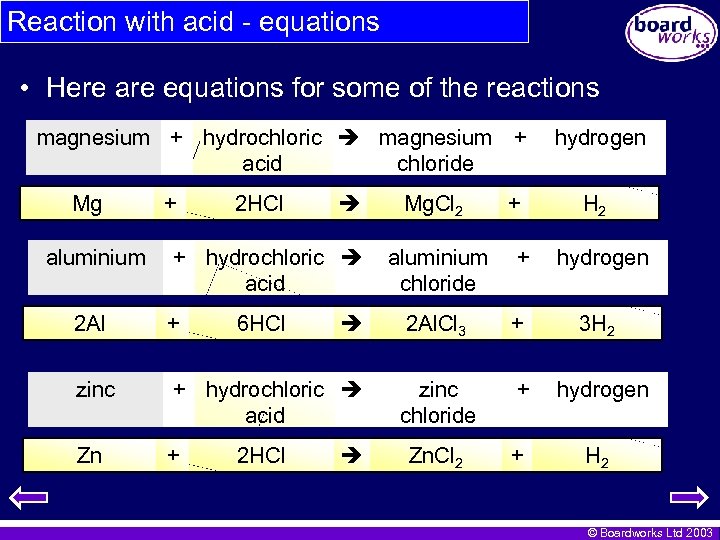
Reaction with acid - equations • Here are equations for some of the reactions magnesium + hydrochloric magnesium + chloride acid Mg aluminium + 2 HCl + hydrochloric acid 2 Al + 6 HCl zinc + hydrochloric acid Zn + 2 HCl Mg. Cl 2 + hydrogen H 2 aluminium chloride + hydrogen 2 Al. Cl 3 + 3 H 2 zinc chloride + hydrogen Zn. Cl 2 + H 2 © Boardworks Ltd 2003
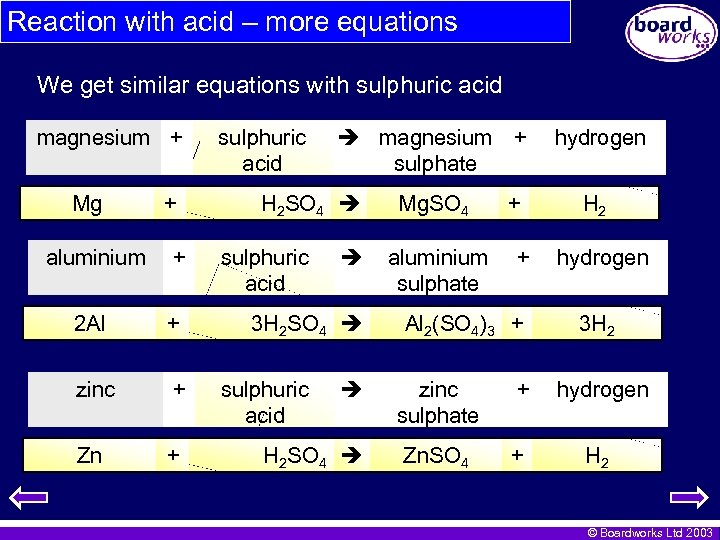
Reaction with acid – more equations We get similar equations with sulphuric acid magnesium + Mg + aluminium + 2 Al + zinc + Zn + sulphuric acid magnesium + sulphate H 2 SO 4 sulphuric acid 3 H 2 SO 4 sulphuric acid H 2 SO 4 Mg. SO 4 aluminium sulphate + + Al 2(SO 4)3 + hydrogen H 2 hydrogen 3 H 2 zinc sulphate + hydrogen Zn. SO 4 + H 2 © Boardworks Ltd 2003
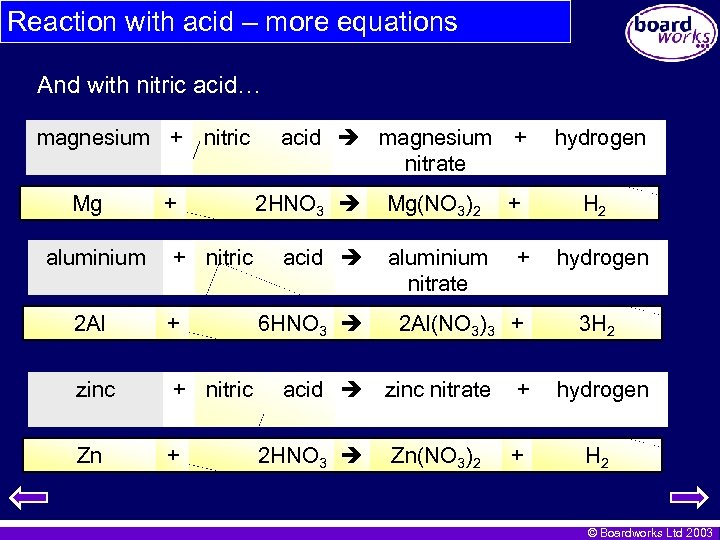
Reaction with acid – more equations And with nitric acid… magnesium + nitric Mg aluminium + + nitric 2 Al + zinc + nitric Zn + acid magnesium + nitrate 2 HNO 3 acid 6 HNO 3 Mg(NO 3)2 aluminium nitrate + 2 Al(NO 3)3 + acid zinc nitrate 2 HNO 3 + Zn(NO 3)2 hydrogen H 2 hydrogen 3 H 2 + hydrogen + H 2 © Boardworks Ltd 2003
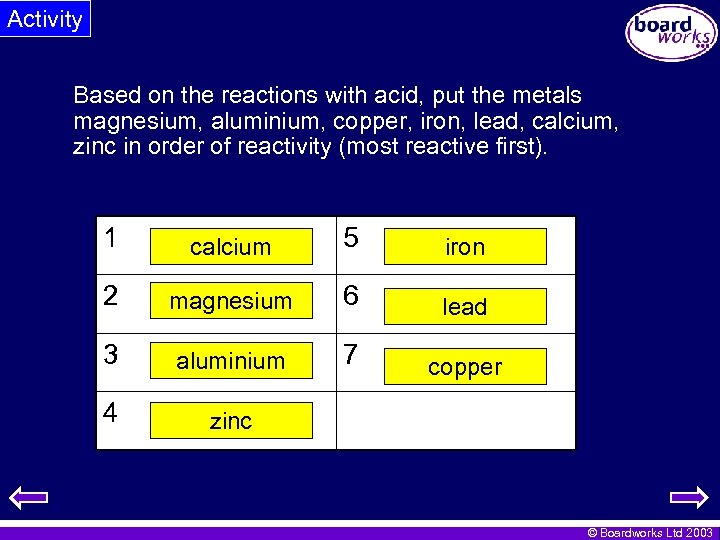
Activity Based on the reactions with acid, put the metals magnesium, aluminium, copper, iron, lead, calcium, zinc in order of reactivity (most reactive first). 1 calcium 5 iron 2 magnesium 6 lead 3 aluminium 7 copper 4 zinc © Boardworks Ltd 2003

The Activity Series • We can combine all the information from the reactions with air, water and acid to get an overall activity series With Oxygen With water With acid magnesium iron oxygen potassium sodium lithium calcium magnesium aluminium zinc Iron Lead copper More complete studies give us the activity series shown on the next slide. © Boardworks Ltd 2003
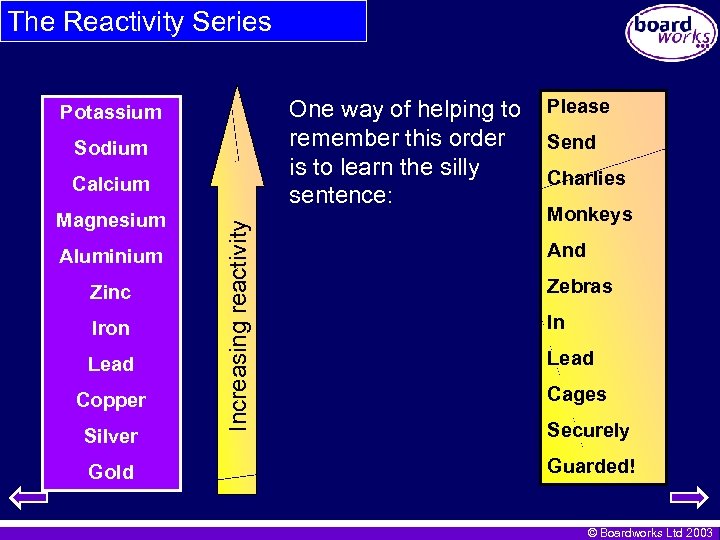
The Reactivity Series One way of helping to remember this order is to learn the silly sentence: Potassium Sodium Magnesium Aluminium Zinc Iron Lead Copper Silver Gold Increasing reactivity Calcium Please Send Charlies Monkeys And Zebras In Lead Cages Securely Guarded! © Boardworks Ltd 2003

The Activity Series - uses • We can use the activity series to make predictions about reactions we have not yet been able to try out. • This will apply both to simple reactions of the metals with oxygen, water and air. • It will also apply to more complex reactions where one metal is competing with another. © Boardworks Ltd 2003
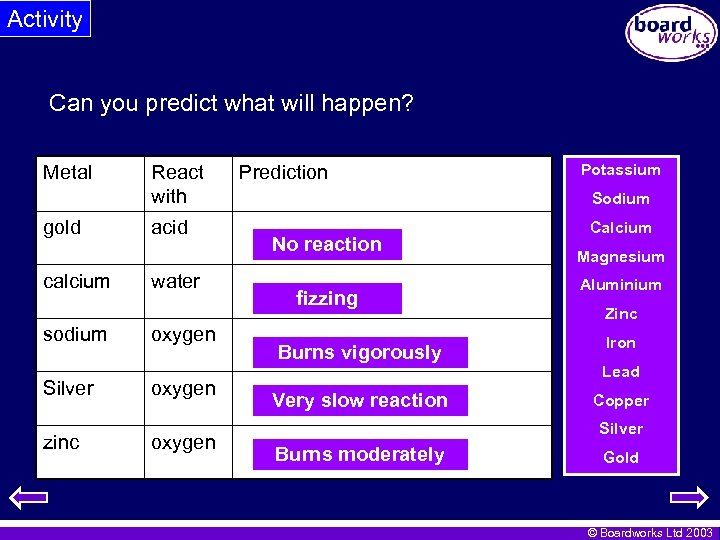
Activity Can you predict what will happen? Metal gold calcium sodium Silver zinc React with acid water oxygen Prediction Potassium Sodium No reaction fizzing Burns vigorously Very slow reaction Calcium Magnesium Aluminium Zinc Iron Lead Copper Silver Burns moderately Gold © Boardworks Ltd 2003

Displaced metals • • • Displaced persons is an old-fashioned word for refugees: people who have lost their homes and possessions - often as a result of wars. In chemistry we sometimes have displaced metals. These are metals that have lost a competition. To start with such metals are bonded to a non-metal as part of a compound. Along comes a more reactive metal and takes the non-metal away. Next we look at predicting the winner of such displacement reactions. © Boardworks Ltd 2003
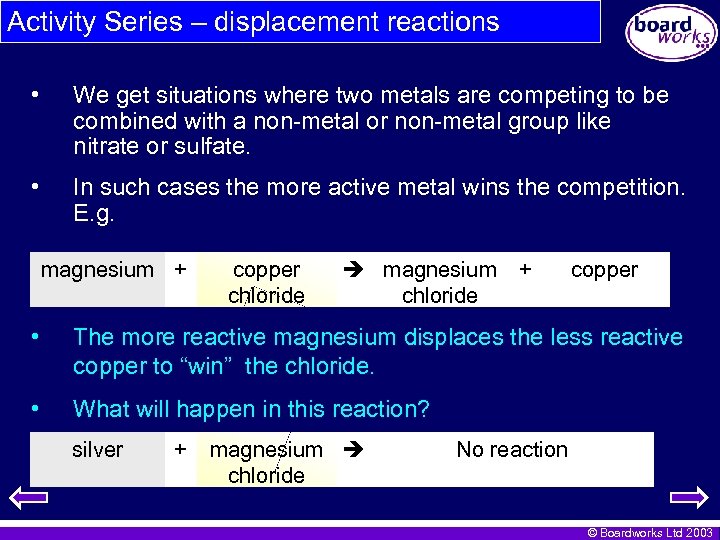
Activity Series – displacement reactions • We get situations where two metals are competing to be combined with a non-metal or non-metal group like nitrate or sulfate. • In such cases the more active metal wins the competition. E. g. magnesium + copper chloride magnesium + chloride copper • The more reactive magnesium displaces the less reactive copper to “win” the chloride. • What will happen in this reaction? silver + magnesium chloride No reaction © Boardworks Ltd 2003
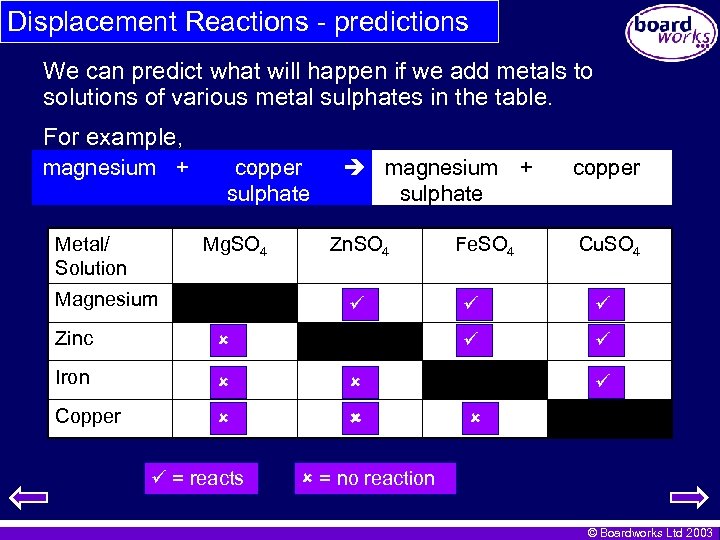
Displacement Reactions - predictions We can predict what will happen if we add metals to solutions of various metal sulphates in the table. For example, magnesium + Metal/ Solution copper sulphate Mg. SO 4 Magnesium magnesium + sulphate Zn. SO 4 Fe. SO 4 Cu. SO 4 Iron Copper Zinc = reacts copper = no reaction © Boardworks Ltd 2003
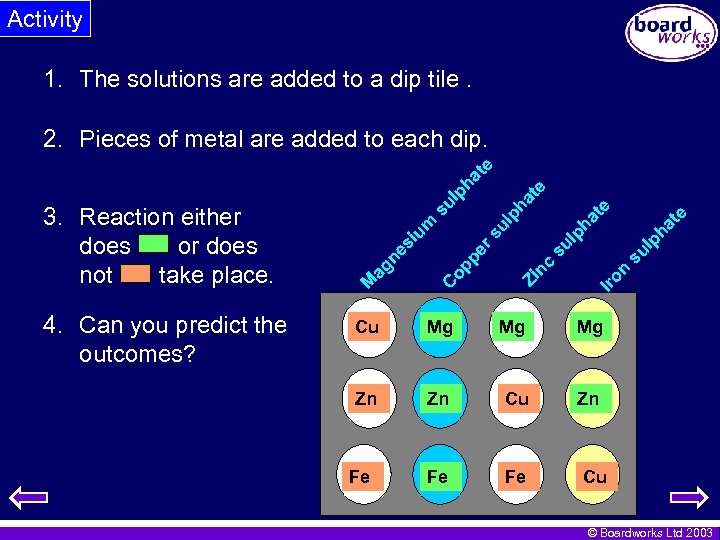
Activity 1. The solutions are added to a dip tile. 4. Can you predict the outcomes? e at lp h ha lp su Iro n Zi nc su er s Co pp at e te ph ul iu m ag ne s M 3. Reaction either does or does not take place. su l ph a te 2. Pieces of metal are added to each dip. Cu Mg Mg Mg Zn Zn Cu Zn Fe Fe Fe Cu © Boardworks Ltd 2003

Displacement Reactions - photos 1. Same reactions can, of course be carried out on a larger scale Reaction between copper sulphate and magnesium. Why does the blue copper sulphate colour gradually disappear? The copper in the copper sulphate is turning into red copper metal. © Boardworks Ltd 2003

Magnesium magnesium + Mg + copper sulphate magnesium + sulphate copper Cu. SO 4 Mg. SO 4 Cu zinc sulphate magnesium + sulphate zinc Zn. SO 4 Mg. SO 4 Zn iron sulphate magnesium + sulphate iron Fe. SO 4 Mg. SO 4 Fe + + + © Boardworks Ltd 2003
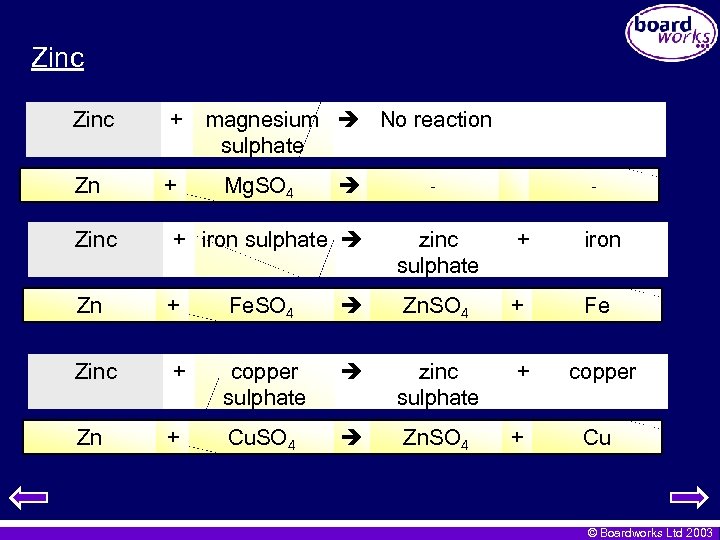
Zinc + Zn + magnesium No reaction sulphate Mg. SO 4 - - zinc sulphate + iron Zn. SO 4 + Fe copper sulphate zinc sulphate + copper Cu. SO 4 Zn. SO 4 + Cu Zinc + iron sulphate Zn + Fe. SO 4 Zinc + Zn + © Boardworks Ltd 2003
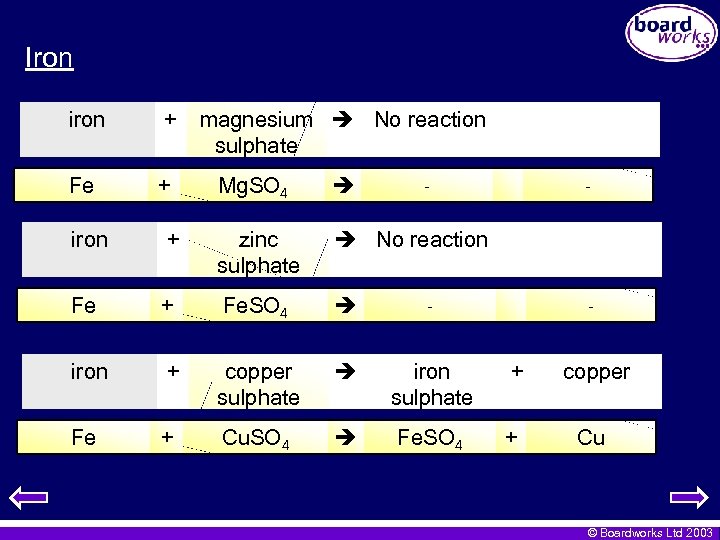
Iron iron + Fe + magnesium No reaction sulphate Mg. SO 4 - - iron + zinc sulphate No reaction Fe + Fe. SO 4 - iron + copper sulphate iron sulphate + copper Fe + Cu. SO 4 Fe. SO 4 + Cu - © Boardworks Ltd 2003
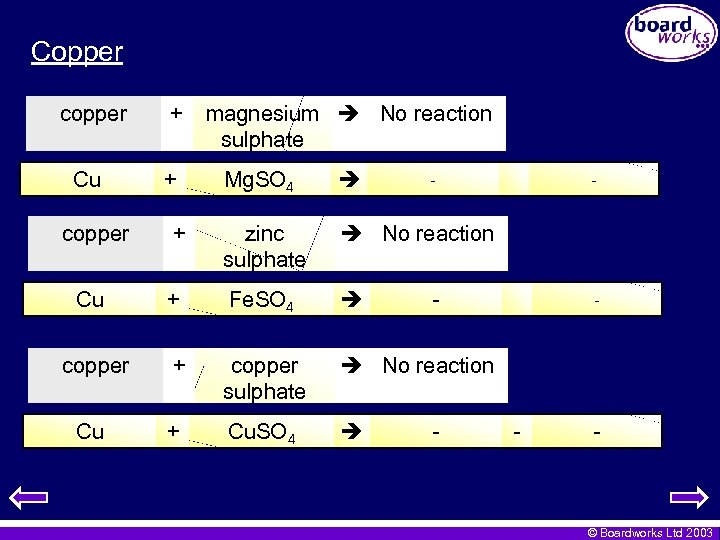
Copper copper + Cu + magnesium No reaction sulphate Mg. SO 4 - copper + zinc sulphate No reaction Cu + Fe. SO 4 copper + copper sulphate No reaction Cu + Cu. SO 4 - - - © Boardworks Ltd 2003

Displacement of oxides • A more reactive metal can displace a less reactive one from a solution of one of its salts. • Similar reactions take place with solid reactants such as the oxides. For example, aluminium • + iron oxide aluminium oxide + iron The more reactive aluminium wins the oxygen from the less reactive iron. This reaction gets so hot that the iron melts! © Boardworks Ltd 2003
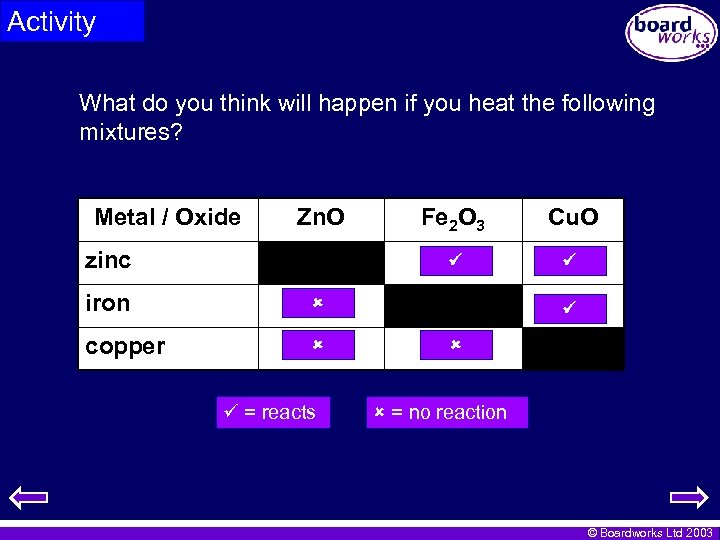
Activity What do you think will happen if you heat the following mixtures? Metal / Oxide Zn. O iron copper Cu. O zinc Fe 2 O 3 = reacts = no reaction © Boardworks Ltd 2003
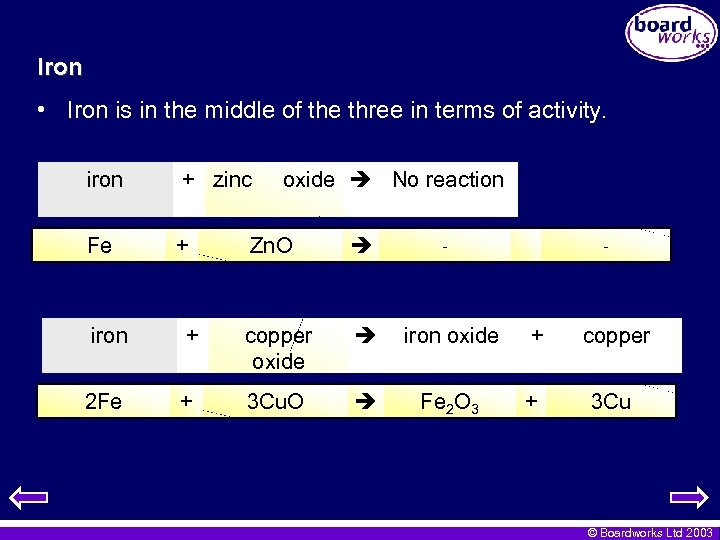
Iron • Iron is in the middle of the three in terms of activity. iron + zinc Fe + oxide No reaction Zn. O - - iron + copper oxide iron oxide + copper 2 Fe + 3 Cu. O Fe 2 O 3 + 3 Cu © Boardworks Ltd 2003

Zinc • Zinc is the most reactive of the three metals. zinc + copper oxide zinc oxide Zn + Cu. O Zn. O zinc + iron 3 Zn + oxide 2 Fe 2 O 3 zinc oxide + copper Cu + iron 3 Zn. O + 2 Fe © Boardworks Ltd 2003
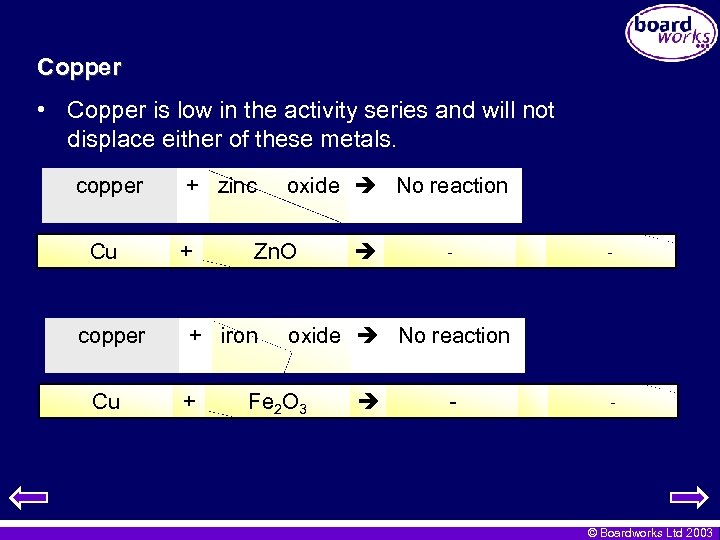
Copper • Copper is low in the activity series and will not displace either of these metals. copper Cu + zinc + Zn. O + iron + oxide No reaction - - oxide No reaction Fe 2 O 3 - - © Boardworks Ltd 2003

Activity Put them in order. Aluminium 1 Potassium Calcium 2 Sodium Copper 3 Calcium Gold 4 Magnesium Iron 5 Aluminium Lead 6 Zinc Magnesium 7 Iron Potassium 8 Lead Silver 9 Copper Sodium 10 Silver Zinc 11 Gold © Boardworks Ltd 2003

Activity Will it react: Yes or no? Reactants Reaction? Potassium Sodium iron oxide magnesium copper sulphate zinc potassium hydrochloric acid iron chloride gold calcium sodium chloride calcium oxygen Yes Calcium Magnesium Yes!! No Aluminium Zinc Iron Lead Copper No Silver Gold Yes © Boardworks Ltd 2003
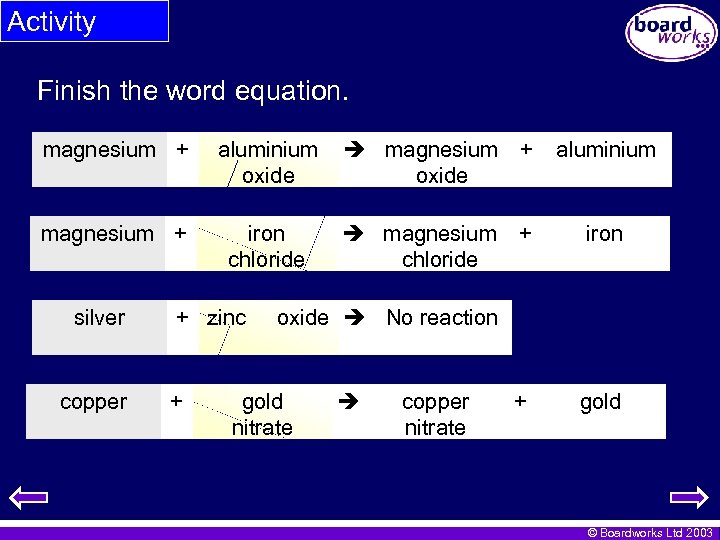
Activity Finish the word equation. magnesium + aluminium oxide magnesium + oxide aluminium magnesium + iron chloride magnesium + chloride iron silver copper + zinc + oxide No reaction gold nitrate copper nitrate + gold © Boardworks Ltd 2003

A useful displacement reaction – thermit reaction When iron (III) oxide reacts with aluminium, a vigorous reaction occurs which produces enough heat to melt any iron which is produced. This reaction was used to weld railway lines together. © Boardworks Ltd 2003

What type of compounds are usually formed when a metal burns in air? A) B) C) D) Metal carbonate Metal hydroxide Metal nitride © Boardworks Ltd 2003

What type of compounds are usually formed when a metal reacts with water? A) B) C) D) Metal carbonate Metal hydroxide Metal nitride © Boardworks Ltd 2003

What type of compounds are usually formed when a metal reacts with nitric acid? A) B) C) D) Metal carbonate Metal chloride Metal sulphate Metal nitrate © Boardworks Ltd 2003

What type of compounds are usually formed when a metal reacts with sulphuric acid? A) B) C) D) Metal carbonate Metal chloride Metal sulphate Metal nitrate © Boardworks Ltd 2003

What number (X) has to be added to balance the equation? Mg + X HCl Mg. Cl 2 + H 2 A) 1 B) 2 C) 3 D) 4 © Boardworks Ltd 2003

What number (Y) has to be added to balance the equation? Mg + Y H 2 SO 4 Mg. SO 4 + H 2 A)1 B)2 C)3 D)4 © Boardworks Ltd 2003

Which metal will fail to displace zinc from a solution of zinc nitrate? A) B) C) D) magnesium calcium potassium copper © Boardworks Ltd 2003

What substances are formed when aluminium reacts with iron oxide? A)Magnesium oxide B)Aluminium oxide and iron C)Aluminium and magnesium oxide D)Aluminium and iron © Boardworks Ltd 2003

Which substances will NOT react? A) Magnesium oxide + sodium B) Aluminium oxide and iron C) Copper oxide and magnesium D) Calcium and iron oxide © Boardworks Ltd 2003
afd832d6773c9adb511046d4dcd5d10a.ppt