48d5ea8d998c55c8a66834027d5266de.ppt
- Количество слайдов: 95
Blood Transfusion Dr Emer Lawlor, IBTS 3 rd February 2003

First Blood Transfusions 1628 1665 -’ 66 Harvey Discovered Circulation of Blood Wilkins & Lower Transfusions from dog to dog 1667 Jean-Baptiste Denis Performed first recorded blood transfusions from animals to humans

19 th Century Transfusions 1818 James Blundell, Obstetrician Blundell First transfusion of human to human

Early transfusion: Paris, France

20 th Century Transfusions 1901 Karl Landsteiner Discovers A, B, O Blood Groups
20 th Century Transfusions 1902 AB Group discovered 1907 Importance of crossmatching blood between donor & recipient 1914 Sodium Citrate proposed as anticoagulant 1936 First Blood Bank: Barcelona, Spanish Civil War 1940 Levine & Landsteiner, Rhesus blood Group System
Bad Blood France, Switzerland, Italy, Netherlands, Germany, Denmark, Ireland, Australia, New Zealand, Canada, USA Japan

Aims of Transfusion Centre • Provision of Blood of the best possible quality and safety for the patient receiving it • To care for the donor - ensure act of donation does not harm donor
Blood Supply Chain • Blood Donor Screening Criteria • Donation Process • Donation Testing • Component preparation • Plasma Products

Donor Screening • Self deferral of ‘At Risk’ groups • Health Questionnaire • Microbiological screening of each donation
Blood Donor Criteria • Age 17 -65 ( new donors until 60) • Weight > 50 kg ( 7 st 12 Ibs) • General health • Specific illnesses • Contact with infection
Blood Donor Criteria • HIV, Hepatitis risk • Medication • CJD • Hb > 13 M; >12 F
Haemoglobin Testing

Alternatives to Voluntary Donors: Autologous • 5 -10% of patients are fit to predeposit autologous blood • Orthopaedic, Plastic Surgery, Gynaecology • Up to 5 units can be predeposited/1 week • Increased donor reactions • Still have risk of : Clerical error, Bacterial Infections

Alternatives to Voluntary Donors: Directed • Relatives or friends • Not demonstrably safer • Not voluntary • Viral marker rates higher as often first time donors • TA-GVHD
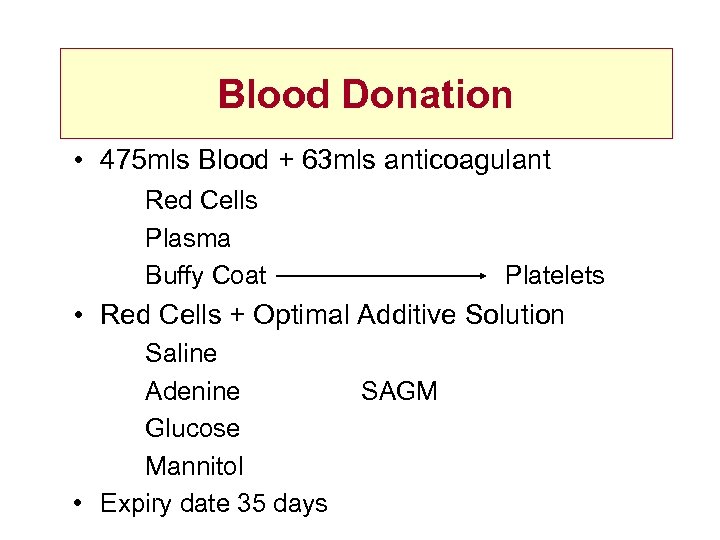
Blood Donation • 475 mls Blood + 63 mls anticoagulant Red Cells Plasma Buffy Coat Platelets • Red Cells + Optimal Additive Solution Saline Adenine Glucose Mannitol • Expiry date 35 days SAGM
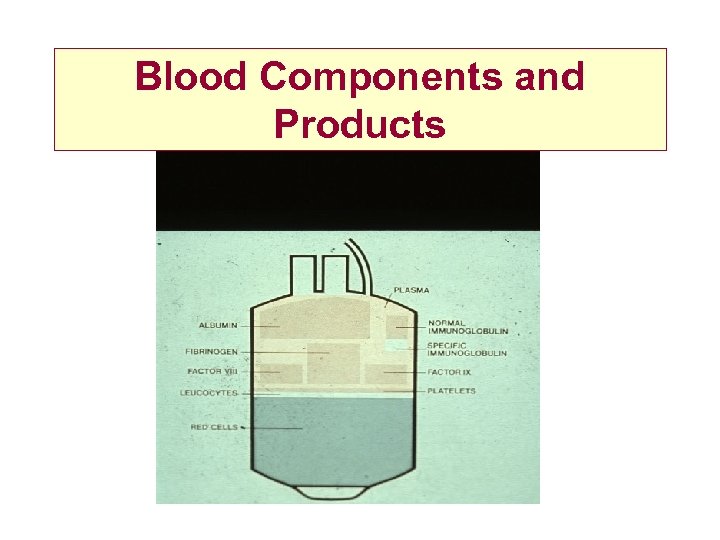
Blood Components and Products
Blood Component Production
Blood Component Production
Leucodepletion • Universal leucodepletion introduced in 1999 to reduce the risk of v. CJD transmission by blood • other benefits - less febrile reactions, less alloimmunisation, less GVHD, ? reduce immunosuppresssive effects
Leucodepletion
Platelets • Pools prepared from buffycoats of whole blood donations (4 donations) • Apheresis concentrates from one donor using a cell separator • pool/apheresis pack (250 mls) = standard adult dose
Blood Donation Testing • Microbiology markers • Blood grouping and screening for high titre antibodies • Quality monitoring
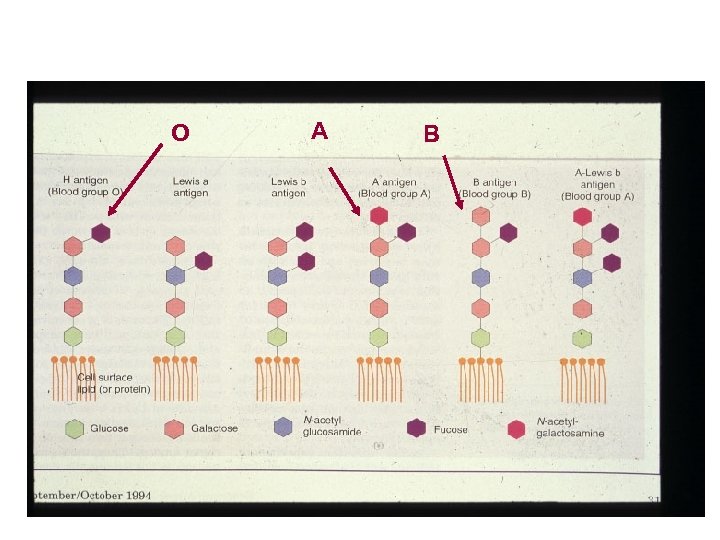
O A B
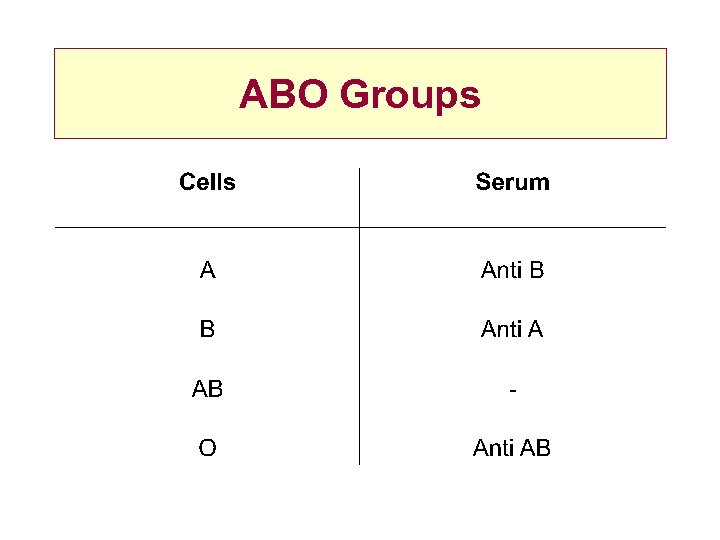
ABO Groups
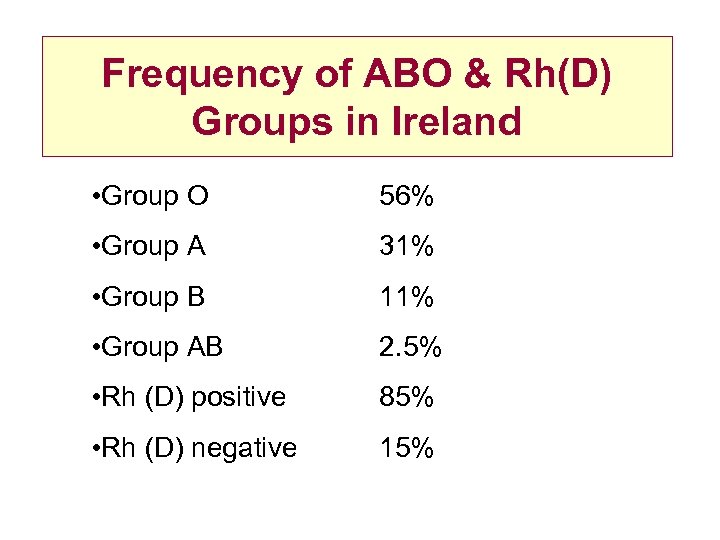
Frequency of ABO & Rh(D) Groups in Ireland • Group O 56% • Group A 31% • Group B 11% • Group AB 2. 5% • Rh (D) positive 85% • Rh (D) negative 15%
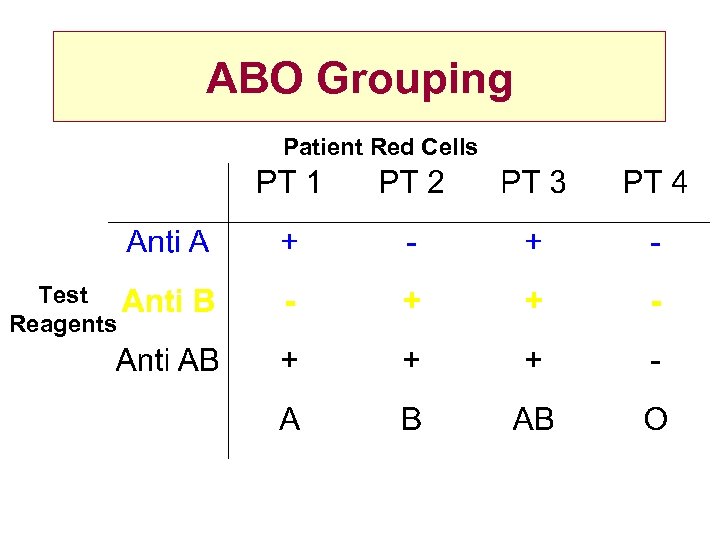
ABO Grouping Patient Red Cells Test Reagents
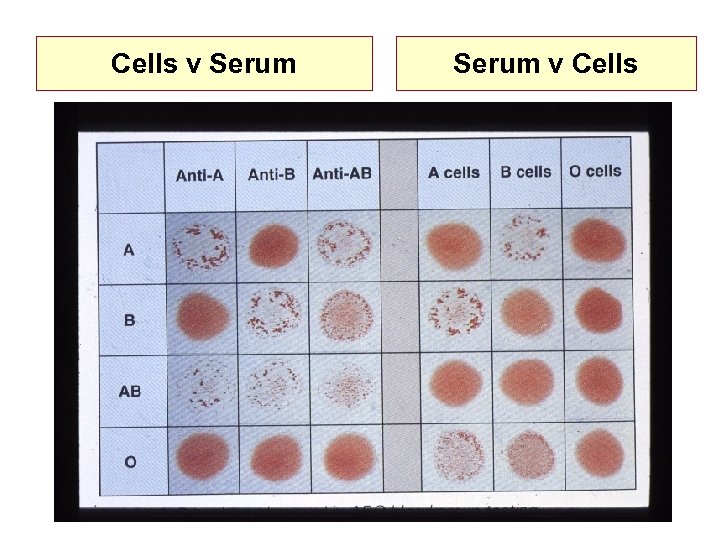
Cells v Serum v Cells
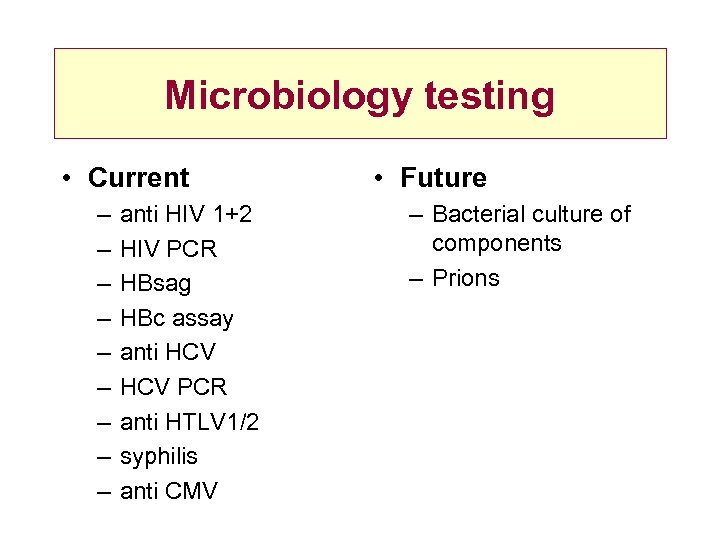
Microbiology testing • Current – – – – – anti HIV 1+2 HIV PCR HBsag HBc assay anti HCV PCR anti HTLV 1/2 syphilis anti CMV • Future – Bacterial culture of components – Prions
Hospital Blood Transfusion Laboratory Patient/donor testing and product selection and issue
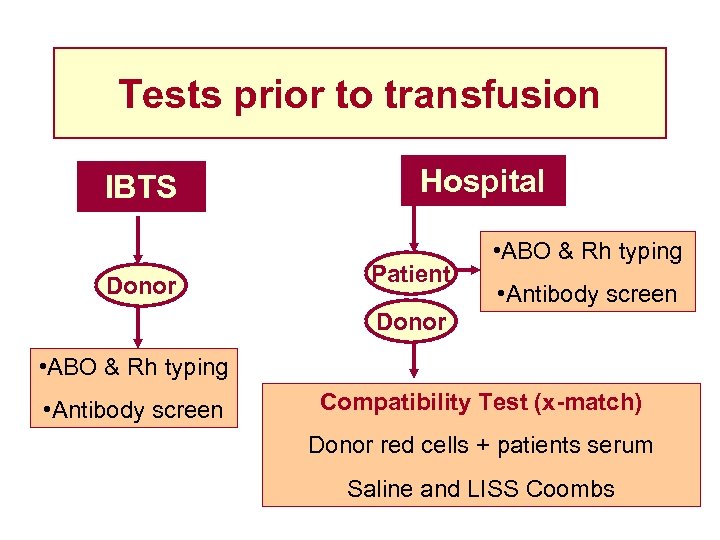
Tests prior to transfusion IBTS Donor Hospital Patient • ABO & Rh typing • Antibody screen Donor • ABO & Rh typing • Antibody screen Compatibility Test (x-match) Donor red cells + patients serum Saline and LISS Coombs

Antigens on Red Cells
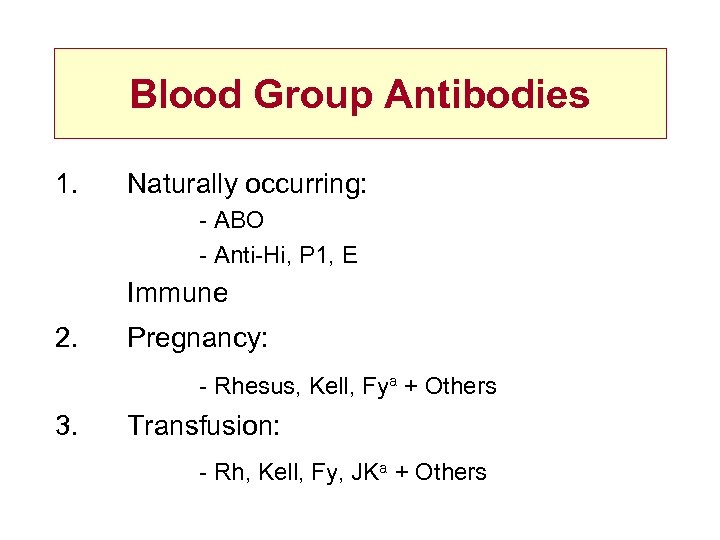
Blood Group Antibodies 1. Naturally occurring: - ABO - Anti-Hi, P 1, E Immune 2. Pregnancy: - Rhesus, Kell, Fya + Others 3. Transfusion: - Rh, Kell, Fy, JKa + Others

Conventional Testing. Weak reactions often difficult to interpret. Can also be downgraded due to shaking the completed test.
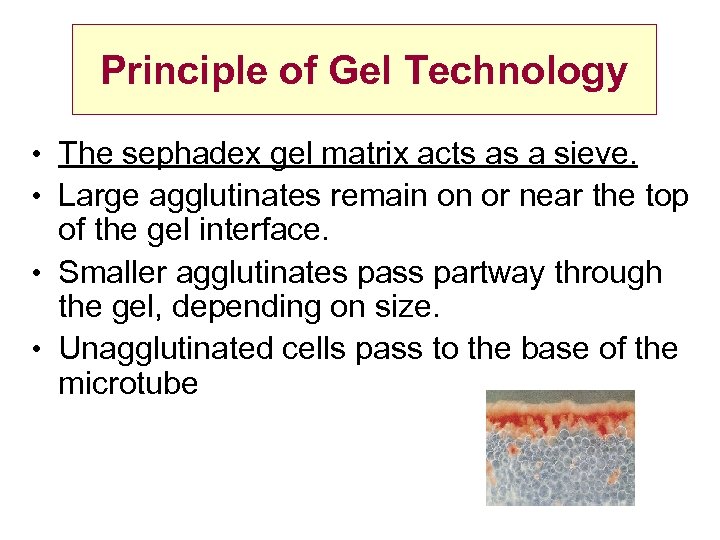
Principle of Gel Technology • The sephadex gel matrix acts as a sieve. • Large agglutinates remain on or near the top of the gel interface. • Smaller agglutinates pass partway through the gel, depending on size. • Unagglutinated cells pass to the base of the microtube
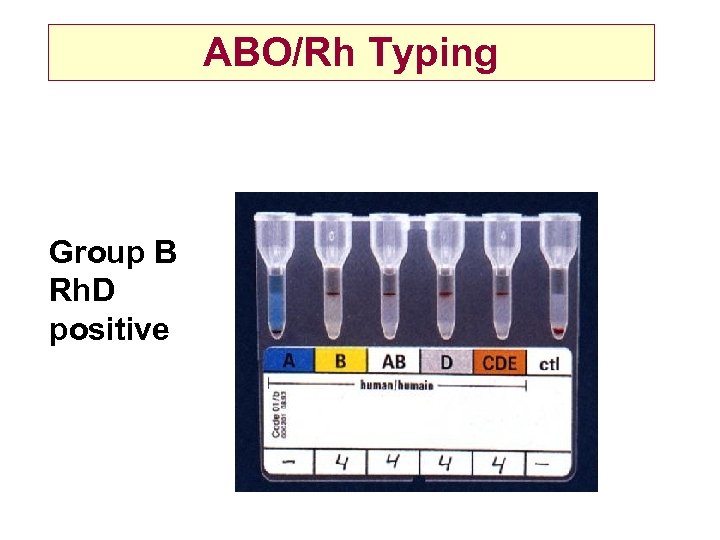
ABO/Rh Typing Group B Rh. D positive
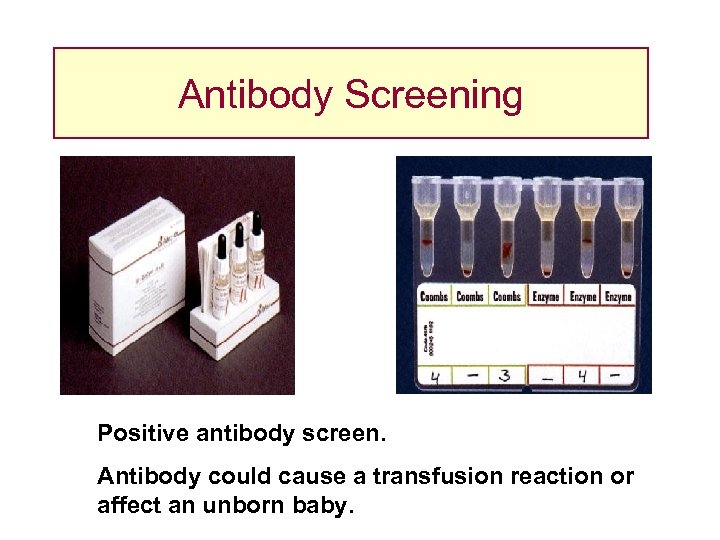
Antibody Screening Positive antibody screen. Antibody could cause a transfusion reaction or affect an unborn baby.
Purpose of Crossmatch • Detect unsuspected ABO incompatibility Donor centre Error in laboratory • Detection of unsuspected antibodies in <1% cases
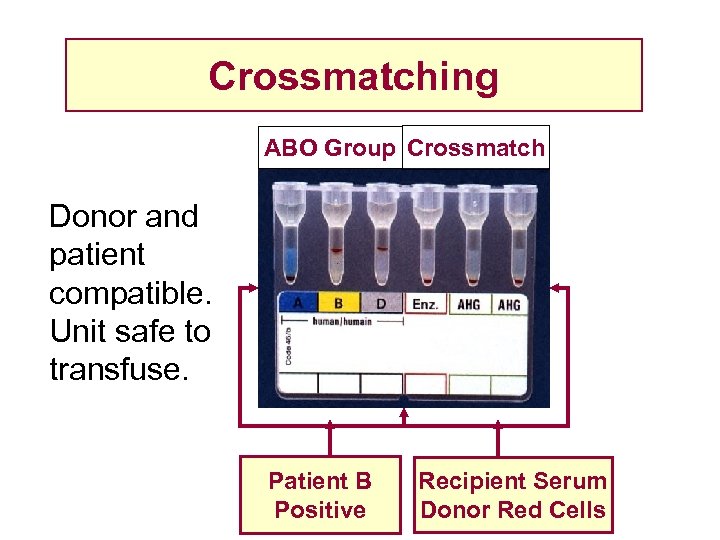
Crossmatching ABO Group Crossmatch Donor and patient compatible. Unit safe to transfuse. Patient B Positive Recipient Serum Donor Red Cells
Electronic Crossmatch • Donor units • Repeat ABO Rh groups performed on all donor units • Automated Grouping • Validated computer software to ensure that ABO incompatible units cannot be selected for patient
Blood Component Storage • Whole blood/red cells - 2 -6 C for up to 35 days use within 5 hours of removal from blood fridge • Platelets -20 -24 C on agitator for 5 days • SD Fresh frozen plasma ( FFP) for 6 months - use within 4 hrs of thawing • Cryoprecipitate -30 C use within 4 hours of thawing
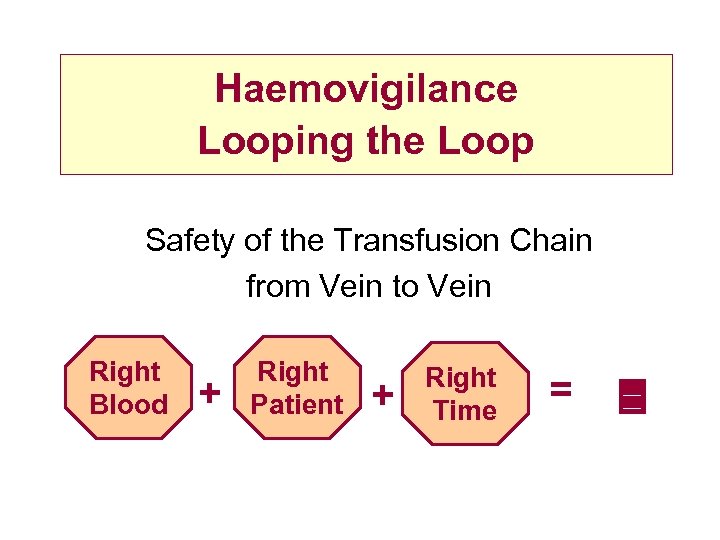
Haemovigilance Looping the Loop Safety of the Transfusion Chain from Vein to Vein Right Blood + Right Patient + Right Time =
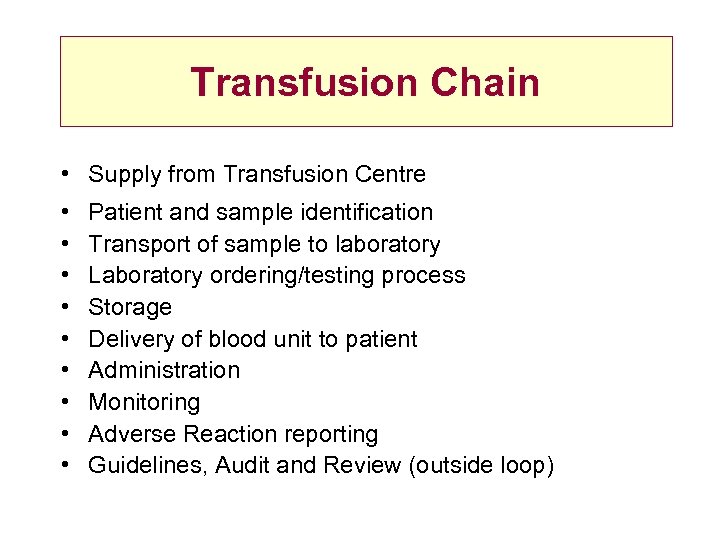
Transfusion Chain • Supply from Transfusion Centre • • • Patient and sample identification Transport of sample to laboratory Laboratory ordering/testing process Storage Delivery of blood unit to patient Administration Monitoring Adverse Reaction reporting Guidelines, Audit and Review (outside loop)
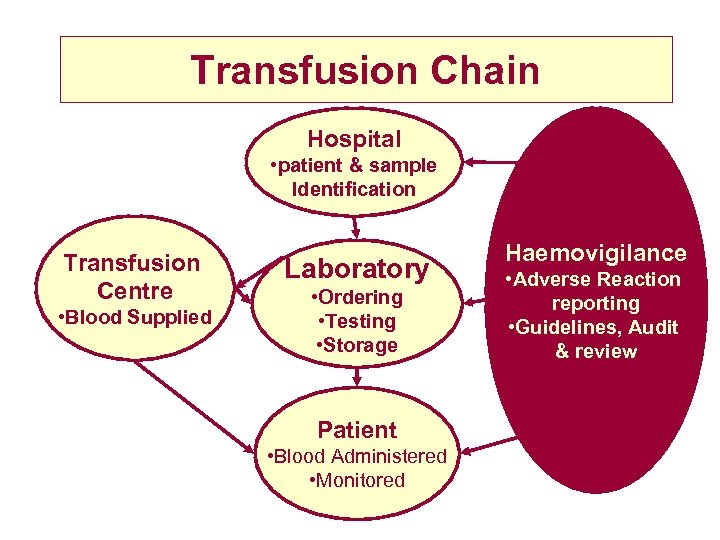
Transfusion Chain Hospital • patient & sample Identification Transfusion Centre • Blood Supplied Laboratory • Ordering • Testing • Storage Patient • Blood Administered • Monitored Haemovigilance • Adverse Reaction reporting • Guidelines, Audit & review
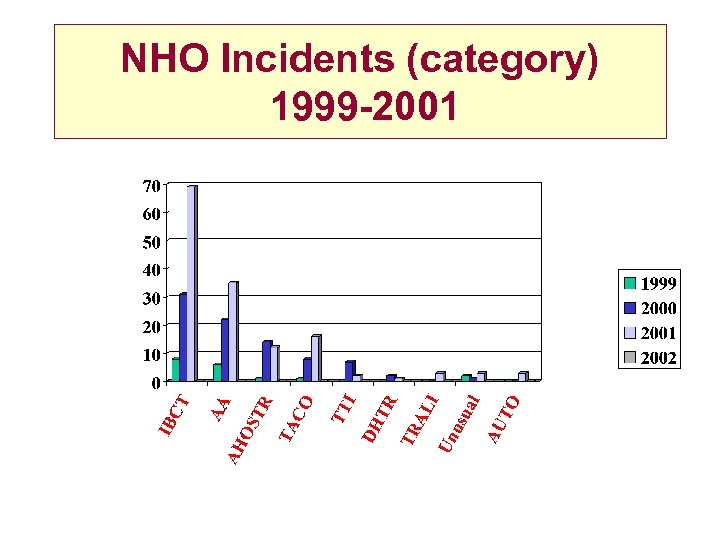
NHO Incidents (category) 1999 -2001
Transfusion Chain • Supply from Transfusion Centre • • • Patient and sample identification Transport of sample to laboratory Laboratory ordering/testing process Storage Delivery of blood unit to patient Administration Monitoring Adverse Reaction reporting Guidelines, Audit and Review
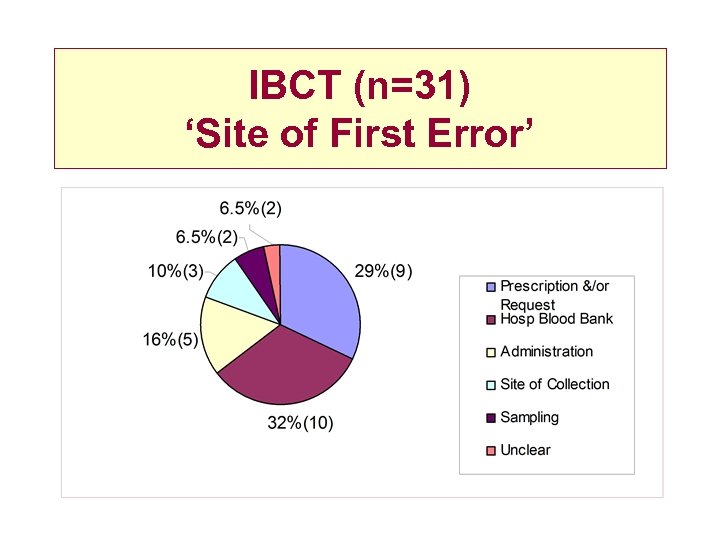
IBCT (n=31) ‘Site of First Error’
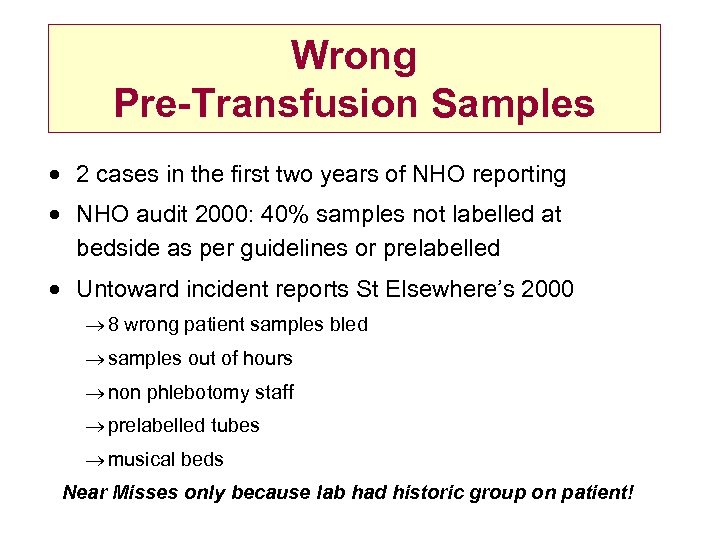
Wrong Pre-Transfusion Samples · 2 cases in the first two years of NHO reporting · NHO audit 2000: 40% samples not labelled at bedside as per guidelines or prelabelled · Untoward incident reports St Elsewhere’s 2000 ® 8 wrong patient samples bled ® samples out of hours ® non phlebotomy staff ® prelabelled tubes ® musical beds Near Misses only because lab had historic group on patient!
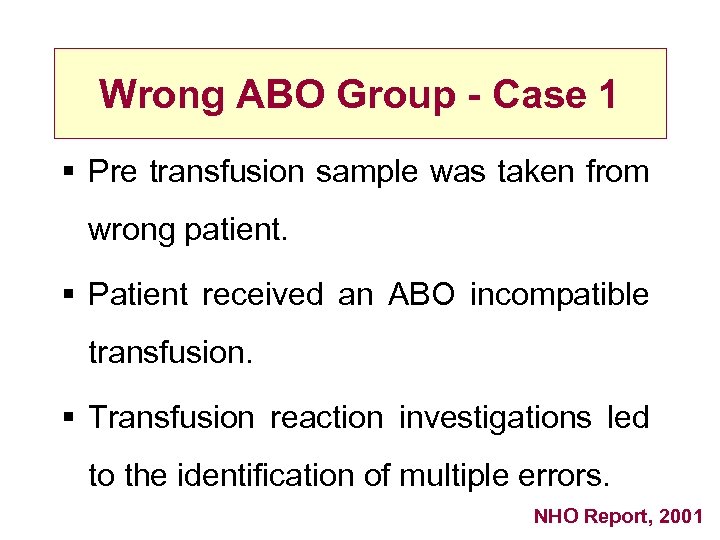
Wrong ABO Group - Case 1 § Pre transfusion sample was taken from wrong patient. § Patient received an ABO incompatible transfusion. § Transfusion reaction investigations led to the identification of multiple errors. NHO Report, 2001

Wrong ABO Group (Cases 5 & 6) • Two patients in the same ward, one Group O, other Group A, received blood crossmatched for each other – Remote checking of units – Failure to positively identify the patients – Error detected by nursing staff following commencement of the transfusion. – Transfusion reaction in O patient who received 100 mls A red cells NHO Report, 2001
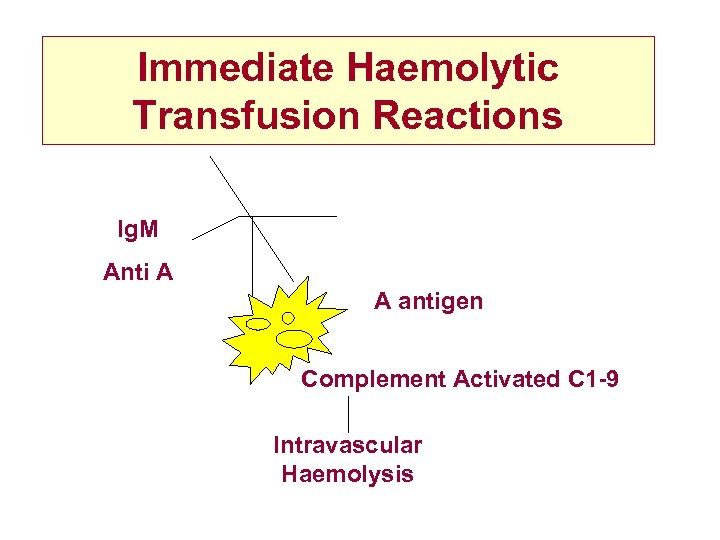
Immediate Haemolytic Transfusion Reactions Ig. M Anti A A antigen Complement Activated C 1 -9 Intravascular Haemolysis
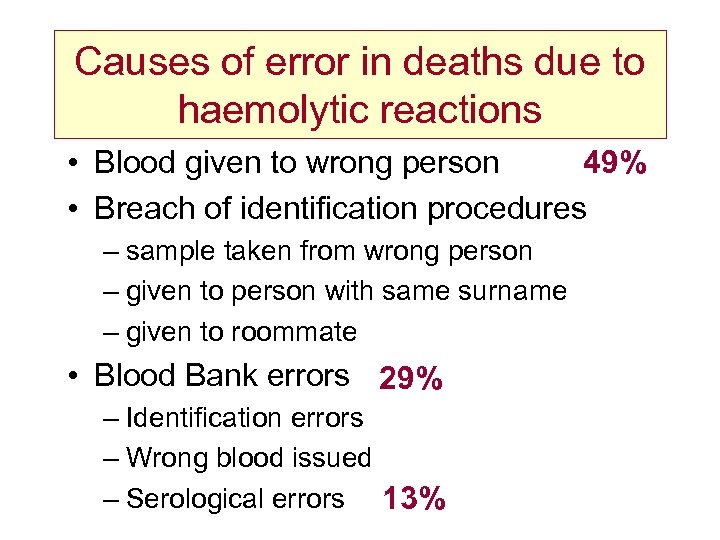
Causes of error in deaths due to haemolytic reactions • Blood given to wrong person 49% • Breach of identification procedures – sample taken from wrong person – given to person with same surname – given to roommate • Blood Bank errors 29% – Identification errors – Wrong blood issued – Serological errors 13%
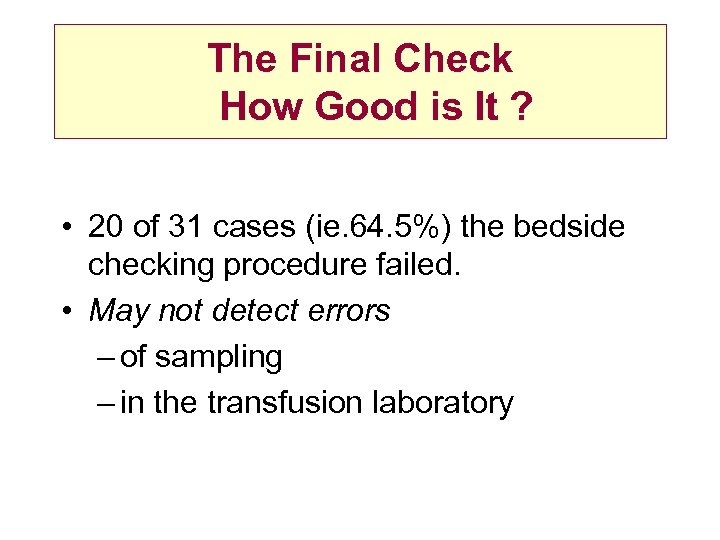
The Final Check How Good is It ? • 20 of 31 cases (ie. 64. 5%) the bedside checking procedure failed. • May not detect errors – of sampling – in the transfusion laboratory
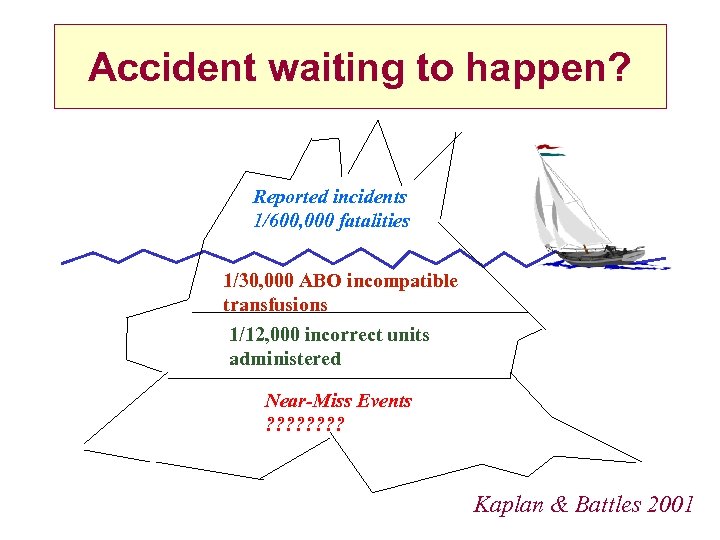
Accident waiting to happen? Reported incidents 1/600, 000 fatalities 1/30, 000 ABO incompatible transfusions 1/12, 000 incorrect units administered Near-Miss Events ? ? ? ? Kaplan & Battles 2001
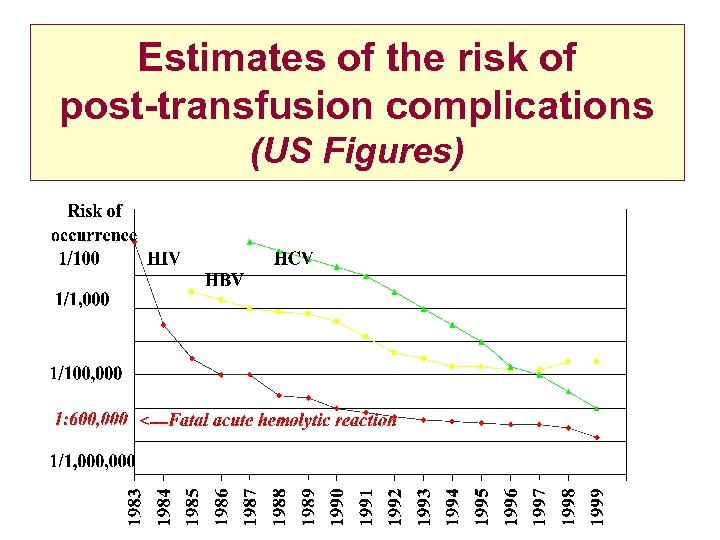
Estimates of the risk of post-transfusion complications (US Figures)
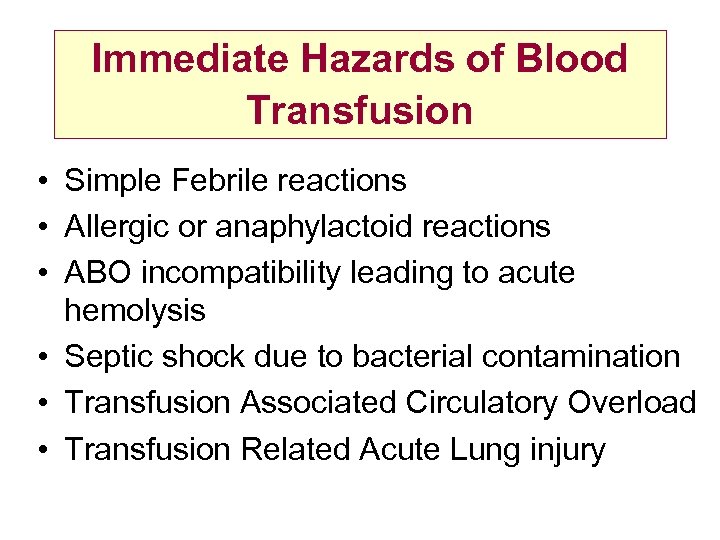
Immediate Hazards of Blood Transfusion • Simple Febrile reactions • Allergic or anaphylactoid reactions • ABO incompatibility leading to acute hemolysis • Septic shock due to bacterial contamination • Transfusion Associated Circulatory Overload • Transfusion Related Acute Lung injury
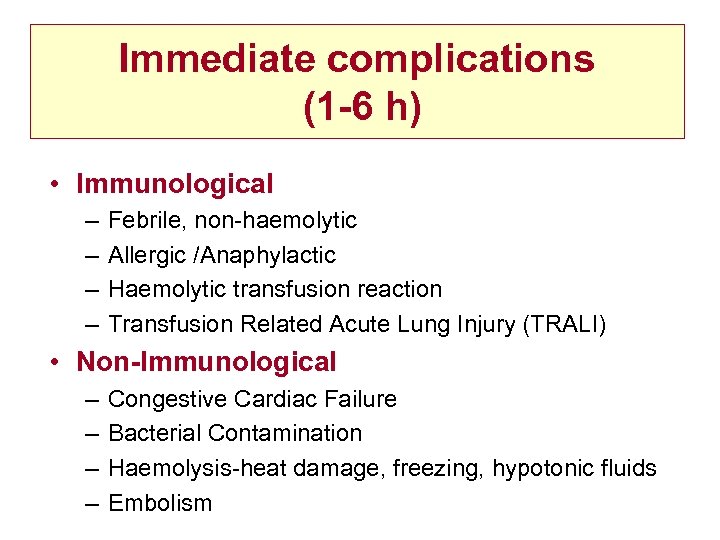
Immediate complications (1 -6 h) • Immunological – – Febrile, non-haemolytic Allergic /Anaphylactic Haemolytic transfusion reaction Transfusion Related Acute Lung Injury (TRALI) • Non-Immunological – – Congestive Cardiac Failure Bacterial Contamination Haemolysis-heat damage, freezing, hypotonic fluids Embolism
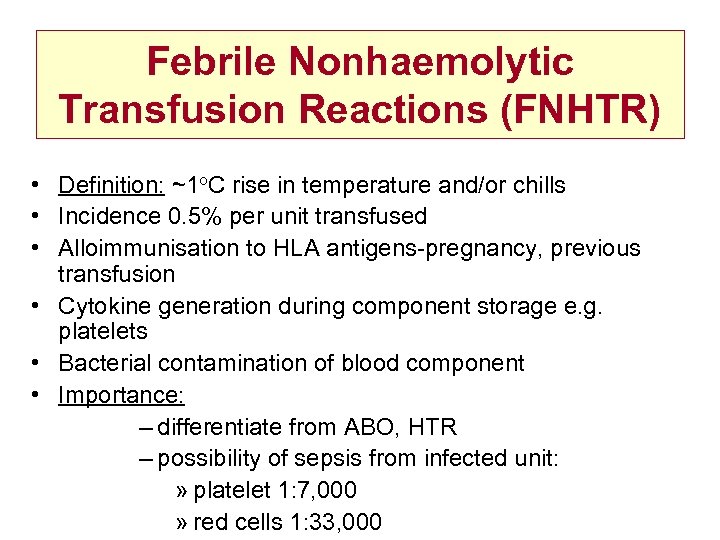
Febrile Nonhaemolytic Transfusion Reactions (FNHTR) • Definition: ~1 o. C rise in temperature and/or chills • Incidence 0. 5% per unit transfused • Alloimmunisation to HLA antigens-pregnancy, previous transfusion • Cytokine generation during component storage e. g. platelets • Bacterial contamination of blood component • Importance: – differentiate from ABO, HTR – possibility of sepsis from infected unit: » platelet 1: 7, 000 » red cells 1: 33, 000
Urticarial Reactions • 1 -3% of transfusions • Slow/Stop the transfusion rate • Administer iv antibodies e. g chlorpheniramine 1020 mg • If no further progression after 30 mins transfusion may proceed normally • Prevention: prophylactic antihistamines
Delayed Hazards • Delayed Hemolytic Transfusion Reactions • Post Transfusion Purpura • Transfusion Associated Graft versus Host Disease • Viral infection - parasitic infections ? prions • Immunosuppression • Iron overload in multi- transfused recipients
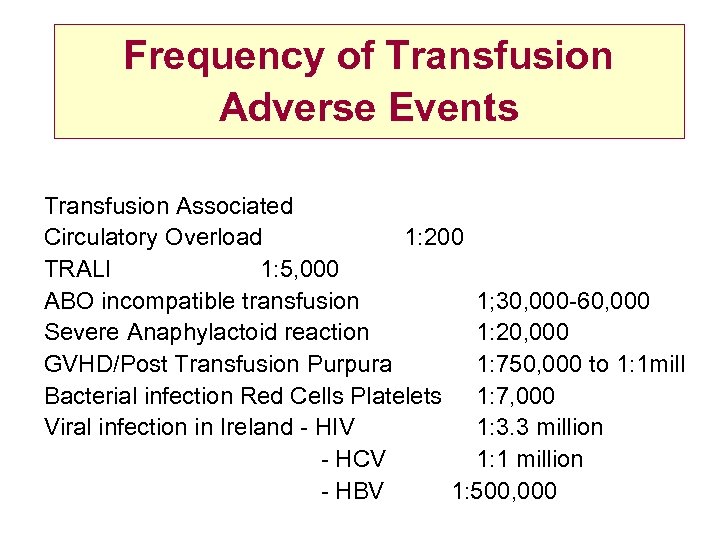
Frequency of Transfusion Adverse Events Transfusion Associated Circulatory Overload 1: 200 TRALI 1: 5, 000 ABO incompatible transfusion 1; 30, 000 -60, 000 Severe Anaphylactoid reaction 1: 20, 000 GVHD/Post Transfusion Purpura 1: 750, 000 to 1: 1 mill Bacterial infection Red Cells Platelets 1: 7, 000 Viral infection in Ireland - HIV 1: 3. 3 million - HCV 1: 1 million - HBV 1: 500, 000
Transfusion Associated Circulatory Overload (TACO) • 1% of transfusions are complicated by TACO • dysnoea, hypertension, crepitations, O 2 sats • Risk of volume overload/respiratory distress especially in the small and/or elderly patient • Largely avoidable by careful attention to fluid balance
Transfusion Related Acute Lung Injury • 3 rd commonest cause of death from transfusion • 89% associated with Granulocyte antibodies or HLA antibodies in donor • Donor antibodies react with patient white cells • Aggregates in lungs • Neutrophil priming by lipid ? Older components • 2 Hit hypothesis-underlying condition ? haematological malignancy, ? cardiac
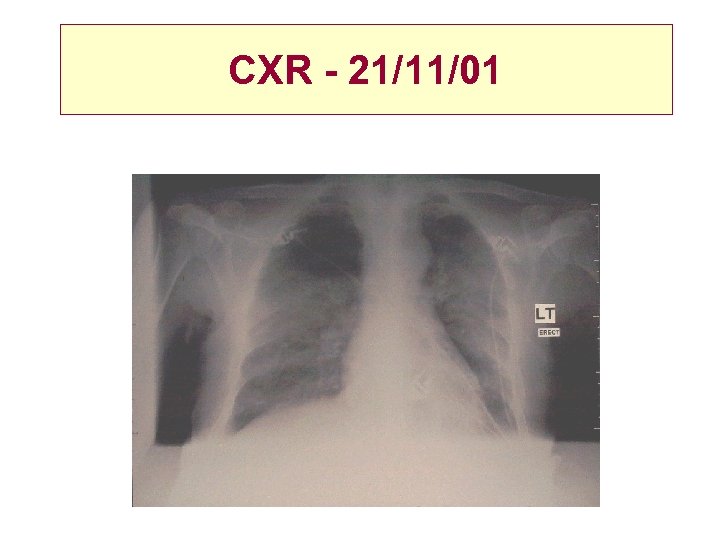
CXR - 21/11/01
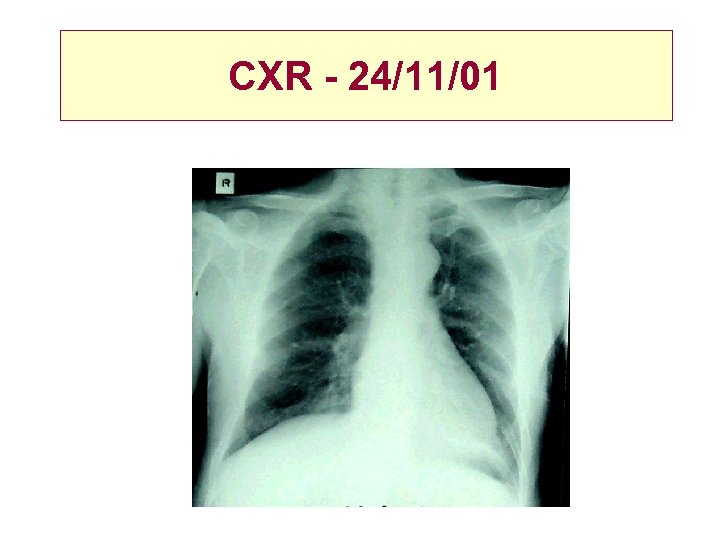
CXR - 24/11/01

Graft versus Host Disease • Rarely reported but virtually always fatal • Occurs in immunosuppressed patients • In normal patients where donor is HLA homozygous and patient shares a haplotype allowing proliferation and expansion of donor lymphocytes • Rare but occurrence commoner where fresh blood, donations from relatives, or where there is a restricted genetic pool i. e. Japan • Prevented by irradiation of product
TA-GVHD • • 1 -6 weeks post transfusion Fever Rash Liver dysfunction Diarrhoea Pancytopenia Skin/bone marrow biopsy Presence circulating donor lymphocytes
Post-Transfusion Purpura • Thrombocytopenia 5 -12 days post transfusion associated with anti HPA antibodies in the patient • Shot 11 cases • All women previous pregnancies • 4 previously transfused • Platelet alloantibody: HPA-1 a in 8/9 cases • Rx Iv. Ig
Bacterial Contamination • Infective shock 1: 2 million units transfused • 15 cases reported to SHOT since 1996 • 5 fatalities • largest cause of infection related deaths from blood transfusion
Prevention of Transmissible Disease • Careful selection of Donors › › Exclude IVDU Homosexuals/bisexuals promiscuity Other exposures e. g. visits to malaria areas • Laboratory Screening › › › HIV 1 + 2 & HIV PCR HCV & HCV PCR HBs. Ag + Core Antibody VDRL HTLV 1+2

Sources of Risk • Infectious, but seronegative window period • Immunosilent infection • Variants of known agents • Laboratory error • New agents for which no test available • Unknown agents
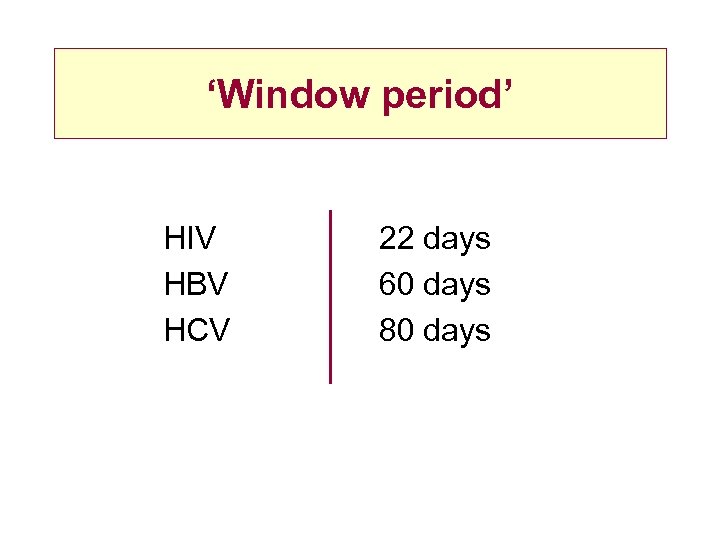
‘Window period’ HIV HBV HCV 22 days 60 days 80 days
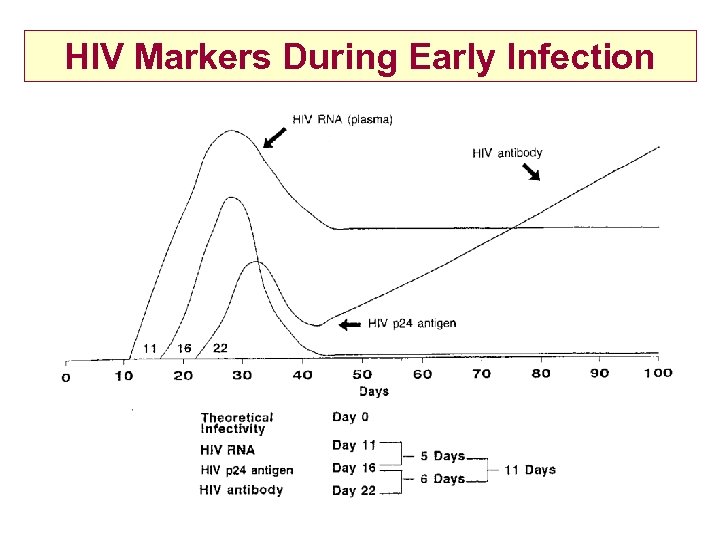
HIV Markers During Early Infection
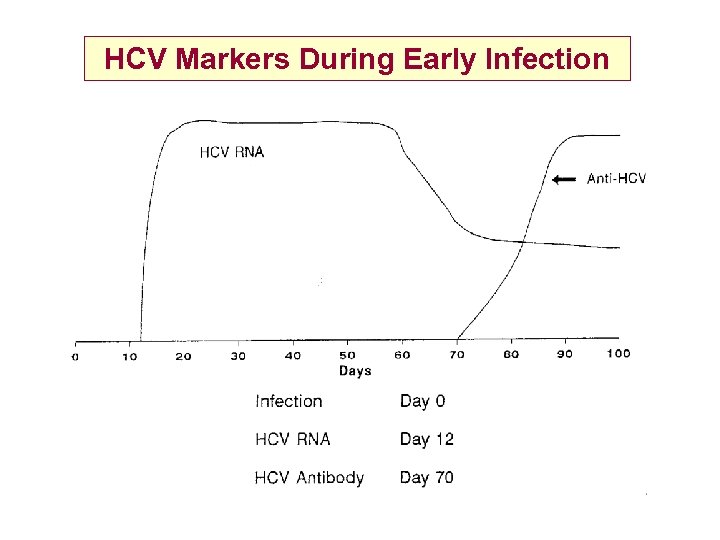
HCV Markers During Early Infection
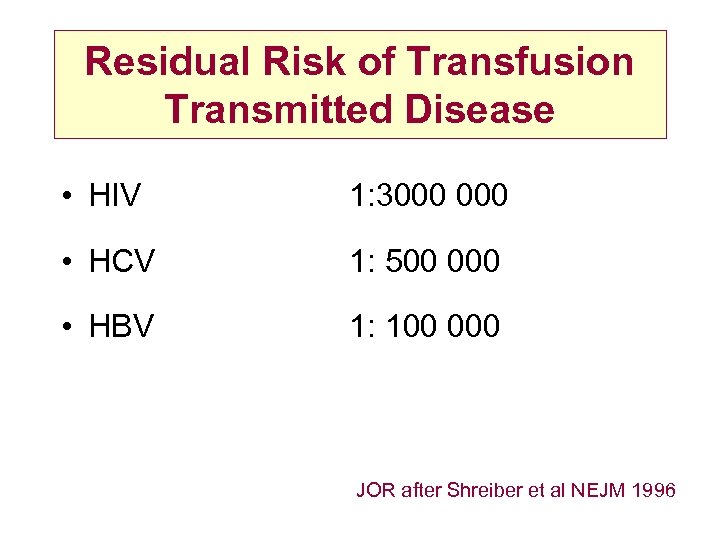
Residual Risk of Transfusion Transmitted Disease • HIV 1: 3000 • HCV 1: 500 000 • HBV 1: 100 000 JOR after Shreiber et al NEJM 1996
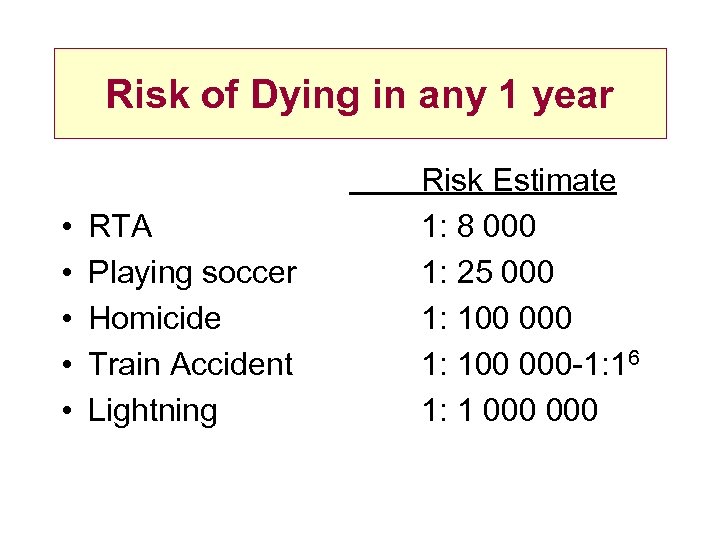
Risk of Dying in any 1 year • • • RTA Playing soccer Homicide Train Accident Lightning Risk Estimate 1: 8 000 1: 25 000 1: 100 000 -1: 16 1: 1 000
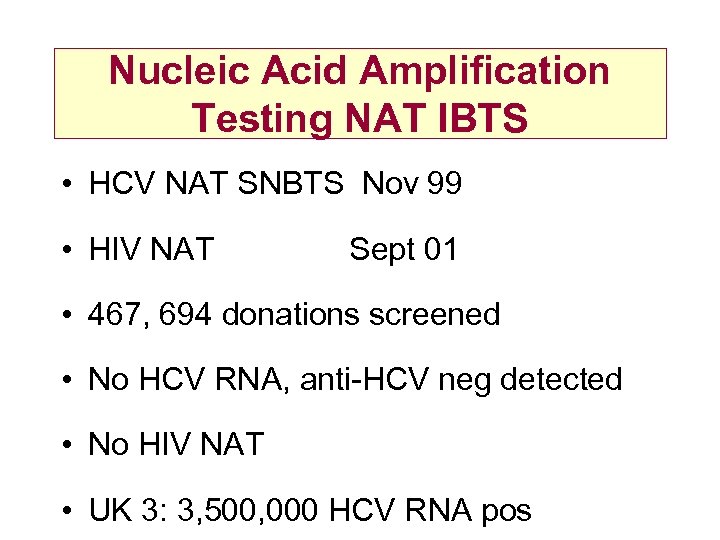
Nucleic Acid Amplification Testing NAT IBTS • HCV NAT SNBTS Nov 99 • HIV NAT Sept 01 • 467, 694 donations screened • No HCV RNA, anti-HCV neg detected • No HIV NAT • UK 3: 3, 500, 000 HCV RNA pos
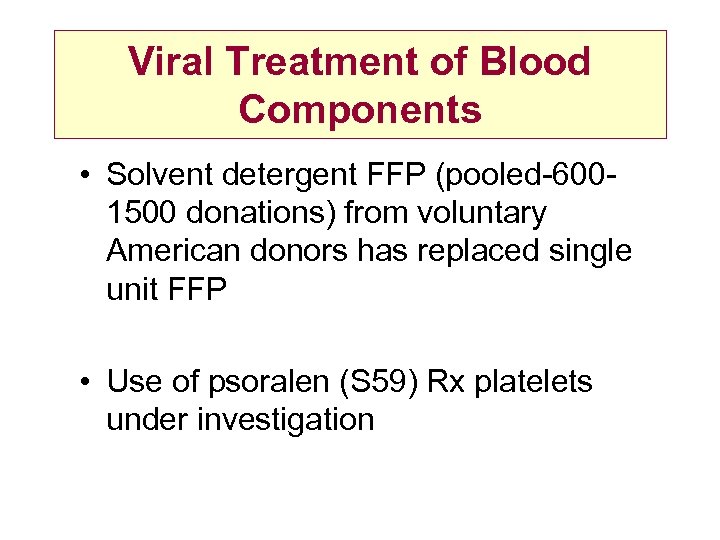
Viral Treatment of Blood Components • Solvent detergent FFP (pooled-6001500 donations) from voluntary American donors has replaced single unit FFP • Use of psoralen (S 59) Rx platelets under investigation

Other Infectious Risks • Viruses – Parvo B 19, CMV, EBV, HAV – West Nile Virus • Parasites – Malaria. – Trypanosomiasis – Babesiosis • Prions-v. CJD
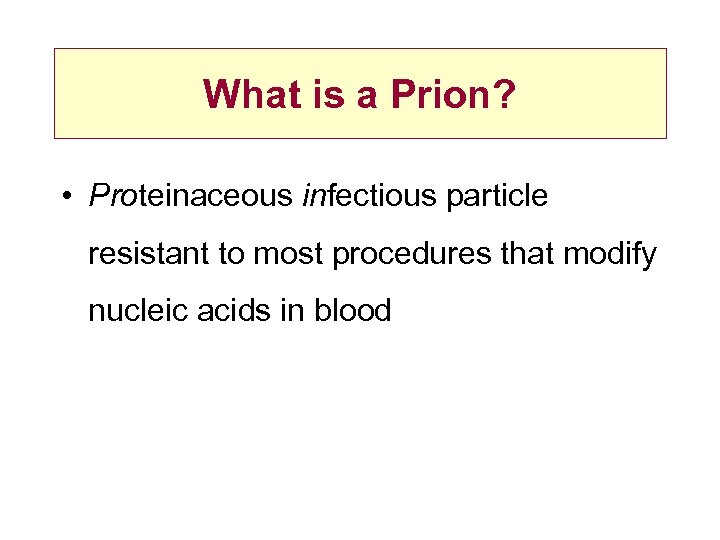
What is a Prion? • Proteinaceous infectious particle resistant to most procedures that modify nucleic acids in blood
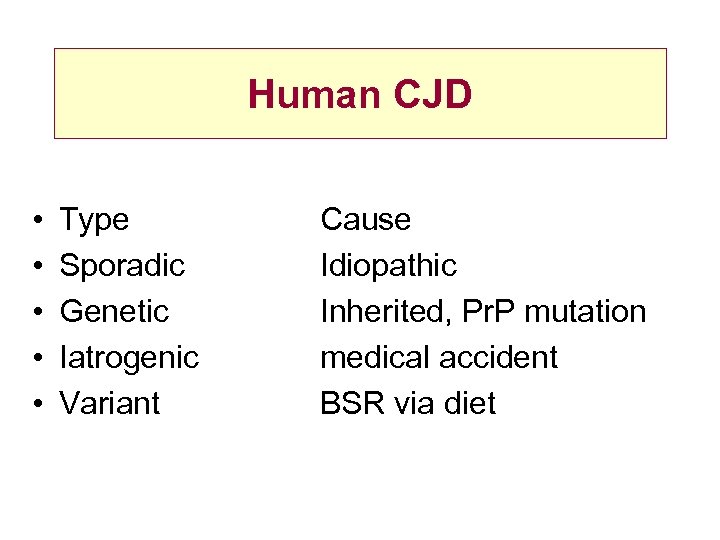
Human CJD • • • Type Sporadic Genetic Iatrogenic Variant Cause Idiopathic Inherited, Pr. P mutation medical accident BSR via diet
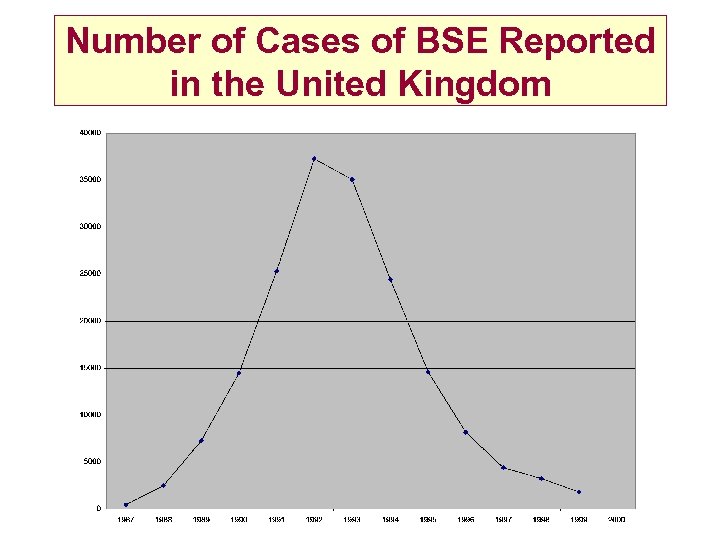
Number of Cases of BSE Reported in the United Kingdom
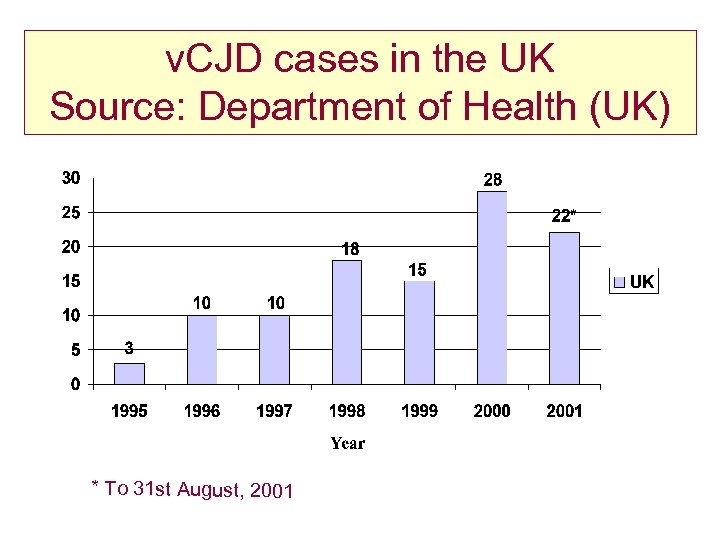
v. CJD cases in the UK Source: Department of Health (UK) * To 31 st August, 2001
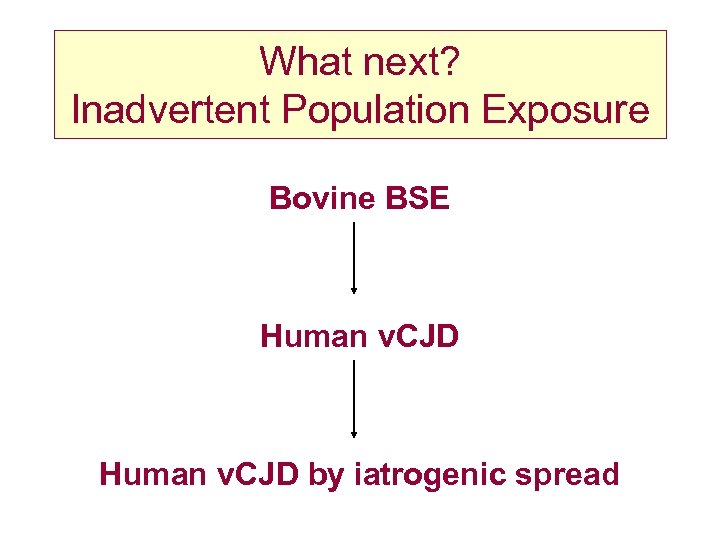
What next? Inadvertent Population Exposure Bovine BSE Human v. CJD by iatrogenic spread
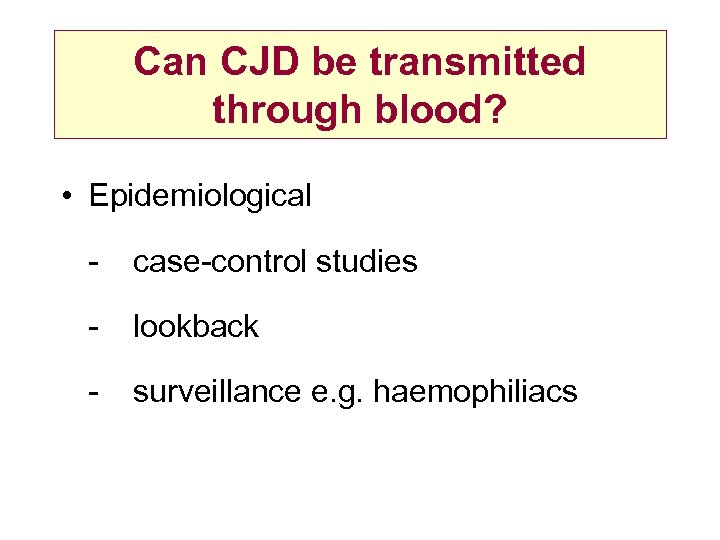
Can CJD be transmitted through blood? • Epidemiological - case-control studies - lookback - surveillance e. g. haemophiliacs

v. CJD and blood transfusion • Is there a risk? – no documented evidence of transfusion transmission in humans – putative infective agent found in lymphoid tissue – BSE transmissible by blood from sheep to sheep
CJD Blood Donor Exclusions • • 5 year Residence in UK 1980 -1996 dementia chronic neurological disorder personal or family hx of CJD recipients of human pituitary hormones corneal transplant recipients brain surgery pre 1992
v. CJD and Blood Transfusion • Current Precautions – Donor exclusion criteria – UK, Irish plasma not used for fractionation – 100% leucodepletion of all components

FDA TSEAC 28 -29 June, 2001 Indigenous risk assessment in European countries – UK 98% of total risk – France 5% of UK risk – Rest of Europe 1. 5% of UK risk
Protective Measures • Sourcing: country • Sourcing: donor • Processing • Testing • Appropriate use of blood/alternatives
West Nile Virus • Flavivirus natural host birds, mammals spread by mosquito • 43 States in US • 80% cases asymptomatic • 20% mild illness • 1% encephalitis • 3989 cases -259 deaths • 30 related to Transplant /Transfusion
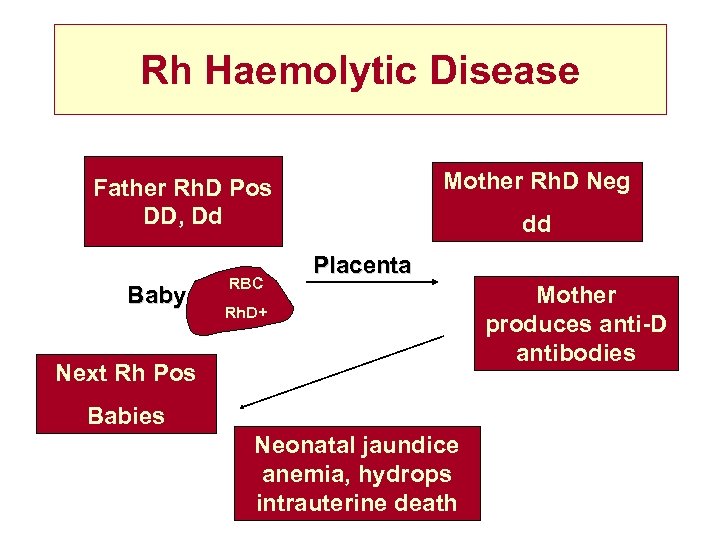
Rh Haemolytic Disease Mother Rh. D Neg Father Rh. D Pos DD, Dd Baby RBC dd Placenta Rh. D+ Next Rh Pos Babies Neonatal jaundice anemia, hydrops intrauterine death Mother produces anti-D antibodies
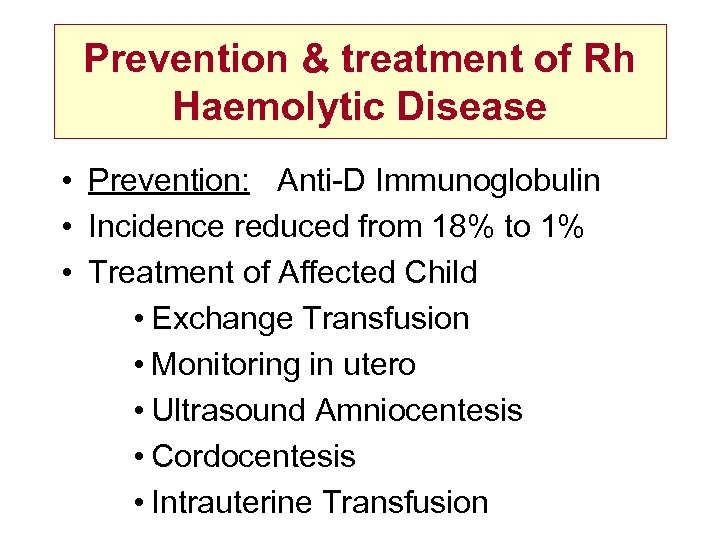
Prevention & treatment of Rh Haemolytic Disease • Prevention: Anti-D Immunoglobulin • Incidence reduced from 18% to 1% • Treatment of Affected Child • Exchange Transfusion • Monitoring in utero • Ultrasound Amniocentesis • Cordocentesis • Intrauterine Transfusion
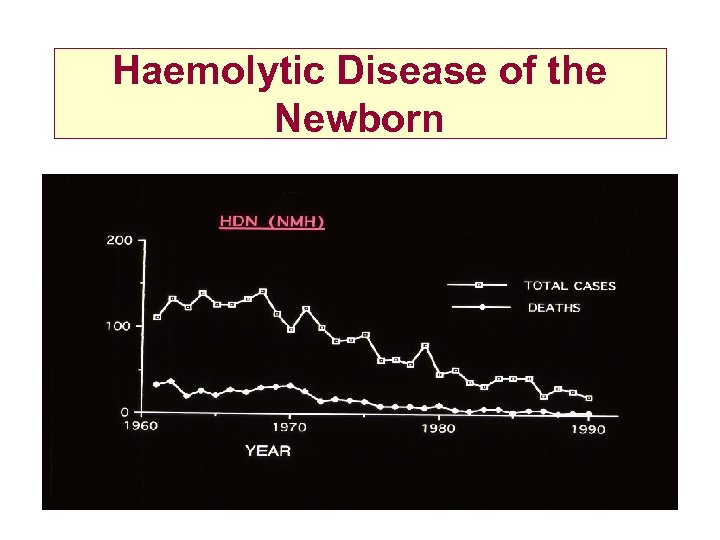
Haemolytic Disease of the Newborn
48d5ea8d998c55c8a66834027d5266de.ppt