d82abbaf526bdedb6c98e9a810913f7b.ppt
- Количество слайдов: 83
Blood Product Transfusion Rational use Complications and its management Dr Chu Po Ngai Alvin 13 Feb 2009

Take home message… Drug Art > solid evidence Look out for complications

Blood components Red blood cells Platelets Fresh Frozen Plasma Cryoprecipitate

Red blood cells • Red cell transfusions increase the oxygen carrying capacity of blood by raising the Hb concentration • Avoid tissue hypoxia • 1 unit – 300 ml volume – Hb concentration 18 -23 g/d. L • 1 unit -> ↑ Hb 1 g/d. L
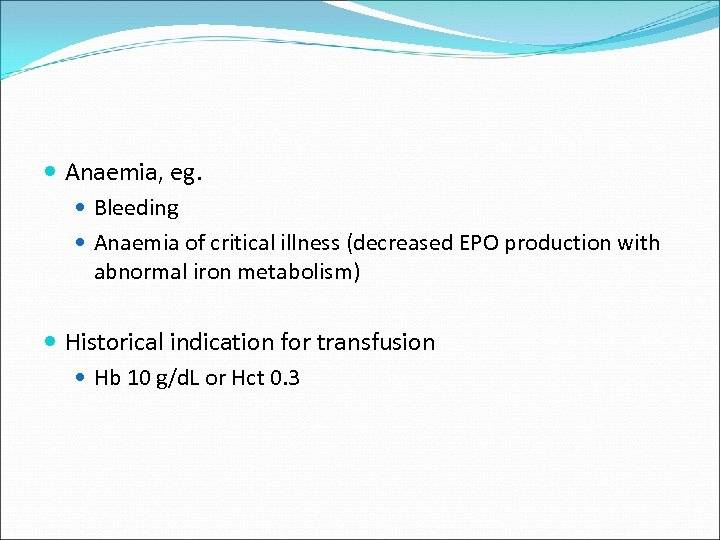
Anaemia, eg. Bleeding Anaemia of critical illness (decreased EPO production with abnormal iron metabolism) Historical indication for transfusion Hb 10 g/d. L or Hct 0. 3

Infection risk (eg. HIV, hepatitis, v. CJD) Adverse reactions Limited supply


• Compensation for anaemia: Acute isovolaemic anaemia to Hb 5 g/d. L in volunteers and patients produced no evidence of inadequate oxygenation (Weiskopf, 1998)
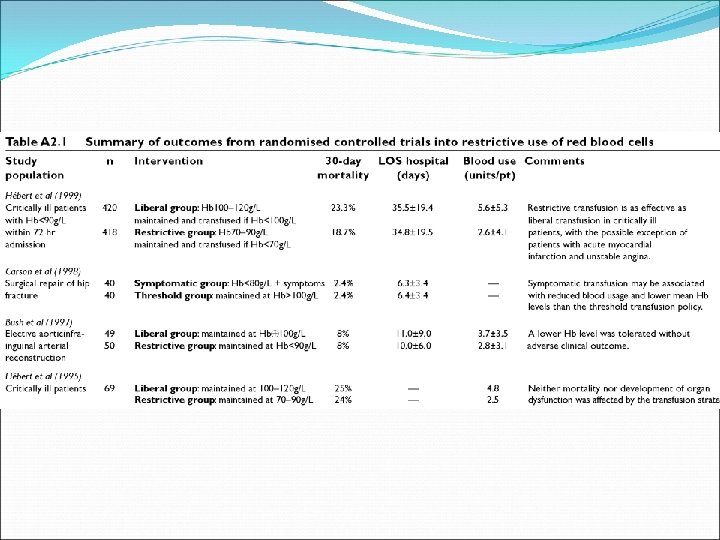

Crit Care Med, 2004, Vol 32: 1
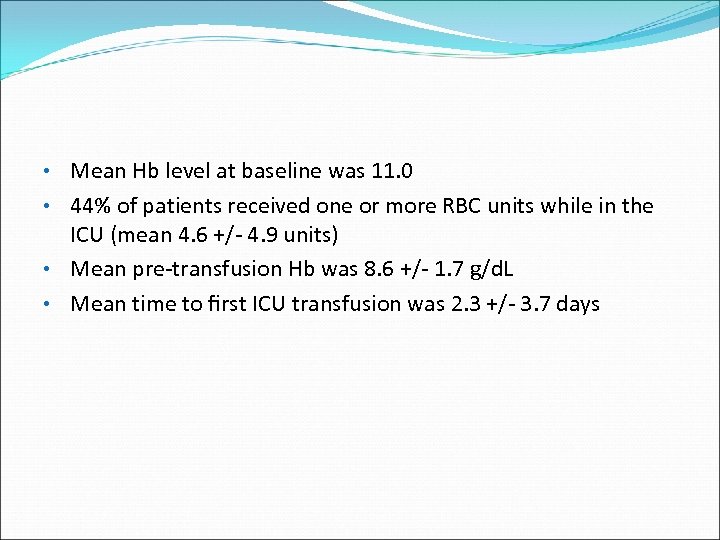
• Mean Hb level at baseline was 11. 0 • 44% of patients received one or more RBC units while in the ICU (mean 4. 6 +/- 4. 9 units) • Mean pre-transfusion Hb was 8. 6 +/- 1. 7 g/d. L • Mean time to first ICU transfusion was 2. 3 +/- 3. 7 days
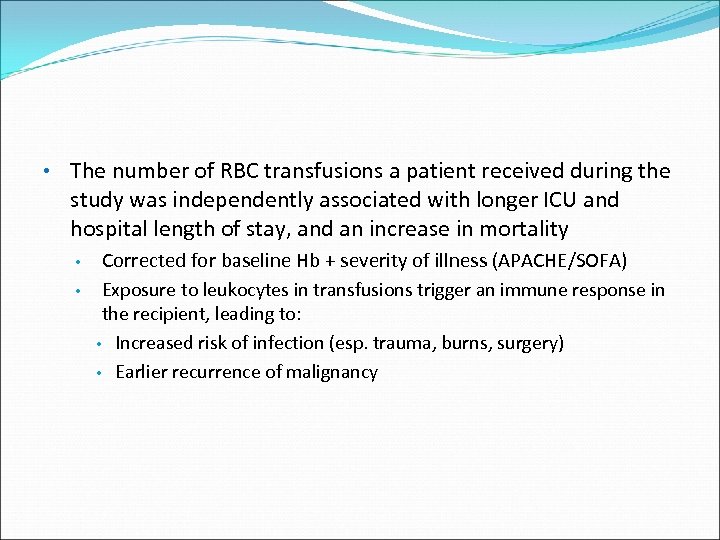
• The number of RBC transfusions a patient received during the study was independently associated with longer ICU and hospital length of stay, and an increase in mortality • • Corrected for baseline Hb + severity of illness (APACHE/SOFA) Exposure to leukocytes in transfusions trigger an immune response in the recipient, leading to: • Increased risk of infection (esp. trauma, burns, surgery) • Earlier recurrence of malignancy
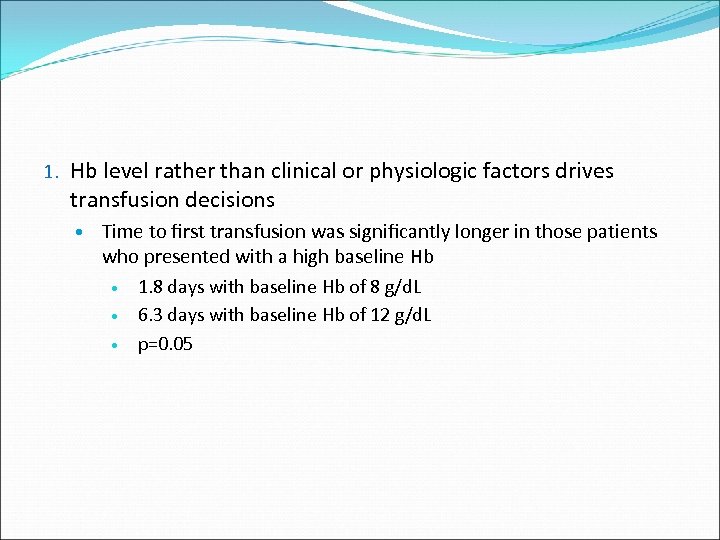
1. Hb level rather than clinical or physiologic factors drives transfusion decisions Time to first transfusion was significantly longer in those patients who presented with a high baseline Hb 1. 8 days with baseline Hb of 8 g/d. L 6. 3 days with baseline Hb of 12 g/d. L p=0. 05
JAMA, 2002, Vol 288: 12

Mean Hb (on admission) 11. 3 g/d. L • 29% had Hb < 10 g/d. L Transfusion rate: 37%
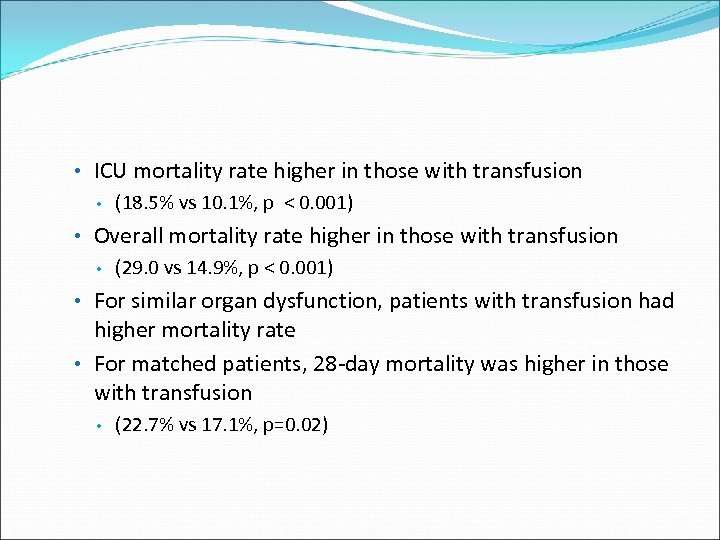
• ICU mortality rate higher in those with transfusion • (18. 5% vs 10. 1%, p < 0. 001) • Overall mortality rate higher in those with transfusion • (29. 0 vs 14. 9%, p < 0. 001) • For similar organ dysfunction, patients with transfusion had higher mortality rate • For matched patients, 28 -day mortality was higher in those with transfusion • (22. 7% vs 17. 1%, p=0. 02)

• British Committee for Standards in Haematology (BCSH), Guidelines for the clinical use of red cell transfusions • National Health and Medical Research Council (NHMRC), Clinical practice guidelines on the use of blood components • American Society of Anesthesiologists, Practice Guidelines for Blood component therapy
• Consideration of the patient’s condition is essential cause of anaemia, severity, chronicity patient’s ability to compensate (age > 65, cardiovascular / pulmonary disease) likelihood of further blood loss need for provision of reserve before onset of tissue hypoxia
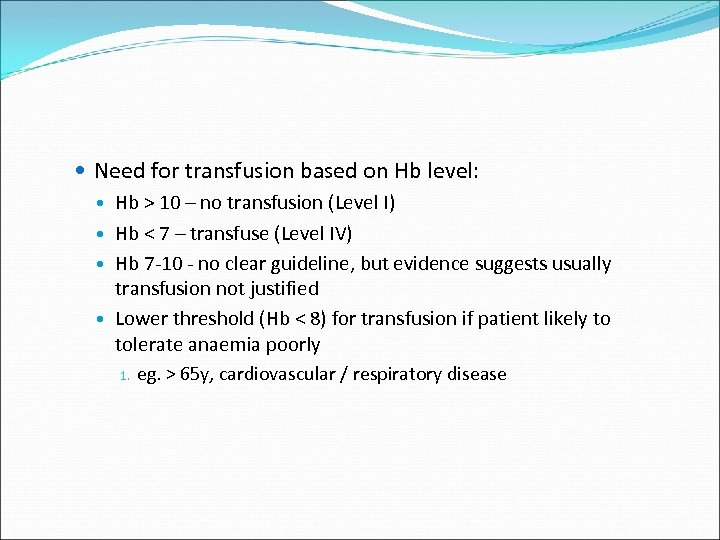
Need for transfusion based on Hb level: Hb > 10 – no transfusion (Level I) Hb < 7 – transfuse (Level IV) Hb 7 -10 - no clear guideline, but evidence suggests usually transfusion not justified Lower threshold (Hb < 8) for transfusion if patient likely to tolerate anaemia poorly 1. eg. > 65 y, cardiovascular / respiratory disease
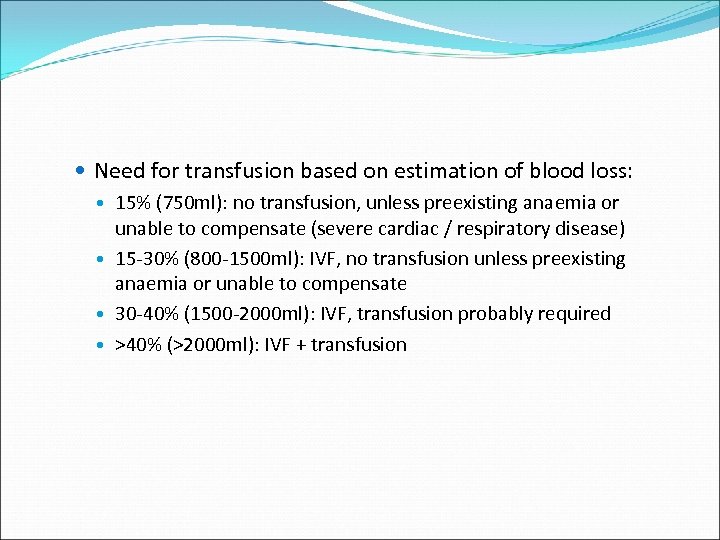
Need for transfusion based on estimation of blood loss: 15% (750 ml): no transfusion, unless preexisting anaemia or unable to compensate (severe cardiac / respiratory disease) 15 -30% (800 -1500 ml): IVF, no transfusion unless preexisting anaemia or unable to compensate 30 -40% (1500 -2000 ml): IVF, transfusion probably required >40% (>2000 ml): IVF + transfusion
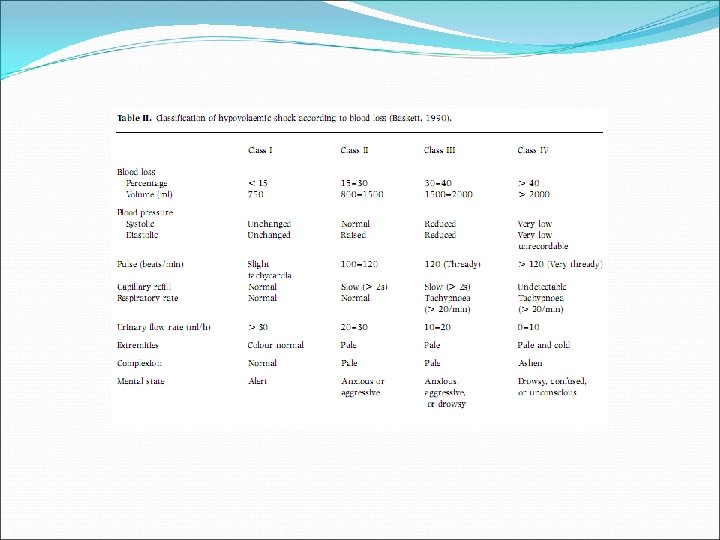

• Similar guidelines for peri-operative patients, but importance of avoiding transfusions Eg. Fe for Fe deficiency, stop anti-platelet drugs (Aspirin), reverse anticoagulation (Warfarin) • Importance of volume replacement, maintenance of BP and cardiac output
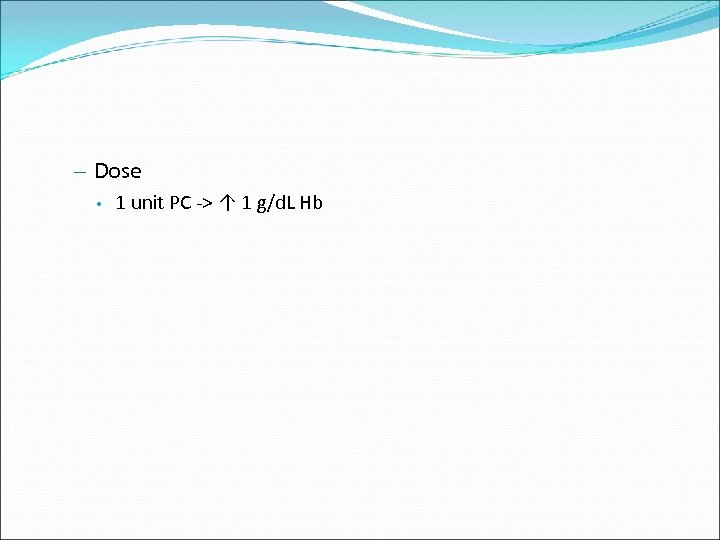
– Dose • 1 unit PC -> ↑ 1 g/d. L Hb

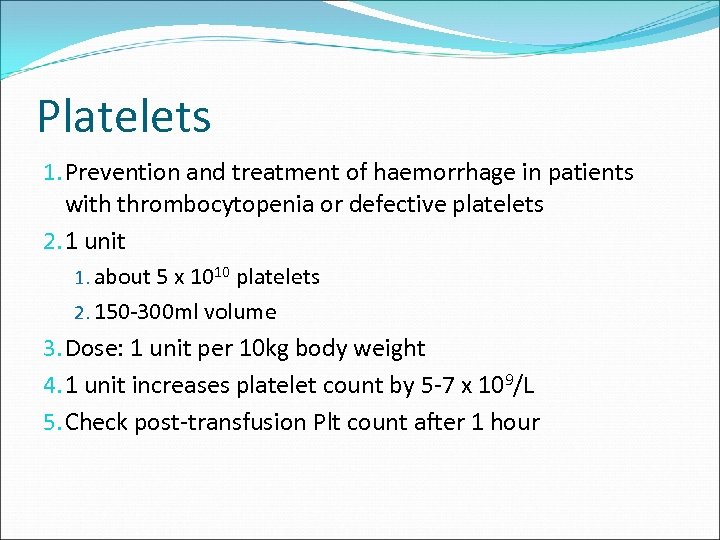
Platelets 1. Prevention and treatment of haemorrhage in patients with thrombocytopenia or defective platelets 2. 1 unit 1. about 5 x 1010 platelets 2. 150 -300 ml volume 3. Dose: 1 unit per 10 kg body weight 4. 1 unit increases platelet count by 5 -7 x 109/L 5. Check post-transfusion Plt count after 1 hour
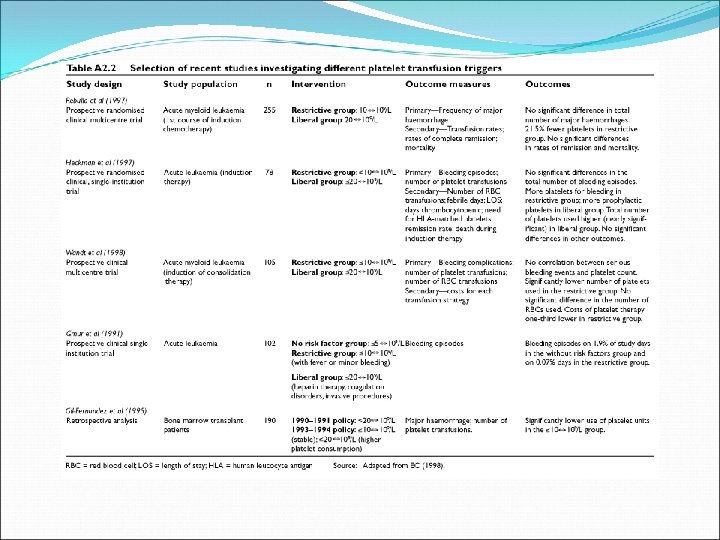

Transfusion, 2006, Vol 46
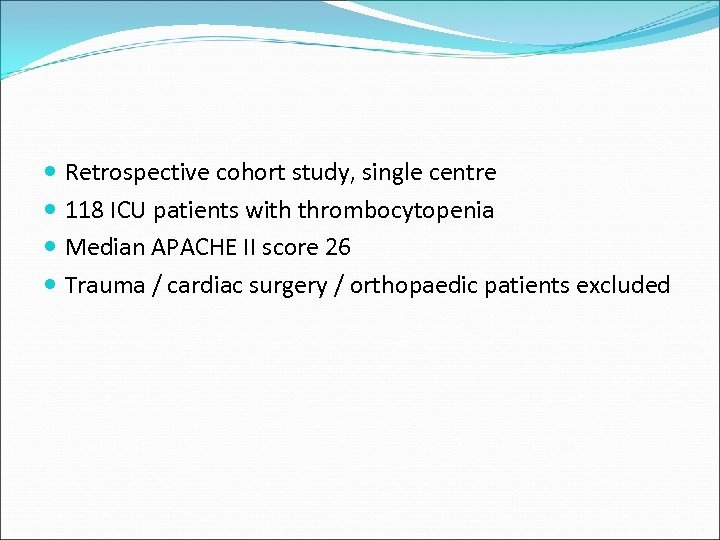
Retrospective cohort study, single centre 118 ICU patients with thrombocytopenia Median APACHE II score 26 Trauma / cardiac surgery / orthopaedic patients excluded
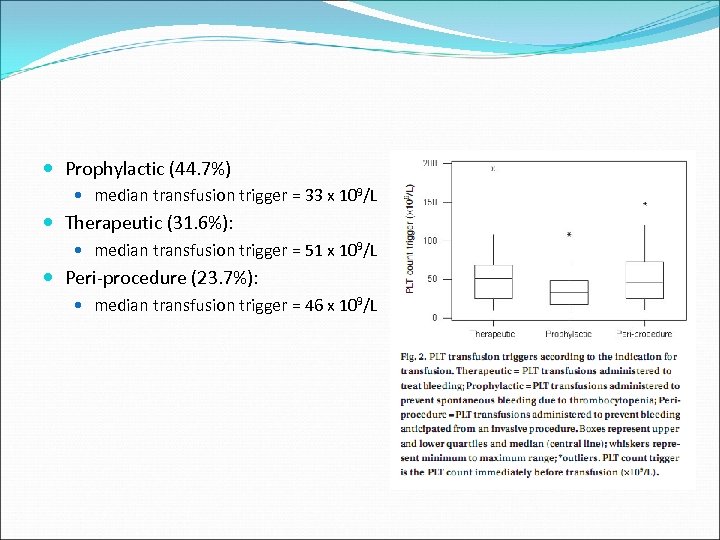
Prophylactic (44. 7%) median transfusion trigger = 33 x 109/L Therapeutic (31. 6%): median transfusion trigger = 51 x 109/L Peri-procedure (23. 7%): median transfusion trigger = 46 x 109/L
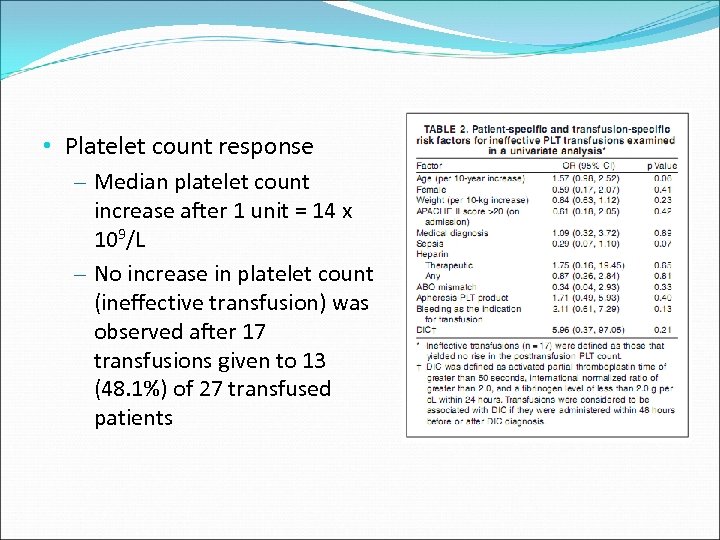
• Platelet count response – Median platelet count increase after 1 unit = 14 x 109/L – No increase in platelet count (ineffective transfusion) was observed after 17 transfusions given to 13 (48. 1%) of 27 transfused patients

BCSH 03 NHMRC 01
• Transfuse if: – < 10 x 109/L, w/o risk factors (bleeding, fever, antibiotics) (Level II) – < 20 x 109/L, w/ risk factors (Level II) – ≤ 50 x 109/L, undergoing surgery / invasive procedure, massive haemorrhage, massive transfusion (1. 5 -2. 0 blood volumes, or > 10 units RBC within 24 hours) (Level IV) – ≤ 100 x 109/L, undergoing neurosurgery / eye surgery, diffuse microvascular bleeding (Level IV)
No benefit from platelet transfusion, may even be harmful (Level IV) Immune-mediated platelet destruction TTP HUS Post-cardiac bypass surgery without bleeding Drug induced thrombocytopenia without bleeding


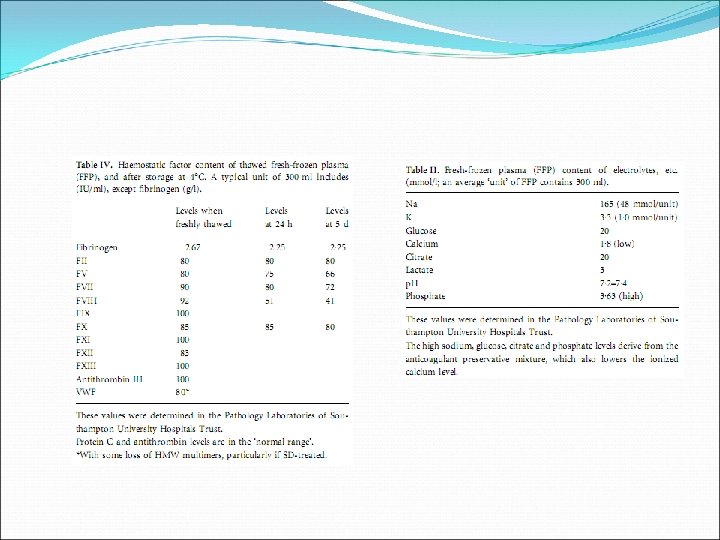
Fresh Frozen Plasma • Treatment of excessive bleeding or prevention of bleeding in patients with abnormal coagulation • Dose: 10 -20 ml FFP/kg body weight • 1 dose expected to increase level of coagulation factors by 15 -20% immediately after transfusion (ie. 1 ml FFP/kg body weight raises coagulation factors by about 1%) • Bolus • Volume: 180 -400 ml/unit

Indications are very limited

Journal of Trauma, 2008; Vol 65(2)

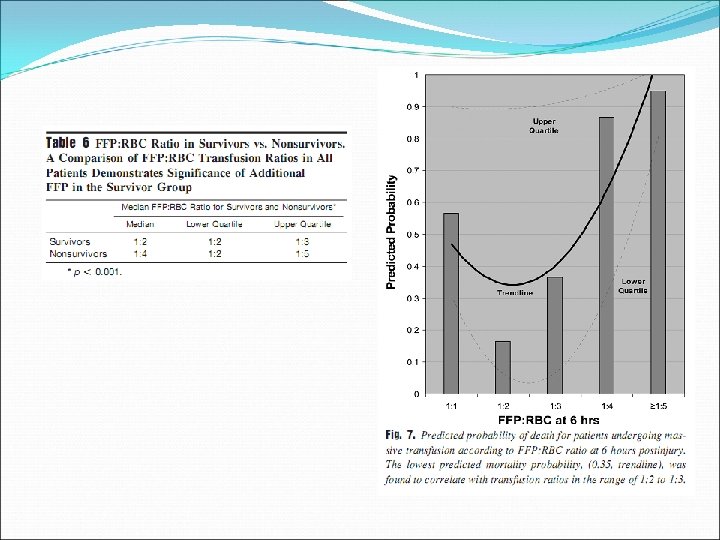
Retrospective review, single centre (Denver Health Medical Center), 2001 -2006 Massive transfusion (> 10 units RBC in initial 6 hours)

Journal of Trauma, 2007; 63: 805 -813

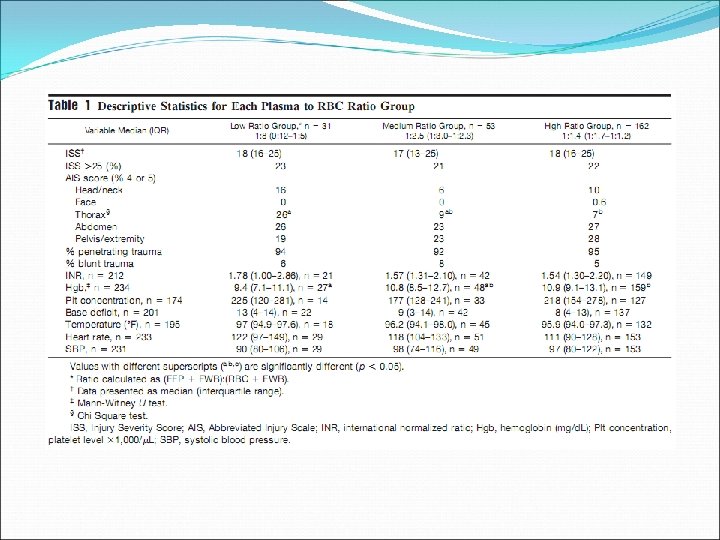
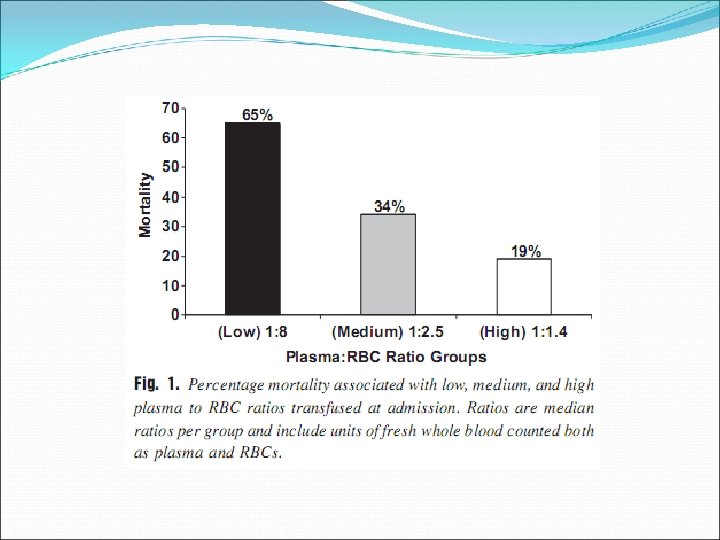
Retrospective chart review, 246 patients, single centre (one US Army combat support hospital in Iraq), 20032005
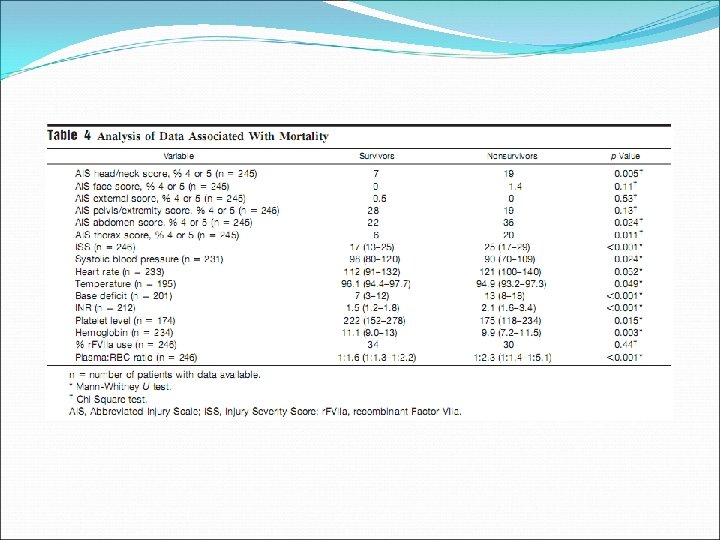
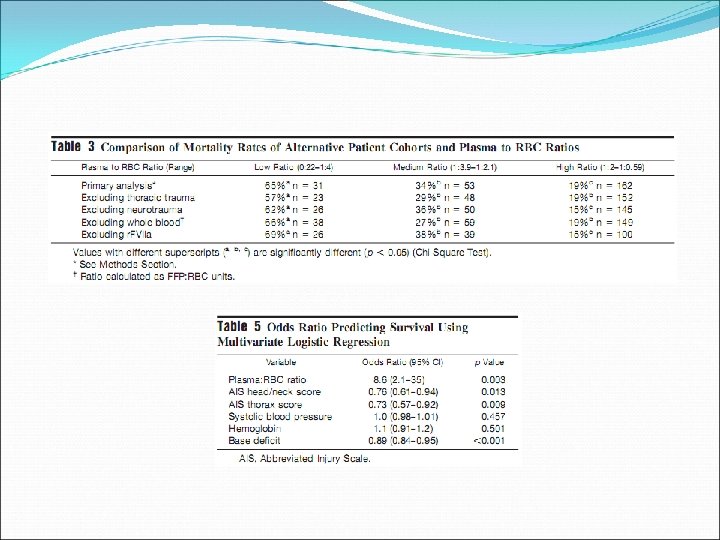

Transfusion, 2006; 46: 1279 -1285

Prospective audit of all FFP transfusions at the Massachusetts General Hospital, 2004 -2005 121 patients Excluded patients on plasma exchange
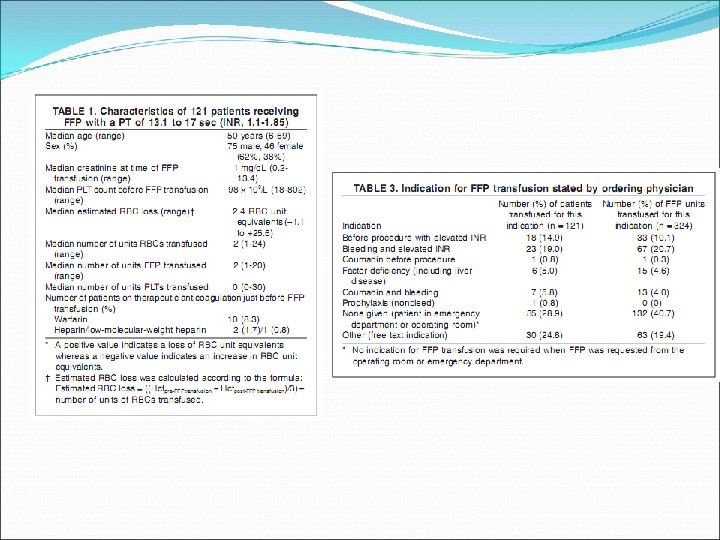
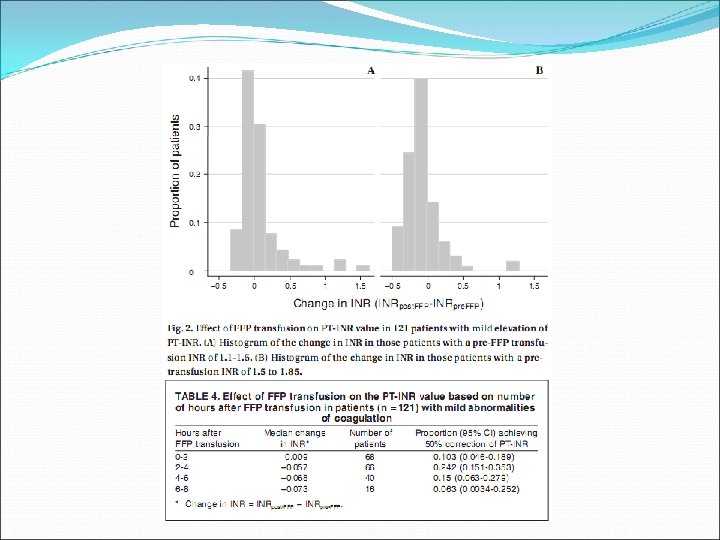
FFP for mild abnormalities of coagulation values results in partial normalisation of PT in a minority of patients, and fails to correct the PT in 99% of patients

NHMRC 01 BCSH 04
Well documented indications limited to: Haemorrhage If massive transfusion required, consider a high FFP: RBC ratio (1: 1 – 1: 1. 4) Patients with known factor deficiencies where specific factor concentrates are not available Factor V Eg. Bleeding, preoperative
• Specific indications (Level IV) – Reversal of Warfarin effect IN THE PRESENCE of potential life- – – threatening bleeding (in conjunction with Vit K +/- Factor IX concentrate) Acute DIC (deficiency of V, VIII, fibrinogen, fibronectin and platelets due to activation of coagulation and fibrinolytic systems) Bleeding and abnormal coagulation parameters (PT/APTT > 1. 5 x mid-normal range) following massive transfusion, liver disease, or cardiac bypass surgery TTP (plasmapheresis) Hereditary angioedema (with C 1 esterase inhibitor deficiency)
• No documented benefit from use of FFP (Level IV) – Following uncomplicated cardiac bypass surgery – Plasma exchange procedures (except plasmapheresis for TTP) • Usually no haemorrhage or infection from reduction of coagulation factors / Ig / complement / fibronectin when plasmafree replacement fluids are used – Reversal of prolonged INR in the absence of bleeding – Volume replacement in acute haemorrhage – Treatment of immunodeficiency states
Alternatives to FFP r. FVIIa (bypasses inhibitors to VIII, IX, v. WF) Massive haemorrhage Prothrombin complex concentrate (PCC) Warfarin reversal, liver disease, haemorrhagic disease of the newborn Specific factor concentrates Recombinant activated Protein C Severe sepsis
Cryoprecipitate • Frozen plasma product thawed at 4°C -> cryoglobulin fraction -> VIII, v. WF, XIII, fibronectin, fibrinogen • Volume: 20 -40 ml, each with at least 150 -250 mg fibrinogen and 60 -100 IU/ml of VIII • Dose: 5 -10 cryoprecipitates (1 -2 g of fibrinogen in a 70 kg patient) • Cryoprecipitate cannot as yet be virally inactivated and therefore should no longer be used for the treatment of bleeding unless other measures have clearly failed (try FFP)
NHMRC 01 May be justified in fibrinogen deficiency (Fibrinogen < 1 g/L) WITH bleeding, invasive procedure, trauma, DIC (Level IV) NOT appropriate in haemophilia, v. WD, XIII / fibronectin deficiencies – unless alternative therapies are not available (level IV)
Further processing 1. Special transfusion requirements 1. Irradiated (RBC / Plt) 1. 2. 3. 4. 5. 6. Severe congenital cellular-mediated immunodeficiencies BMT / stem cell recipients after conditioning Prior to or during autologous bone marrow harvest Transfusion from first-degree relatives Transfusion of HLA-selected platelets Optional 1. Hodgkin’s disease 2. Lymphoma patients receiving purine analogues
CMV –ve (RBC / Plt) CMV –ve patients BMT / organ recipients of CMV –ve donors Leukocyte-depleted via centrifugation or filter (RBC / Plt) 2 or more Febrile Non-haemolytic transfusion reactions Decrease risk of transfusion-related CMV transmission Prevent platelet alloimmunisation in certain patients

Blood warming Cold haemagglutinin disease Large volume of blood infused at > 50 ml/kg/hr Rapid transfusions through central venous catheters
Complications and its management Acute transfusion reactions Management STOP transfusion Monitor patient’s vital signs closely Check labels on blood bag and patient’s ID Maintain IV NS infusion Determine the type of reaction
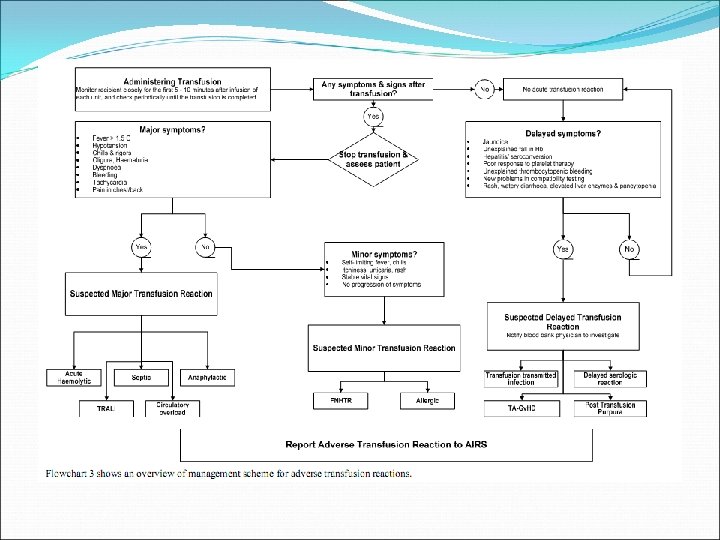
• Acute haemolytic transfusion reaction (1 in 250, 000 – 1, 000) – Caused by ABO incompatibility – S/S within 5 -15 mins • SOB • Chest / back pain • Fever, chills, rigors • Tachycardia • Hypotension • Oliguria • Restlessness • Haemoglobinuria • Generalised bleeding
Management Change IV drip set Give NS Maintain U/O > 100 ml/hr Send used blood packs, administration set and sample of patient’s blood to Blood Bank Blood C/ST
• Febrile, non-haemolytic transfusion reactions (1 in 100) – Caused by reaction of recipient’s antibodies to donor white cells – S/S within 30 mins – 2 hours • Fever, chills, rigors – Management • Antipyretic • Restart transfusion at a slower rate • Consider leucocyte-poor products for patients with recurrent severe reactions
• Allergic reactions (1 in 100 – 300) – Caused by recipient’s reaction to donor plasma proteins – S/S within 1 hr • • Urticaria Bronchospasm – Management • Antihistamine • Resume transfusion if no progression of S/S after 30 mins • Discontinue if no response to antihistamine or bronchospasm
Anaphylactic reactions Caused by interaction between recipient’s pre-existing antibody and protein / allergen in donor’s blood S/S early (NB. No fever) Chest tightness Hypotension Bronchospasm

Management IV Piriton 10 -20 mg IM Adrenaline 0. 5 -1 mg Ventolin
• Acute bacteraemia (RBC: 1 in 500, 000 / Plt: 1 in 10, 000) – Caused by bacterial contamination of blood component – S/S immediately (difficult to differentiate from Acute Haemolytic Transfusion Reaction) • • High fever, chills Tachycardia Hypotension Vomiting

Management Blood C/ST Send used blood packs to Blood Bank Start broad spectrum antibiotics

• Volume overload – Higher risk in elderly (poor cardiopulmonary reserve) and chronic renal failure – S/S within 1 day • • SOB Cough Chest creps Oedema – Management • O 2 • Diuretics
• Transfusion-related acute lung injury (TRALI) (1 in 50, 000 – 200, 000) – Caused by reaction between patient’s white cells and donor’s white cell antibodies causing leuko-agglutination in the pulmonary microcirculation and pulmonary damage – S/S from within 2 hours during transfusion up to 4 hours after transfusion • • • SOB Hypotension Fever Chest creps Cyanosis

– Management • Respiratory support – FFP + TRALI • TRALI is significantly, but not solely, associated with presence of leukocyte alloantibodies in donor plasma. Such antibodies are found most frequently in women after pregnancy, and are not present in plasma from men unless they have been transfused (where such antibodies seem less active than those found in women who have been pregnant). Restricting the source of plasma for FFP production to men seems likely to reduce the incidence of TRALI.
Fever > 1. 5°C Bronchospasm Angioedema SOB Hypotension Tachycardia Chills / Rigors Oliguria Haematuria Chest / back pain Bleeding

Take home message… Drug Art > solid evidence Look out for complications

Why transfuse? Why not transfuse? RBC (7, 8, 10) Platelet (10, 20, 50, 100) FFP (1. 5, 1: 1)

References American Society of Anesthesiologists, Practice Guidelines for Blood Component Therapy: A Report by the American Society of Anesthesiologists Task Force on Blood Component Therapy, Mar 1996; 84(3): 732 -747 Transfusion guideline for HA hospitals, Version 1. 5, Jun 2008 National Health and Medical Research Council / Australasian society of blood transfusion, Clinical practice guidelines on the use of blood components, Sep 2001 British Committee for Standards in Haematology (Blood transfusion task force), Guidelines for the clinical use of red cell transfusions, British Journal of Haematology, 2001; 113: 24 -31 British Committee for Standards in Haematology (Blood transfusion task force), Guidelines for the use of platelet transfusions, British Journal of Haematology, 2003; 122: 10 -23 British Committee for Standards in Haematology (Blood transfusion task force), Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant, British Journal of Haematology, 2004; 126: 11 -28 Corwin HL et al, The CRIT study: anaemia and blood transfusion in the critically ill-current clinical practice in the United States, Crit Care Med, 2004; 32: 1

Vincent JL et al, Anemia and blood transfusion in critically ill patients, JAMA, 2002; 288: 12 Arnold DM et al, Utilization of platelet transfusions in the intensive care unit: indications, transfusion triggers, and platelet count responses, Transfusion, 2006; 46: 1286 -1291 Kashuk JL et al, Postinjury life threatening coagulopathy: is 1: 1 fresh frozen plasma: packed red blood cells the answer? , J Trauma, Aug 2008; 65(2): 261 -270 Borgman MA et al, The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital, Trauma, 2007; 63: 805 -813 Abdel-Wahab OI et al, Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities, Transfusion, 2006; 46: 1279 -1285
d82abbaf526bdedb6c98e9a810913f7b.ppt