17e03983ecf7edd6e68eb62ddbd09e38.ppt
- Количество слайдов: 53

Blood Culture in Sepsis Prof. MD. Ahmet Başustaoğlu Girne American University Faculty of Health Sciences Kyrenia/Northern Cyprus

Anand Kumar, et. All. , Chest 2009; 136; 1237 -1248;

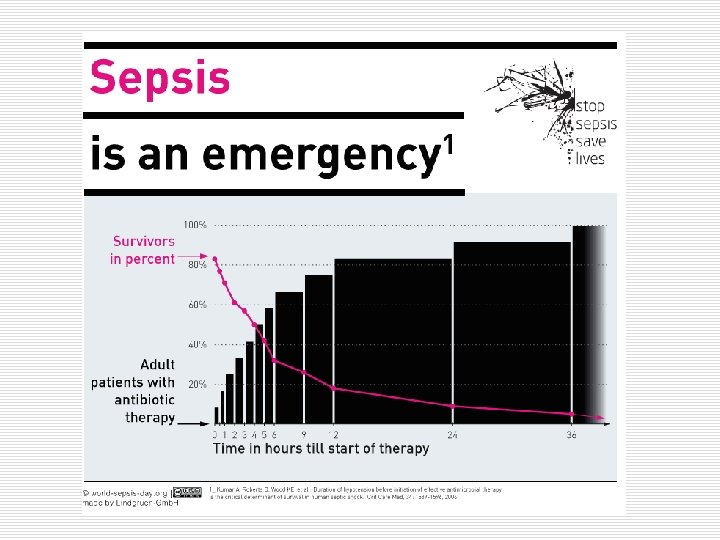
Each hour of delay in initiation of effective antimicrobials can increase mortality rates by 7. 6%.
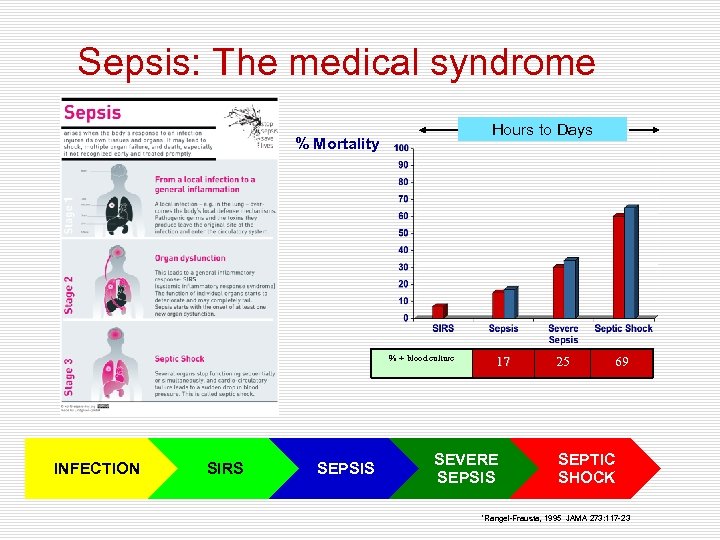
Sepsis: The medical syndrome Hours to Days % Mortality % + blood culture INFECTION SIRS SEPSIS 17 SEVERE SEPSIS 25 69 SEPTIC SHOCK *Rangel-Frausta, 1995 JAMA 273: 117 -23

Standards and Guidelines ü WHO guidelines on drawing blood: best practices in phlebotomy ü Blood Culture Ordering and Collection Guidelines Vanderbilt University Medical Center ü Johns Hopkins Medical Microbiology Specimen Collection Guidelines ü Principles and Procedures for Blood Cultures; Approved Guideline M 47 -A CLSI (Clinical Laboratories Standards Institude) ü Cumitech 1 C; Blood Cultures, ASM (American Society for Microbiology)

Key Points from CLSI 47 -A and Cumitech 1 C ü Timing of drawing blood cultures ü Skin disinfection ü Volume of blood to inoculate ü Number of blood culture sets ü How long to incubate bottles?
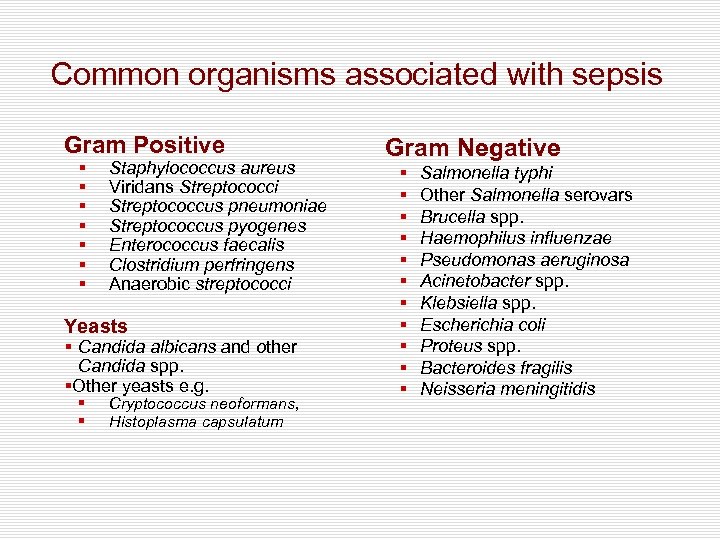
Common organisms associated with sepsis Gram Positive § § § § Staphylococcus aureus Viridans Streptococci Streptococcus pneumoniae Streptococcus pyogenes Enterococcus faecalis Clostridium perfringens Anaerobic streptococci Yeasts § Candida albicans and other Candida spp. §Other yeasts e. g. § § Cryptococcus neoformans, Histoplasma capsulatum Gram Negative § § § Salmonella typhi Other Salmonella serovars Brucella spp. Haemophilus influenzae Pseudomonas aeruginosa Acinetobacter spp. Klebsiella spp. Escherichia coli Proteus spp. Bacteroides fragilis Neisseria meningitidis
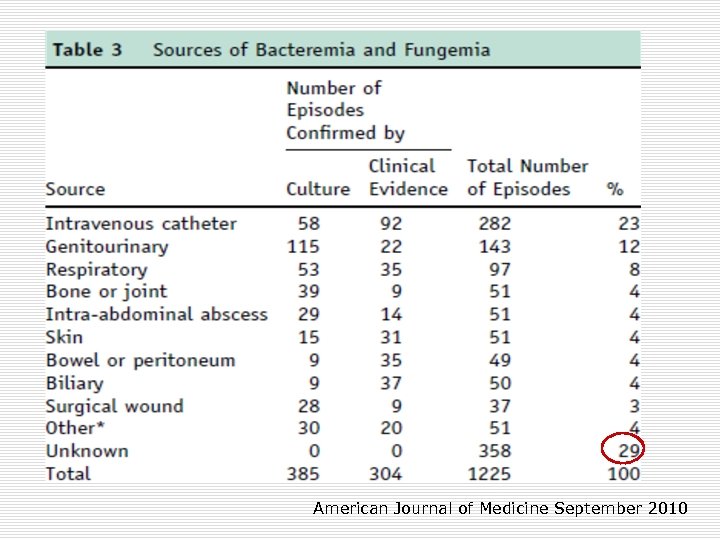
American Journal of Medicine September 2010
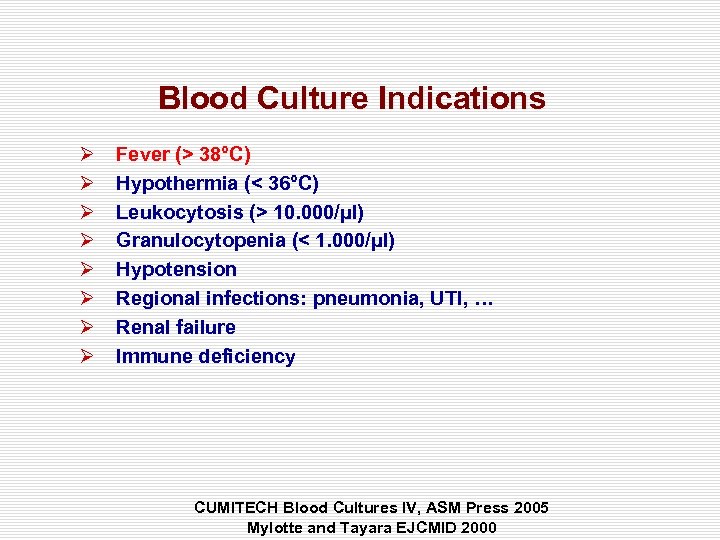
Blood Culture Indications Ø Ø Ø Ø Fever (> 38°C) Hypothermia (< 36°C) Leukocytosis (> 10. 000/µl) Granulocytopenia (< 1. 000/µl) Hypotension Regional infections: pneumonia, UTI, … Renal failure Immune deficiency CUMITECH Blood Cultures IV, ASM Press 2005 Mylotte and Tayara EJCMID 2000
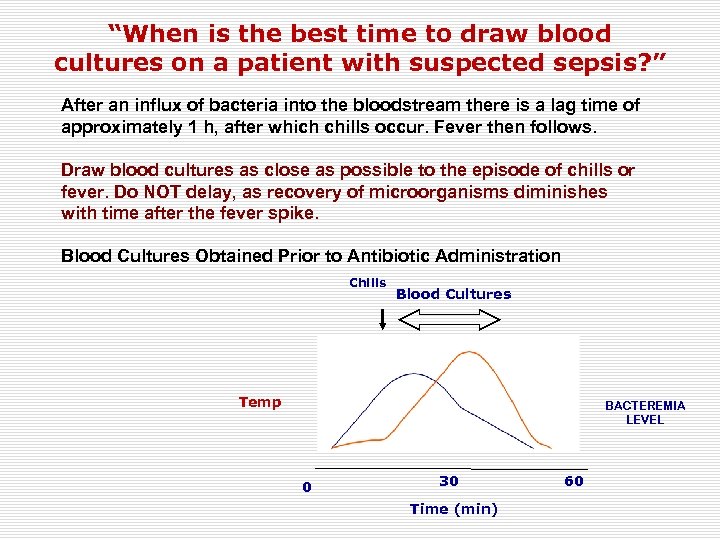
“When is the best time to draw blood cultures on a patient with suspected sepsis? ” After an influx of bacteria into the bloodstream there is a lag time of approximately 1 h, after which chills occur. Fever then follows. Draw blood cultures as close as possible to the episode of chills or fever. Do NOT delay, as recovery of microorganisms diminishes with time after the fever spike. Blood Cultures Obtained Prior to Antibiotic Administration Chills Blood Cultures Temp BACTEREMIA LEVEL 0 30 Time (min) 60
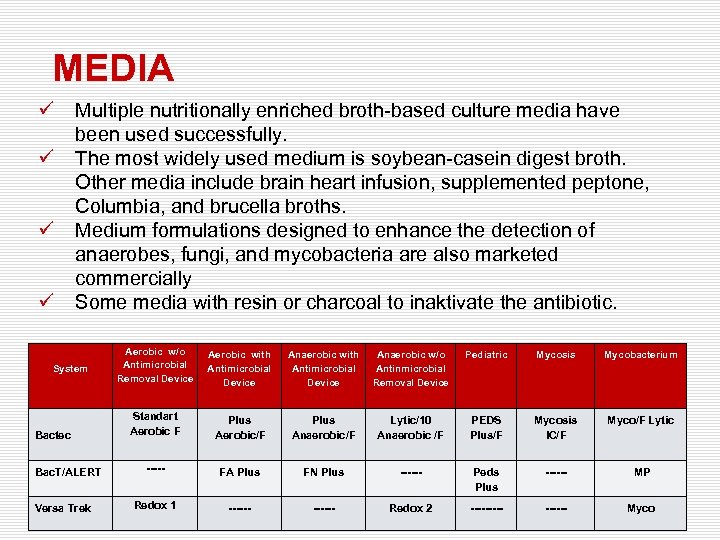
MEDIA ü ü Multiple nutritionally enriched broth-based culture media have been used successfully. The most widely used medium is soybean-casein digest broth. Other media include brain heart infusion, supplemented peptone, Columbia, and brucella broths. Medium formulations designed to enhance the detection of anaerobes, fungi, and mycobacteria are also marketed commercially Some media with resin or charcoal to inaktivate the antibiotic. System Bactec Bac. T/ALERT Versa Trek Aerobic w/o Antimicrobial Removal Device Aerobic with Antimicrobial Device Anaerobic w/o Antinmicrobial Removal Device Pediatric Mycosis Mycobacterium Standart Aerobic F Plus Aerobic/F Plus Anaerobic/F Lytic/10 Anaerobic /F PEDS Plus/F Mycosis IC/F Myco/F Lytic ----- FA Plus FN Plus ------ Peds Plus ------ MP Redox 1 ------ Redox 2 ------ Myco

Methods of Obtaining Blood for Culture ü Venipuncture ü Intravascular devices ü ü ü Venipuncture remains the technique of choice for obtaining blood for culture Blood cultures obtained from intravascular access devices are associated with greater contamination rates than obtained by venipuncture through a properly prepared skin site If needed to be obtained from intravenous lines and similar access devices, should be paired with another culture of blood obtained by venipuncture to assist in interpretation in the event of a positive result.

Skin Disinfection ü A positive blood culture represents ü Cause of infection ü Contamination ü Failure to adequately cleaning of the skin increases the risk that microbial flora of the skin, will contaminate the blood culture. ü Health care workers who obtain blood cultures are often in a hurry, do not understand the importance of antiseptic preparation contact time. Cumitech; Blood Cultures, ASM
Skin Disinfection ü ü Povidone-iodine 2% Tincture of iodine, 0, 2% Chloride peroxide, 5% Chlorhexidine gluconate ü Tincture of iodine, chloride peroxide, and chlorhexidine gluconate are superior to povidone-iodine preparations Tincture of iodine and chlorhexidine gluconate are probably equivalent Chlorhexidine gluconate is recommended for older infants, children, and adults 30 seconds to 1. 5 - 2 min of contact time Chlorhexidine preparations have the advantage of being both colorless and less irritating to skin. ü ü

Blood-to-Broth Ratio ü Human blood contains substances capable of inhibiting microbial growth. ü Diluting blood in culture broth reduces the concentrations of these inhibitory substances and concentrations of any antimicrobial agents that may be administered to patients. ü Studies that have addressed the blood-to-broth ratio have recommended 5 - to 10 -fold dilution ü Dilutions less than 1: 5 may result in reduced yield
Methods of Obtaining Blood for Culture ü ü ü “two-needle” method “single-needle” method “transfer set” or “a double-ended needle” method ü Higher contamination rates with the single-needle method (3. 7%) compared with the two-needle method (2. 0%), ü the higher rates are tolerated in order to reduce the risk of occupational needle-stick injuries
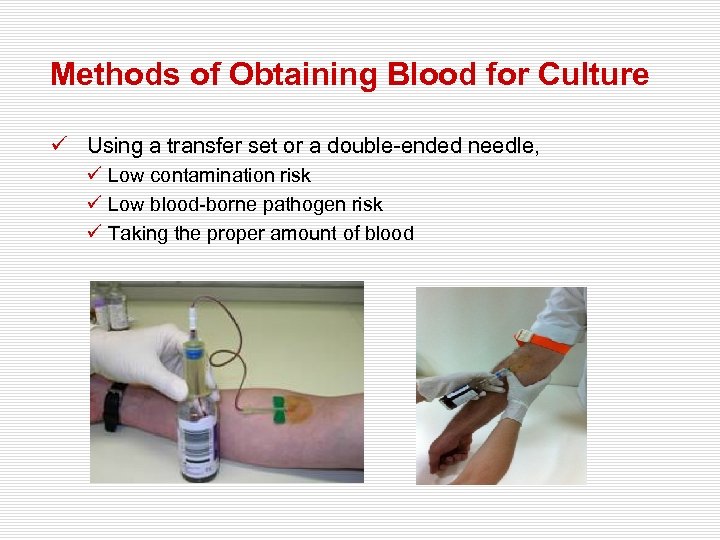
Methods of Obtaining Blood for Culture ü Using a transfer set or a double-ended needle, ü Low contamination risk ü Low blood-borne pathogen risk ü Taking the proper amount of blood

Blood Culture ü ü At least 2 blood culture sets should be drawn in critically ill patients with suspected sepsis Catheter-drawn blood cultures ü Catheter-drawn blood cultures are equally likely to be truly positive (associated with sepsis), but more likely to be colonized (J Clin Microbiol 38: 3393, 2001. ) ü Both drawn through vein PPV of 98% ü One drawn through catheter and other though vein PPV of 96% ü Both drawn from catheter PPV 0 f 50% Study of positive coagulase negative Staphylococcus cultures and sepsis (Clin Infect Dis. 39: 333, 2004. )

Use of Anearobic blood culture bottles; ü When one aerobic and one anaerobic bottle was used, a significantly increased yield of some agents was demonstrated; ü Streptococci, Staphylococci, Enterococci ü Enterobacteriaceae, Anaerobes ü In a study by Akyar et al. (Acıbadem University) out of all the positive cultures; ü 172 (%24. 3) was only in anerobic bottles, ü 310 (%43. 7) was only in aerobic bottles, ü 227 (%32) was both aerobic and anerobic bottles
“The patient is already on antibiotics, would resin-based media help to recover the organism? ” ü Approximately 28 -62% of patients are already on antibiotics when the first blood culture is obtained!!! ü In patients overall, resin media are superior to media without resins ü The difference is greatest in those patients already on antibiotics…and too many patients are on antibiotics when blood cultures are obtained! ü Therefore, best practice would be to use resin media routinely!
Checklist for Blood Cultures before Leaving the Patient’s Bedside As with all specimens submitted to the laboratory, blood culture vials should be labeled with the appropriate identification information showing ü date and time of collection, ü the identification of the patient (barcode), ü All bottles or tubes and requisitions are labeled with collector’s employee name, number, or code. ü The specific site of collection (which vein, which arm, etc. ) is recorded for each set of bottles or tubes obtained ü Examples of such a code are as follows: ü ü ü AL, arterial line; DIALL, dialysis catheter left; and MEDL, mediport left

Transport to the Laboratory and Handling and Moving within the Laboratory ü Timing. Blood should be transported as quickly as possible to the laboratory, preferably within 2 h, and should be placed into the incubator as quickly as possible. ü Temperature. Blood culture should never be refrigerated or allowed to cool. Keeping the bottles warm (no warmer than 37°C) is preferable to leaving them at room temperature. ü Safety for transport. Blood culture bottles should be carried in some sort of container that will protect them from dropping and from knocking against each other.

Rejection Criteria ü Staff who receive blood cultures in the laboratory should check the bottles and requisitions carefully to detect a number of problems or errors; ü Depending on laboratory and hospital guidelines, specimens with improper labeling may need to be rejected ü Bottles with no labels are usually rejected at all times ü Patient identification data on the culture bottles and requisition must match, and mismatched specimens may have to be rejected ü Whenever rejection is being considered, the physician and/or nursing unit must be notified immediately so they can recollect the samples or discuss the options with a laboratory director or supervisor.
Length of Incubation of Blood Cultures ü ü In routine circumstances, using automated continuous monitoring systems, blood cultures need not be incubated for longer than 5 days For laboratories using manual blood culture systems, 7 days should be sufficient. 99. 5% of nonendocarditis BSIs and 100% of endocarditis episodes were detected within 5 days of incubation Extended incubation periods recommended in the past for Brucella, Capnocytophaga, Campylobacter spp. and the HACEK group (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella spp. ) are not necessary for the new continious monitoring systems.
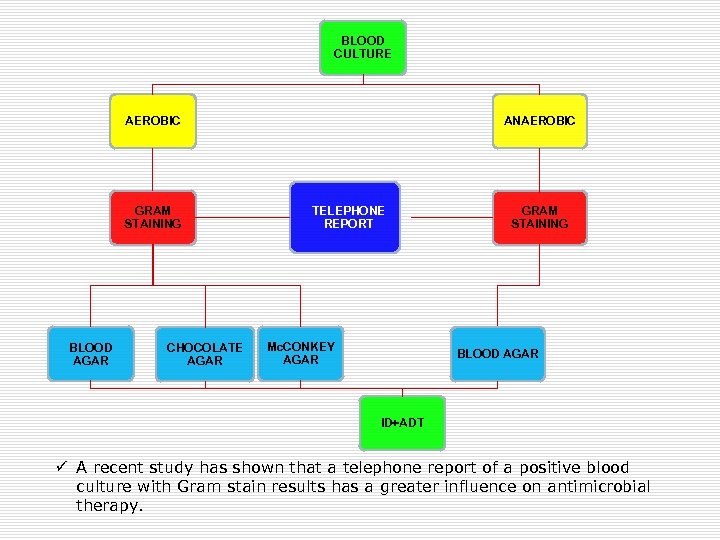
BLOOD CULTURE AEROBIC GRAM STAINING BLOOD AGAR CHOCOLATE AGAR ANAEROBIC TELEPHONE REPORT Mc. CONKEY AGAR GRAM STAINING BLOOD AGAR ID+ADT ü A recent study has shown that a telephone report of a positive blood culture with Gram stain results has a greater influence on antimicrobial therapy.

Interpretation of Culture Results ü Time to detection of positive cultures ü ü Spectrum of organisms recovered in blood cultures ü ü ü Most positive cultures detected in first 48 hours of incubation S. aureus detected in <24 hours; other staph >24 hours Culture routinely held 5 -7 days 10 -15% of cultures typically positive Most common isolates are: CNS, S. aureus, E. coli, Enterococcus, Klebsiella, S. pneumoniae Significant vs. contaminated culture ü ü ü Most isolates of S. aureus, S. pneumoniae, ß-hemolytic streptococci, Enterococcus, Enterobacteriaceae, Ps. aeruginosa, gram-neg. anaerobes, and yeasts are significant Most isolates of CNS, Corynebacterium, Proprionibacterium, and Bacillus are insignificant Significant isolates of CNS are typically associated with a contaminated line or other foreign body.
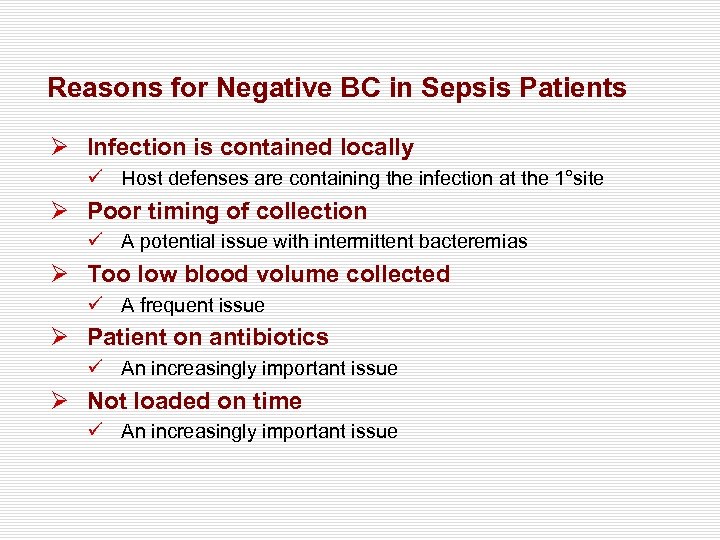
Reasons for Negative BC in Sepsis Patients Ø Infection is contained locally ü Host defenses are containing the infection at the 1°site Ø Poor timing of collection ü A potential issue with intermittent bacteremias Ø Too low blood volume collected ü A frequent issue Ø Patient on antibiotics ü An increasingly important issue Ø Not loaded on time ü An increasingly important issue
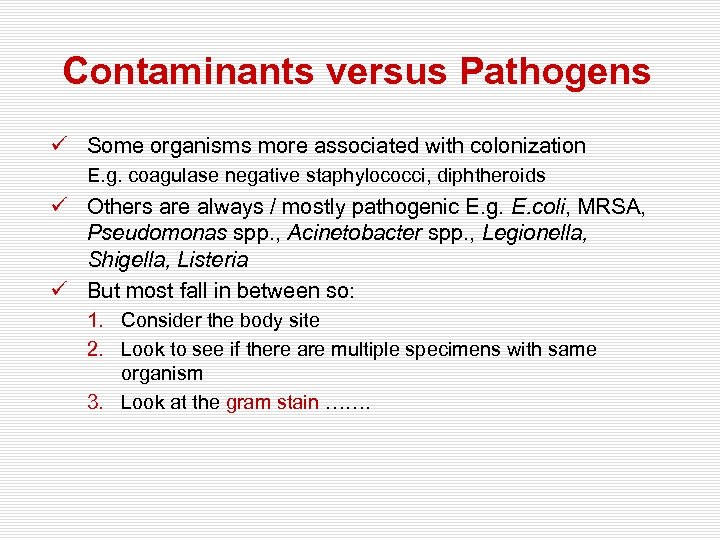
Contaminants versus Pathogens ü Some organisms more associated with colonization E. g. coagulase negative staphylococci, diphtheroids ü Others are always / mostly pathogenic E. g. E. coli, MRSA, Pseudomonas spp. , Acinetobacter spp. , Legionella, Shigella, Listeria ü But most fall in between so: 1. Consider the body site 2. Look to see if there are multiple specimens with same organism 3. Look at the gram stain …….

False-Positive Blood Cultures (“Contaminants”) Contamination True pathogen ü Contamination is “a single culture positive for coagulase-negative staphylococci or coryneform gram-positive rods or Micrococcus or Propionibacterium, or a single bottle with Bacillus species, not anthracis. ” ü These isolates are also perfectly capable of causing serious infections, catheterassociated septicemia. ü Differentiating “contaminants” from true pathogens is extremely difficult, especially because the same species can easily be found in either situation. ü Obviously, this can be accomplished most effectively by paying strict attention to the process of skin antisepsis, venipuncture, and specimen transfer to blood culture bottles.
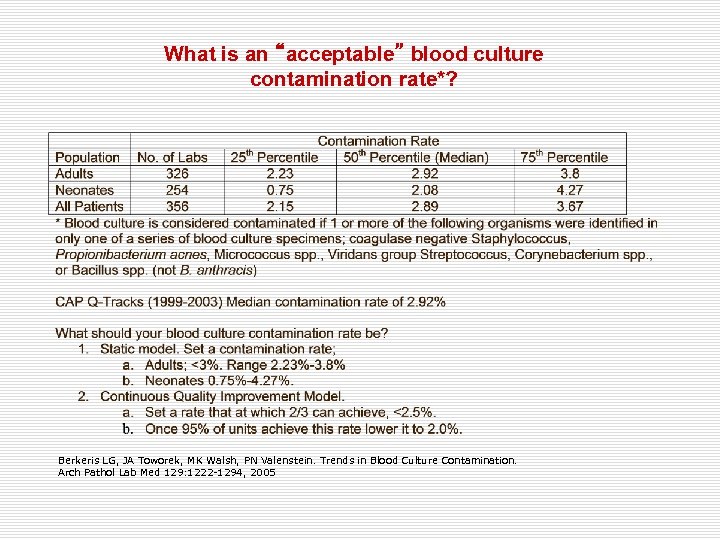
What is an “acceptable” blood culture contamination rate*? Berkeris LG, JA Toworek, MK Walsh, PN Valenstein. Trends in Blood Culture Contamination. Arch Pathol Lab Med 129: 1222 -1294, 2005
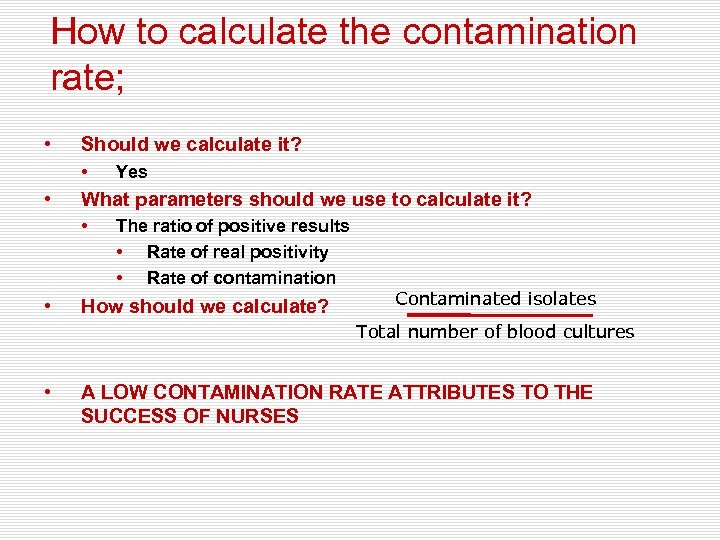
How to calculate the contamination rate; • Should we calculate it? • • What parameters should we use to calculate it? • • Yes The ratio of positive results • Rate of real positivity • Rate of contamination How should we calculate? Contaminated isolates Total number of blood cultures • A LOW CONTAMINATION RATE ATTRIBUTES TO THE SUCCESS OF NURSES

False-Positive Blood Cultures (“Contaminants”) ü ü ü If, a potential contaminant is recovered from one or both bottles and without a second blood culture for comparison, interpretation of the clinical relevance of that positive culture is impossible. Physicians may be forced to initiate treatment for practical and legal reasons if susceptibilities are reported by the laboratory. Therefore, the evaluation of an isolate with low virulence potential recovered from a single blood culture set (one or both bottles) should be limited to the extent to which phenotypically similar but medically important organisms can be safely excluded from the identification. Routine susceptibility testing is not necessary for suspected contaminants, but all isolates should be saved so that additional studies can be performed if an identical organism is recovered from a subsequent blood culture from the same patient. At that point, full identification of both isolates along with susceptibility testing should be initiated.

QUALITY CONTROL / QUALITY ASSURANCE ü ü ü CLIA and the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) both state that commercially produced blood culture media do not require additional in-laboratory QC testing beyond visual inspection if the manufacturer follows CLSI guidelines. Users should, however, keep accurate records of lot numbers, dates received and expired, and the product inserts certifying the proper QC by the manufacturer. Blood culture accessioning, processing, incubating, and all the activities that occur within the laboratory for identification of isolates, susceptibility testing, and results formatting are activities for which QC parameters should be measured and monitored

EVALUATION OF BLOOD CULTURE PRACTICES: USE OF EPICENTER DATA Ahmet Başustaoğlu 1, Serap Süzük 2, İpek Mumcuoğlu 3, Z. Ceren Karahan 4, Dilara Öğünç 5, İlknur Kaleli 6, Şenol Kurşun 3, Ebru Evren 4, Betil Özhak Baysal 5, Melek Demir 6, Nermin Hamurcu 7 Patrick Murray 8 üGirne American University, Girne, Northern Cyprus ü MOH. Public Health Agency of Turkey, Ankara, Turkey üAnkara Numune Training and Research Hospital, Ankara, Turkey üAnkara Uni. Faculty of Medicine İbni Sina Training and Research Hosp. , Ankara, Turkey üAkdeniz Uni. Faculty of Medicine , Antalya, Turkey üPamukkale Uni. Hospital, Denizli, Turkey ü BD Diagnostic Systems , Istanbul, Turkey ü BD Diagnostic Systems , Maryland , ABD.

EVALUATION OF BLOOD CULTURE APPLICATIONS BY EPICENTER DATA

The aim of the study : To use the data obtained from proper blood culture practices for; ü Demonstrating of the traceability of the data via the statistical analysis program of the Epi. Center operating system üRevealing the efficacy of proper blood culture practices on correct diagnosis through the data obtained and offer areas for improvement within this process üRaising awareness about the use of this program.
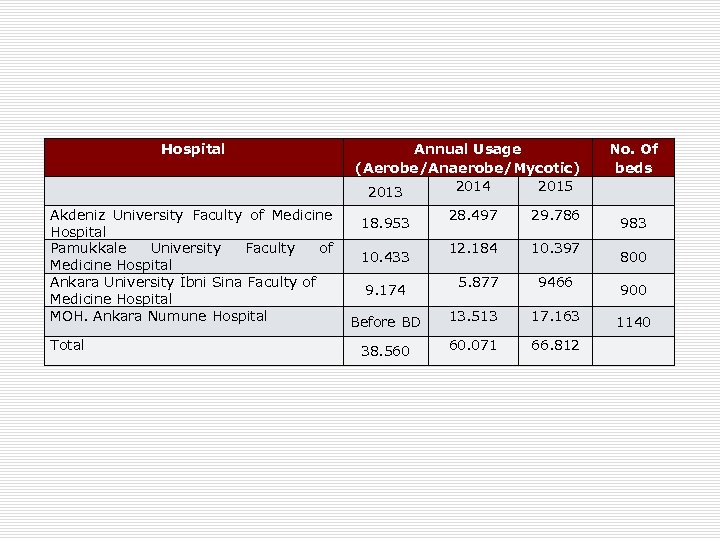
Hospital Annual Usage (Aerobe/Anaerobe/Mycotic) 2014 2015 2013 No. Of beds Akdeniz University Faculty of Medicine 28. 497 18. 953 Hospital Pamukkale University Faculty of 12. 184 10. 433 Medicine Hospital Ankara University İbni Sina Faculty of 5. 877 9. 174 Medicine Hospital MOH. Ankara Numune Hospital 13. 513 Before BD 29. 786 17. 163 1140 Total 66. 812 38. 560 60. 071 10. 397 9466 983 800 900
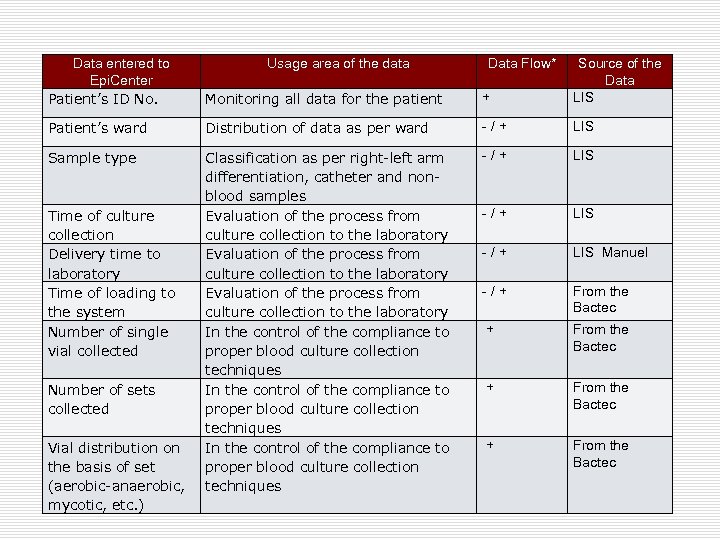
Data entered to Epi. Center Patient’s ID No. Monitoring all data for the patient + Source of the Data LIS Patient’s ward Distribution of data as per ward - / + LIS Sample type Classification as per right-left arm differentiation, catheter and nonblood samples Evaluation of the process from culture collection to the laboratory In the control of the compliance to proper blood culture collection techniques - / + LIS Manuel - / + From the Bactec Time of culture collection Delivery time to laboratory Time of loading to the system Number of single vial collected Number of sets collected Vial distribution on the basis of set (aerobic-anaerobic, mycotic, etc. ) Usage area of the data Data Flow*
Important points that arouse upon the analysis of Epi. Center data; Preanalytical phase: üData requested on Epi. Center that can support hospital quality systems/practices such as ü ü the name of the clinic, the name of the requesting physican the name of the person that collected the sample, the patient’s room number which are important with respect to monitoring hospital infections and epidemiology have not been entered into the HIS and not transferred to the system. üTimes of sample collection for blood culture were mostly not entered into the HIS and/or not written on the request form and consequently were not transferred to the Epi. Center database.
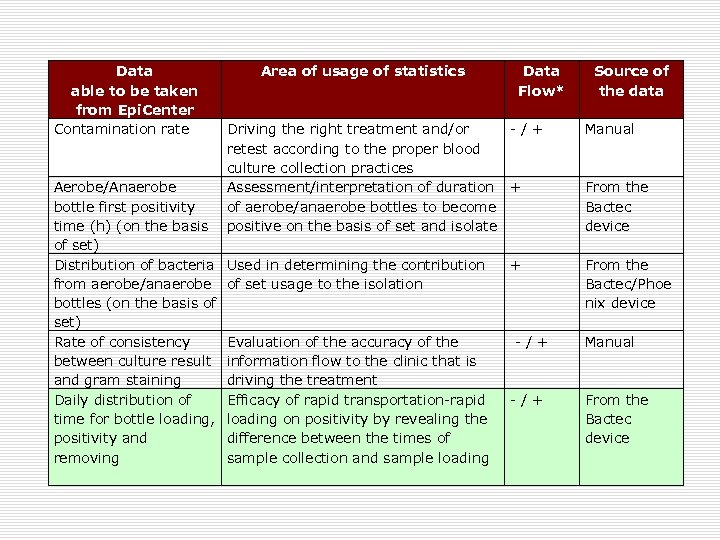
Data able to be taken from Epi. Center Contamination rate Area of usage of statistics Data Flow* Driving the right treatment and/or - / + retest according to the proper blood culture collection practices Aerobe/Anaerobe Assessment/interpretation of duration + bottle first positivity of aerobe/anaerobe bottles to become time (h) (on the basis positive on the basis of set and isolate of set) Distribution of bacteria Used in determining the contribution + from aerobe/anaerobe of set usage to the isolation bottles (on the basis of set) Rate of consistency Evaluation of the accuracy of the - / + between culture result information flow to the clinic that is and gram staining driving the treatment Daily distribution of Efficacy of rapid transportation-rapid - / + time for bottle loading, loading on positivity by revealing the positivity and difference between the times of removing sample collection and sample loading Source of the data Manual From the Bactec device From the Bactec/Phoe nix device Manual From the Bactec device
ü ü ü Adopting of appropriate sample collection techniques through necessary trainings ensures drop in contamination rate for blood cultures and increases the chance of obtaining correct results. . Contamination rates for the hospitals that were included in this study were (6, 2 - 8, 9%), The reasons behind not being able to calculate contamination rates were ; ü ü ü the site of blood collection was not specified, more than one vial/set was not taken, mistakes in the interpretation of the isolated agent were made there was a lack of clinic-laboratory cooperation Awareness should be raised in hospitals of the importance of monitoring these rates
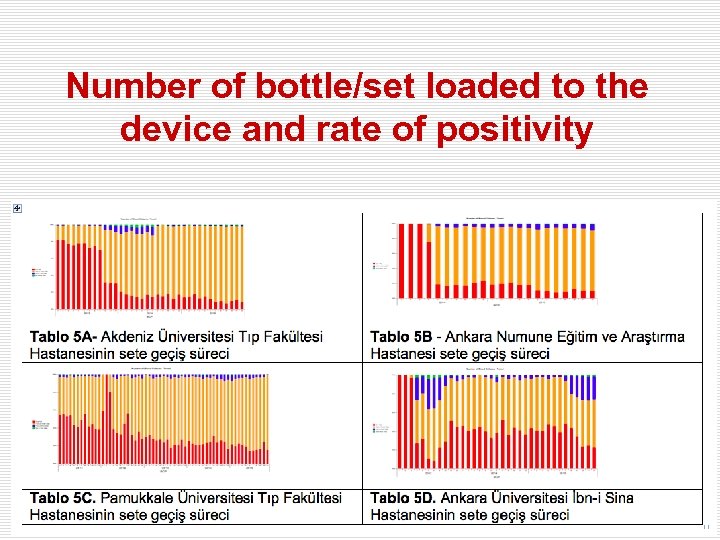
Number of bottle/set loaded to the device and rate of positivity
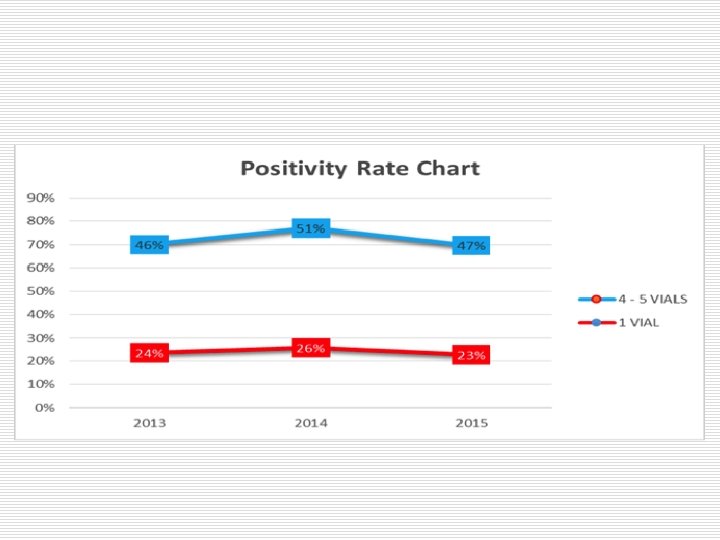
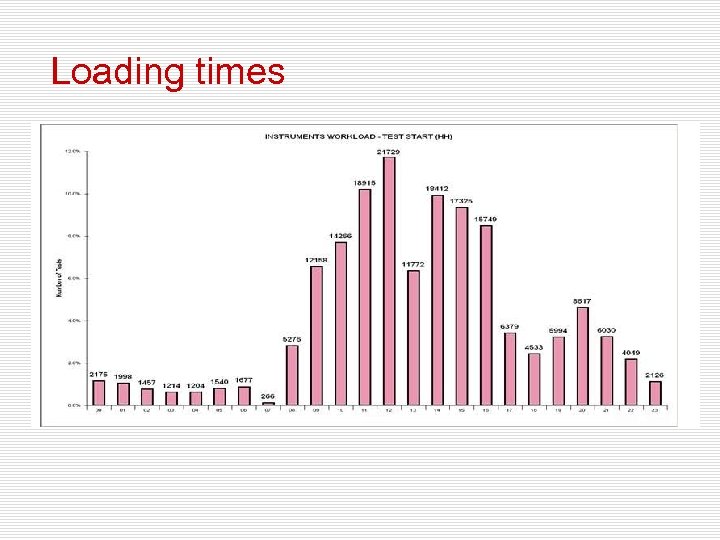
Loading times
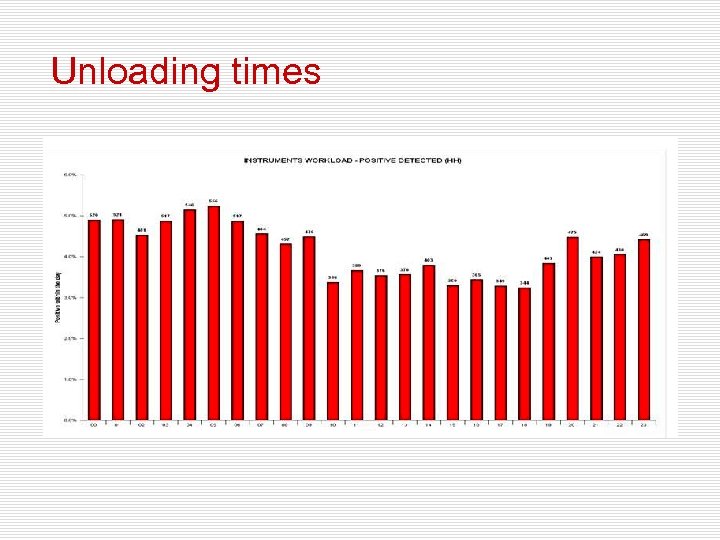
Unloading times
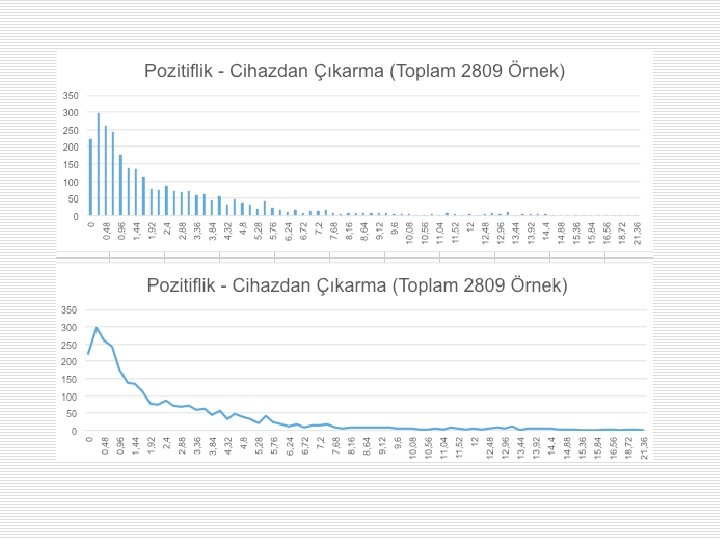
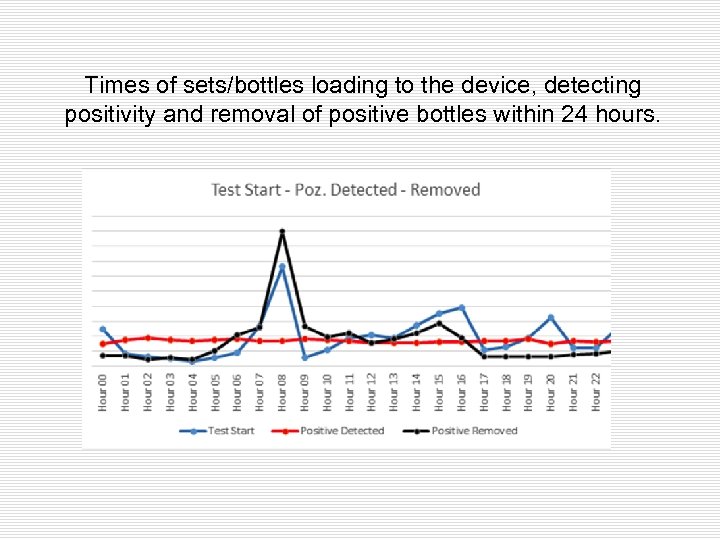
Times of sets/bottles loading to the device, detecting positivity and removal of positive bottles within 24 hours.

Each hour of delay in initiation of effective antimicrobials can increase mortality rates by 7. 6%. Anand Kumar, et. All. , Chest 2009; 136; 1237 -1248;
17e03983ecf7edd6e68eb62ddbd09e38.ppt