c6a5a3f108aa0deadb6a3c8af0a3cb75.ppt
- Количество слайдов: 38

Biowarfare to Biodefense: Operation Whitecoat & USAMRIID History Arthur O. Anderson MD Office of Human Use and Ethics CD-22 Exp 25 Jan 1955, USAMU Est 20 Jun 1956, USAMRIID Est 27 Jan 1969

U. S. Was Unprepared For BW U. S. Concerned About The Possibility That The Nazis Were Preparing For Bio. Warfare As Entry into WW II Approaches

Detrick Field Was An Air National Guard Training Center It Became A Bio. Warfare Center as Camp Detrick 1931 April 1943 Scientific Director, Dr. Ira Baldwin, [U. Wisc] planned operations 1942 -44 LTC William S. Bacon, CMLC was first Commander of Camp Detrick

Biosafety invented at Camp Detrick before bioweapons developed A. G. Wedum MD - Safety “S” Division - first division to be activated in 1943
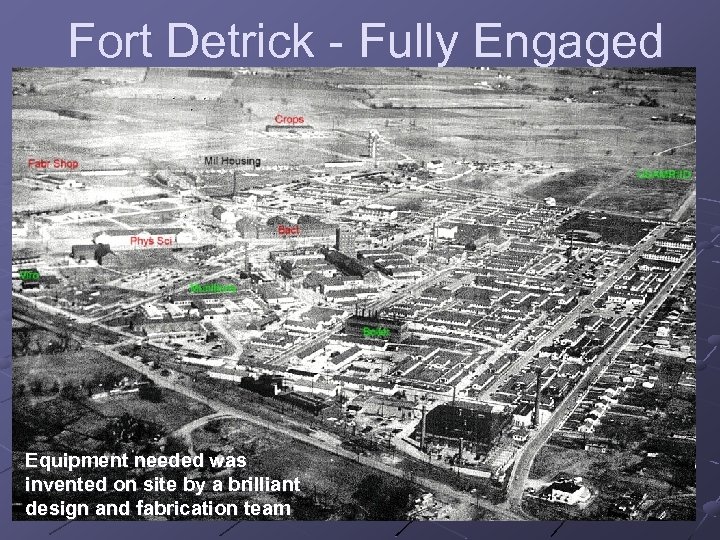
Fort Detrick - Fully Engaged Equipment needed was invented on site by a brilliant design and fabrication team

1943 -46 Human Experimentation Camp Detrick - Safety Practices Occupational Safety by “S” Division Training Program, Posters, Constant Survey of Safety Practices Personnel Inspection and First Aid n In the First Aid room near hot suite change rooms, nurses inspected workers leaving hot suites for breaks in skin or clinical signs of disease as they prepared to shower out.

1943 -46 Human Experimentation Camp Detrick - Safety Practices Immunization* n n n Vaccination routine called “special procedures” instituted by Biological Protection Branch of S Division Primary Objective was to protect workers Secondary Objectives: Determine most efficacious immunization methods Determine effectiveness of available preparations No existing vaccines for some agents Only experimental vaccines available for others * Ltr (S), CO CD to Post Surgeon CD, 22 Jan 44, Sub: Immunization. SPCYF 400. 12. In Hq CD (720. 3) * Sp Rpt 15, Immunological Protection of Personnel Against B. W. Agents (Oct 46), p. 2

Special Procedures - SIP Most 1950’s Fort Detrick alumni remember Nurse Betty Grable, Dr. Paul Kadull & the “shot shop”, which is what they called the building where all workers and research subjects received their vaccines prescribed by “Special Procedures”. Both groups got the same IND vaccines but only the research volunteers were given the choice with informed consent

Human Subjects Research at Fort Detrick 1943 - 1946 The station hospital, originally activated in 1943, provided a “unique opportunity to study the inception, course and therapeutics of many rare diseases in patients whose baseline health data was known” LTC Abram Benenson MC This was primarily “opportunist” research that depended upon occupational incidents among workers in the various biowarfare facilities

Nuremberg Trial - 1947 Liberation of Auschwitz 27 Jan 1945 The Nuremberg war crimes trials convicted 23 Nazi doctors for murder Mengele (left) escaped By 1946 Andrew Ivy released his list of ten conditions required for “permissible medical experiments” in healthy subjects which became the Nuremberg Code

Human Vulnerability to BW Aerosols Not Previously Tested In 1952, the Armed Forces Medical Policy Council wanted information on human vulnerability and countermeasures to biological warfare Army SG met with Chief Chemical Officer at the same time that Secretaries of Defense & Army, Army Chief of Staff and Chemical and Medical elements were meeting to discuss possible use of humans in biowarfare defense research.

Wilson Memorandum of 1953 The Nuremberg Code principles* were incorporated into the Wilson Memorandum to the Secretaries of the Army, Navy and Air Force 26 Feb 1953 * Use of these principles for non-clinical research related to warfare defense was promoted before experiments were planned and funded Army Directive CS-385 issued 30 June 1953 added “consent in writing” and additional safeguards

Human Vulnerability to BW Aerosols Between April 1953 and 25 January 1955, an ad hoc committee that included civilian & military advisory groups planned a research program aimed at responding to the AFMPC requirements of 1952. Human Volunteer Studies, CD-22 and Operation Whitecoat, were planned along with design and construction of the USAMU (later renamed USAMRIID).
Objectives of Multifaceted CD-22 were designed to determine: Human vulnerability in realistic BW scenarios. Effective prevention and treatment of BW casualties. Determination of minimal infective doses. Effectiveness of vaccines and drugs. Serological responses to infections, and. Clinical effects of various doses of infectious agents.

The CD-22 program focused on Human responses to prototypes: Q fever and Tularemia were regarded by CES of AFEB as acceptable prototypical BW agents for testing in Humans that satisfied limiting characteristics* of low lethality, no serious chronicity anticipated, effective therapy available and there was adequate animal experimental data on safety and protective efficacy *[also described in cs-385].

The US Army Medical Unit at Fort Detrick USAMU was created to develop the means to diagnose, treat and prevent diseases caused by biological warfare agents. Approval of the cs-385 directive for ethical operation, plans for organization of the institute, preparation of protocols and initiation of the first human studies took place within 3 -6 months of its creation.
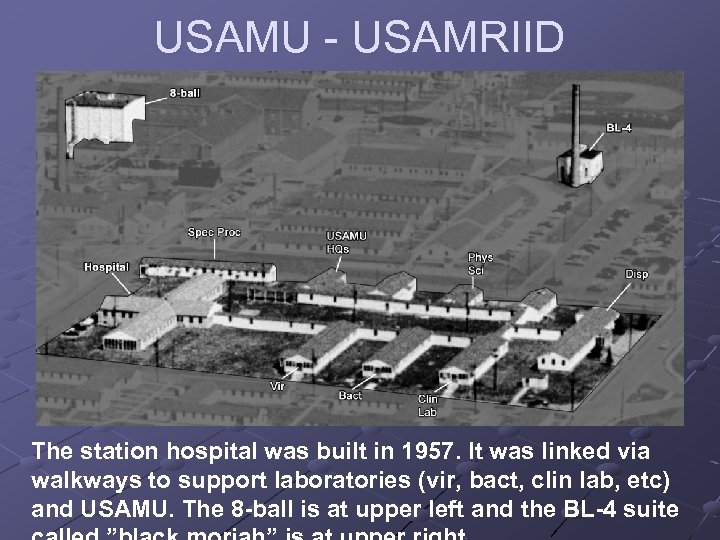
USAMU - USAMRIID The station hospital was built in 1957. It was linked via walkways to support laboratories (vir, bact, clin lab, etc) and USAMU. The 8 -ball is at upper left and the BL-4 suite

COL W. D. Tigertt USAMU October 1954 Colonel Tigertt contacted Dr. Theodore R. Flaiz of Seventh Day Adventist General Conference about seeking 1 A-O SDA volunteer subjects. General Conference of the SDA Church approved recruitment of drafted SDA volunteer subjects into Operation Whitecoat program

Operation Whitecoat Volunteers were SDA “Conscientious Objectors” recruited from Medic Training school at Fort Sam Houston Operation Whitecoat Volunteers at Forest Glenn Ballroom 1956 Between 1954 and 1973 2, 300 Seventh Day Adventist participants of Operation Whitecoat served at Fort Detrick and associated locations.

Protocol Review during Operation Whitecoat • The Medical investigator prepared protocol. • Reviewed for approval at a “Protocol Meeting” attended by Commander, Scientific Advisor, and the Research Division Chiefs. • Approved protocols were forwarded to HQDA (SGRD-DR) for further approval by CES of AFEB (before 1962) or HSRRB (after 1962 *). • After final approval, Whitecoat volunteers were briefed, attended a project interview, and informed consent documents were signed only after at least 24 hours. • *AR 70 -25 published in 1962 was identical to cs-385.
Review and Approval Process USAMU 1955 -73 vs USAMRIID 1955 – 73 USAMU Protocol Meeting Minutes were one page long with only one sentence for the committee decision. Issues were not documented. 1976 – USAMRIID IRB Minutes 5 pages long, 4 of which were Q&A that documented issues. Presently – USAMRIID IRB Minutes are > 14 pages long with 2 pages of narrative summary, 2. 5 pages of Q&A per protocol with decision plus 9 pages of expedite approval ratifications, continuing review and SAE discussion.

Protocol Briefing

Operation Whitecoat served as a model of the ethical use of human subjects in research. The three step process of informed consent - by which research subjects become familiar with the purpose of a study in order to understand the risks and potential benefits involved before agreeing to participate - was successfully implemented from the program’s inception. The soldiers were not required to participate in any of the studies, only to be present for briefings by principal investigators seeking volunteers. Two more steps occurred before subjects were asked to consent. About 20 percent of the men did not participate in any studies during their tenure at Fort Detrick.
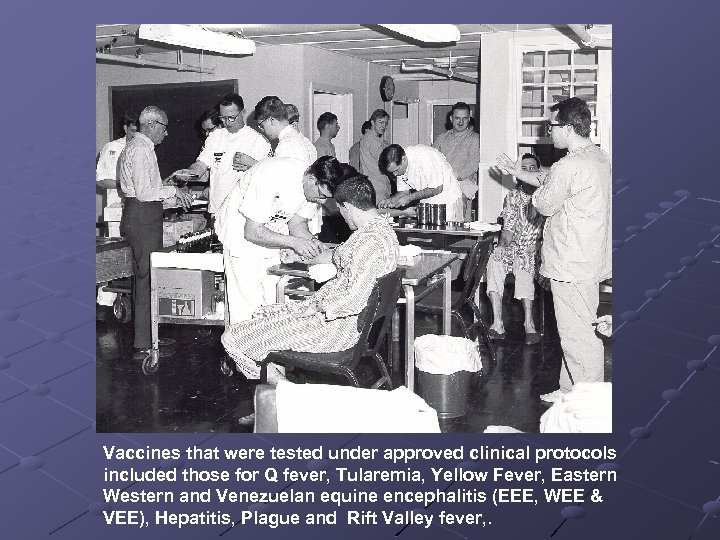
Vaccines that were tested under approved clinical protocols included those for Q fever, Tularemia, Yellow Fever, Eastern Western and Venezuelan equine encephalitis (EEE, WEE & VEE), Hepatitis, Plague and Rift Valley fever, .

Aerosol Efficacy Studies in 8 Ball Q-fever and Tularemia were approved for these studies because safety criteria were met and cure was assured.

Volunteer from the Aerosol Q-fever Study at Dugway Proving Grounds

Experimental Risks Minimized by Availability of Effective Treatment

In addition to the advances made in vaccine and drug development, Operation Whitecoat volunteers contributed to a better understanding of the signs, symptoms, and clinical diagnostic parameters in human disease associated with Q-fever, Tularemia, Sandfly fever, and staphylococcal enterotoxins.

Ethical Accomplishments: Operation Whitecoat Effectively Used Nuremberg Code Principles Created Effective Informed Consent Process Involved “Community” of the SDA Volunteers Local and Extramural Oversight / Monitoring

Biosafety and Engineering Accomplishments Containment – Glove Boxes, Laminar flow Hoods, Biohazard suit with air supply Ambient Air Pressure Controls - Positive Staff Areas, Negative Laboratories and Filtered or incinerated exhaust Education of workers on Safety Biosurety procedures, Vaccines, Occupational Follow-up Laminar flow / light scatter technology, that was developed aerosol for particle sizing became the basis of the Fluorescence Activated Cell Sorter & other flow cytometry systems in use today

Medical Accomplishments: Operation Whitecoat Licensed vaccines were developed, including yellow fever, hepatitis, and plague. Investigational New Drug (IND) vaccines were developed, including those for Venezuelan equine encephalitis (VEE), Rift Valley fever, Q fever, and tularemia. Effective systems for biological hazard containment were developed Rift Valley Fever Virus vaccine; used in 1977 outbreak in Egypt, effected 200, 000 humans (2, 000 deaths) and entire sheep population.

RVF Vaccine caused Peace to break out in the middle east Emissaries from Egypt and Israel requested RVFV as Sadat & Begin met at Camp David. Therefore, a little known benefit that Operation Whitecoat Volunteers provided was to enable peace between Egypt and Israel to “break out” because obtaining RVF Vaccine was an important bargaining chip to both parties. N Meyers - Nature 1986 Jan 9; v 319(6049): p 91

Research Programs Influenced by News Events and World Affairs “Cold War” imperatives continued into the 1980’s and we saw growth in Virology Research under Dr. Jerry Eddy with COL Huxsoll as commander. The Anthrax outbreak in Sverdlovsk, (Ekaterinburg) re-started Anthrax Research here. “Yellow Rain” in Cambodia and Laos signaled the need to increase focus on Toxins and Toxinology was created from Physical Sciences Division. And Aum Shinrikyo warned that Bioterrorism was on the horizon just as the “Evil Empire” was crumbling. This prompted expansion of the means for Rapid Diagnosis just as PCR miniaturization was developing along with other dx technologies.

Bioterrorism: Changing Priorities Bioterrorism in the US was no longer theoretical after 2001 DHS, was created for domestic security and countermeasures R&D for Biodefense medical countermeasures under DHHS Present Bioterrorism response resembles US response to threat of Bio. Warfare during World War II
Moral Dilemma: Comply with FDA law vs Intent to Benefit in BW Emergency 1962 Amendments to the FD&C Act required proof of efficacy of drugs and vaccines for BW created a moral dilemma. Risk killing subjects in a valid clinical trial, versus withholding potentially life saving drugs or vaccines because they lacked substantial evidence of human clinical efficacy.

Project Bio. Shield : CDC, HHS & Do. D may use HHS Project Bio. Shield as specified in the following legislation: n Passed: H. R. 2122 Project Bioshield Act - July 16 2003 n Passed: S. 15 Project Bio. Shield Act - May 19 2004 n President Signed : Public Law No: 108 -276 July 21 2004 n Presented: H. R. 4258 Rapid Pathogen Identification to Delivery of Cures Act - May 3, 2004 These legislative Acts resolve a dilemma and the dichotomy that is associated with the need for widespread use of FDAunapproved products in civilians for national biodefense or in soldiers facing war hazards when it is unethical to do FDA mandated human clinical efficacy studies. Re-Capitalization of USAMRIID and creation of a “Business Model” became linked intrinsically because of increasing diversity of our funding sources

USAMU - USAMRIID Evolution Over the past 50 years since the start of Operation Whitecoat, the U. S. Army Medical Research Institute of Infectious Diseases has grown considerably from what it was as the U. S. Army Medical Unit, yet It continues to conduct basic and applied research on biological threats resulting in medical solutions to protect military service members. n The present building was planned by COL Dan Crozier, and is named in his honor.

COL Arthur O. Anderson MC Chief, Human Use and Ethics US Army Medical Research Institute of Infectious Diseases Arthur. Anderson 2@amedd. army. mil
c6a5a3f108aa0deadb6a3c8af0a3cb75.ppt