5ec6efe7feef7c0235880acf7b0b5583.ppt
- Количество слайдов: 66

Biotechnology Chapter 17
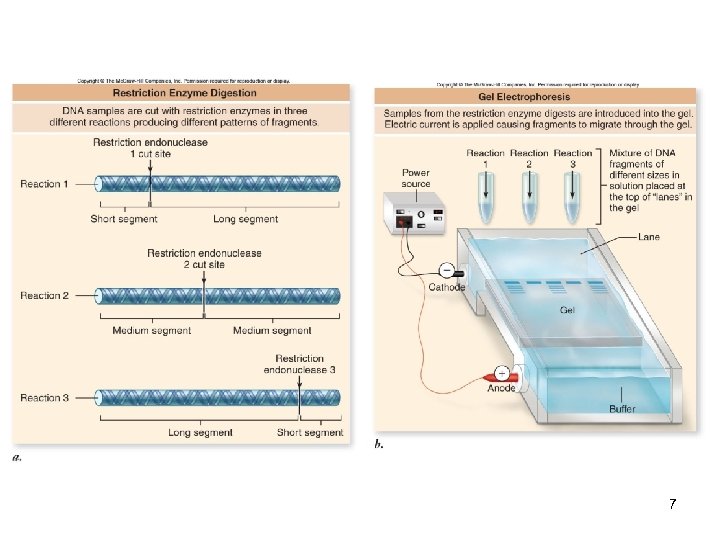
DNA Manipulation The molecular biology revolution started with the discovery of restriction endonucleases -Enzymes that cleave DNA at specific sites These enzymes are significant in two ways 1. Allow a form of physical mapping that was previously impossible 2. Allow the creation of recombinant DNA molecules (from two different sources) 2
DNA Manipulation Restriction enzymes recognize DNA sequences termed restriction sites There are two types of restriction enzymes: -Type I = Cut near the restriction site -Rarely used in DNA manipulation -Type II = Cut at the restriction site -The sites are palindromes -Both strands have same sequence 3 when read 5’ to 3’

DNA Manipulation Type II enzymes produce staggered cuts that generate “sticky ends” -Overhanging complementary ends Therefore, fragments cut by the same enzyme can be paired DNA ligase can join the two fragments forming a stable DNA molecule 4
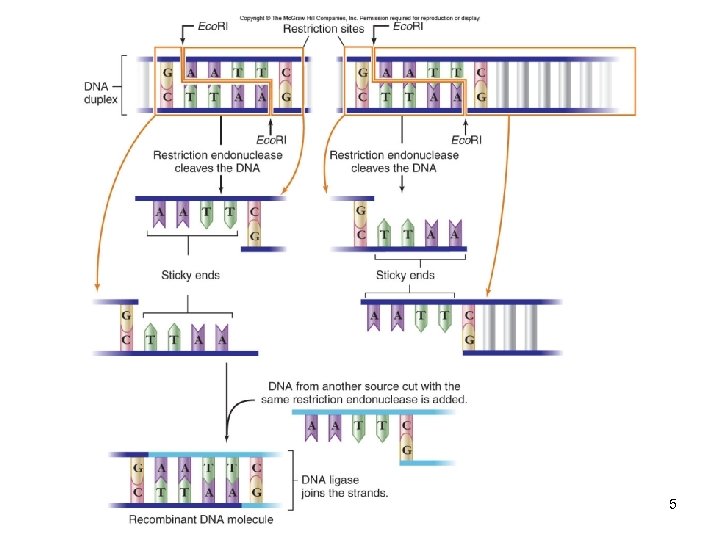
5
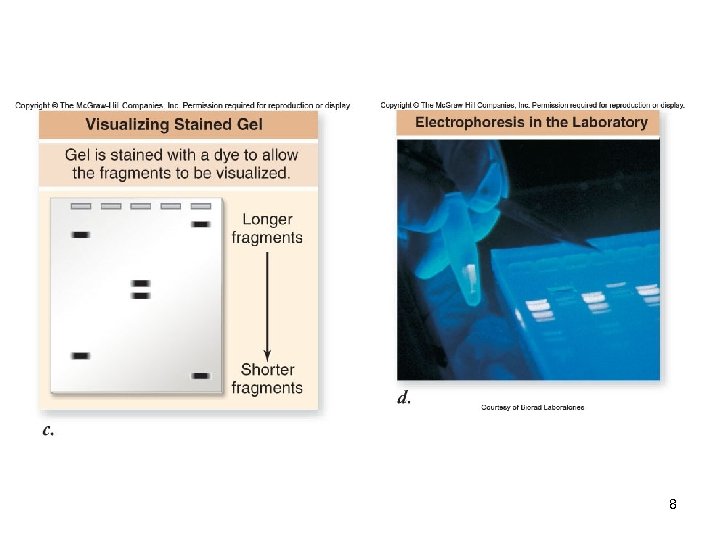
Gel Electrophoresis A technique used to separate DNA fragments by size The gel (agarose or polyacrylamide) is subjected to an electrical field The DNA, which is negatively-charged, migrates towards the positive pole -The larger the DNA fragment, the slower it will move through the gel matrix DNA is visualized using fluorescent dyes 6
7
8
Transformation is the introduction of DNA from an outside source into a cell Natural transformation occurs in many species -However, not in E. coli, which is used routinely in molecular biology labs -Artificial transformation techniques have been developed to introduce foreign DNA into it 9
Molecular Cloning A clone refers to a genetically identical copy Molecular cloning is the isolation of a specific DNA sequence (usually protein-encoding) -Sometimes called gene cloning The most flexible and common host for cloning is E. coli Propagation of DNA in a host cell requires a vector 10
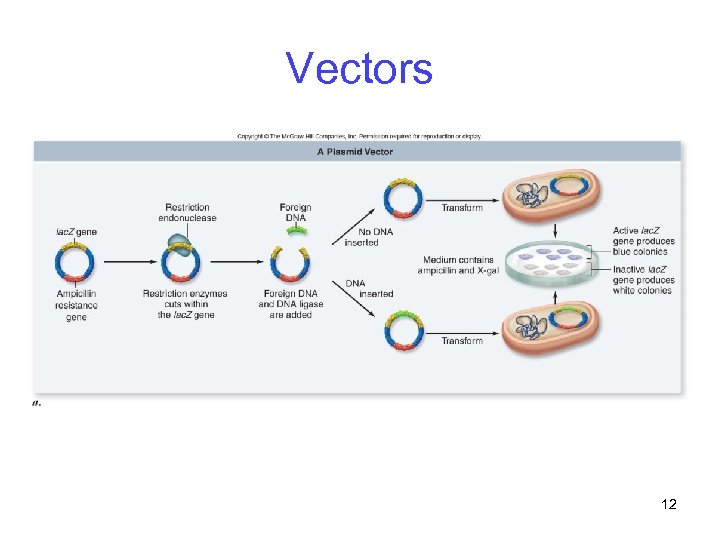
Vectors Plasmids are small, circular extrachromosomal DNA molecules -Used for cloning small pieces of DNA -Have three important components 1. Origin of replication 2. Selectable marker 3. Multiple cloning site (MCS) 11
Vectors 12
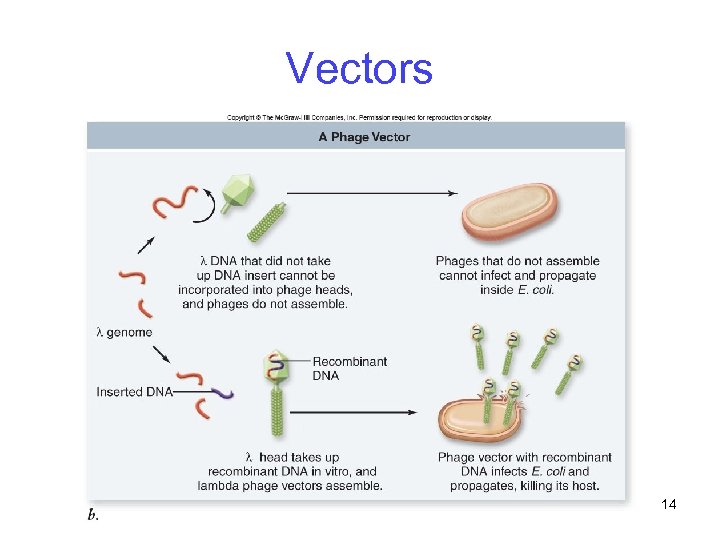
Vectors Phage vectors are modified bacterial viruses -Most based on phage lambda (l) of E. coli -Used to clone inserts up to 40 Kbp -Have two features not shared with plasmid vectors -They kill their host cells -They have linear genomes -Middle replaced with inserted DNA 13
Vectors 14
Vectors Artificial chromosomes -Used to clone very large DNA fragments -Bacterial artificial chromosomes (BACs) -Yeast artificial chromosomes (YACs) 15
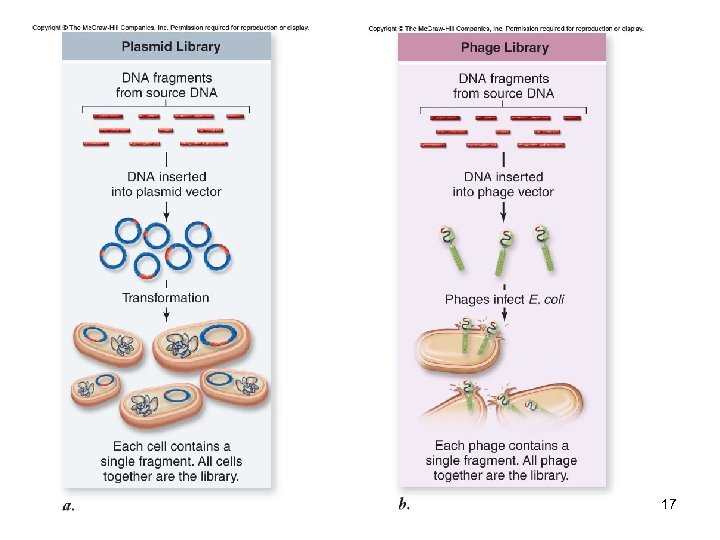
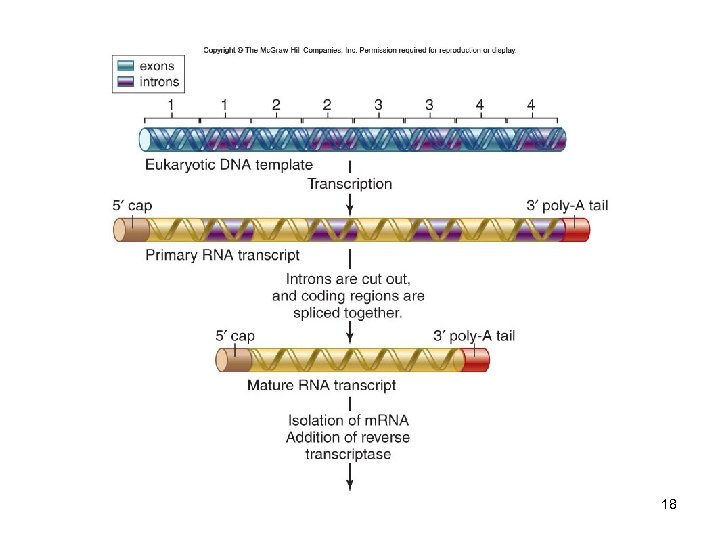
DNA Libraries A collection of DNA fragments from a specific source that has been inserted into host cells A genomic library represents the entire genome A c. DNA library represents only the expressed part of the genome -Complementary DNA (c. DNA) is synthesized from isolated m. RNA using the enzyme reverse transcriptase 16
17
18
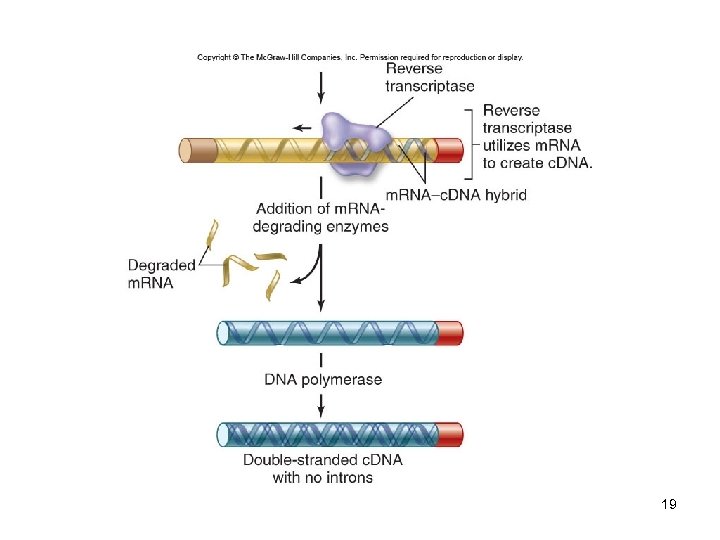
19
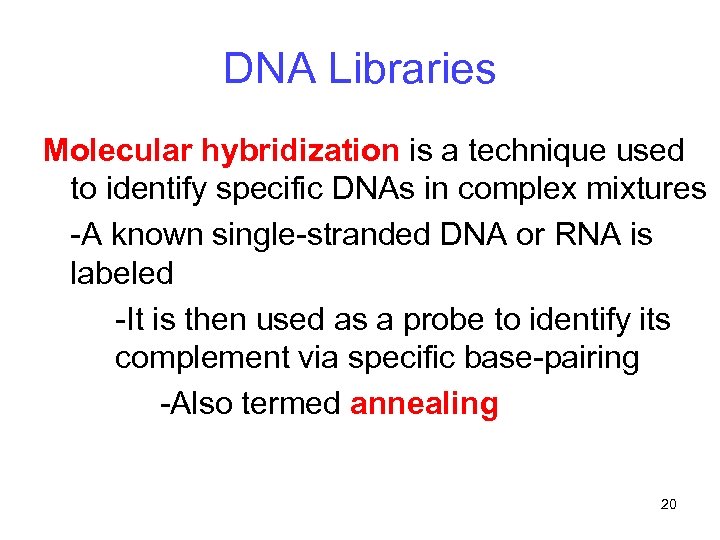
DNA Libraries Molecular hybridization is a technique used to identify specific DNAs in complex mixtures -A known single-stranded DNA or RNA is labeled -It is then used as a probe to identify its complement via specific base-pairing -Also termed annealing 20

DNA Libraries Molecular hybridization is the most common way of identifying a clone in a DNA library -This process involves three steps: 1. Plating the library 2. Replicating the library 3. Screening the library 21
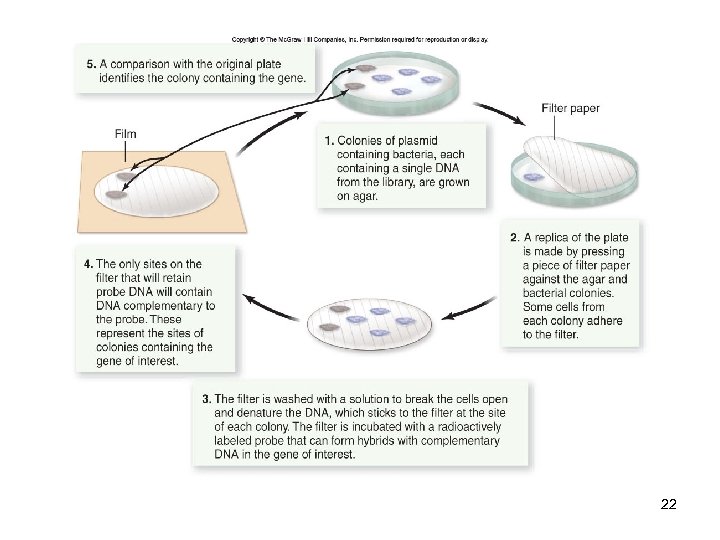
22
DNA Analysis Restriction maps -Molecular biologists need maps to analyze and compare cloned DNAs -The first maps were restriction maps -Initially, they were created by enzyme digestion & analysis of resulting patterns -Many are now generated by computer searches for cleavage sites 23
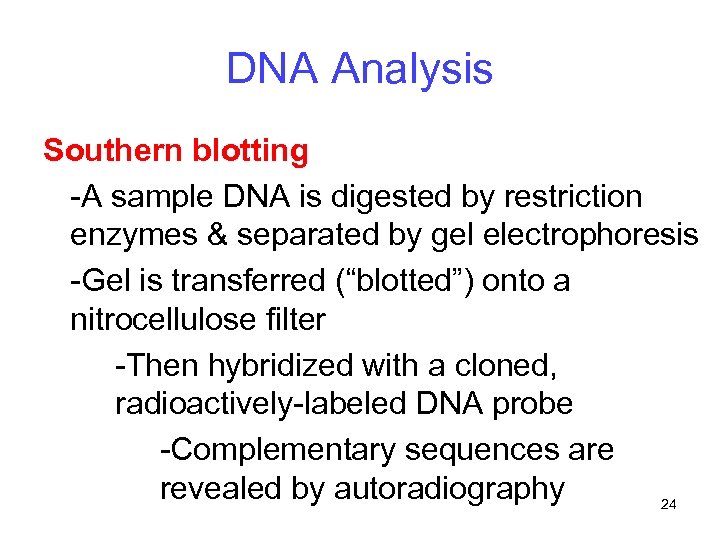
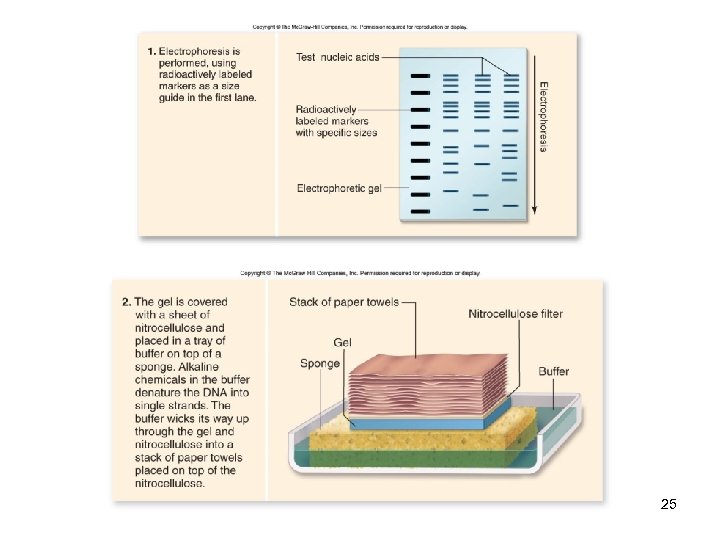
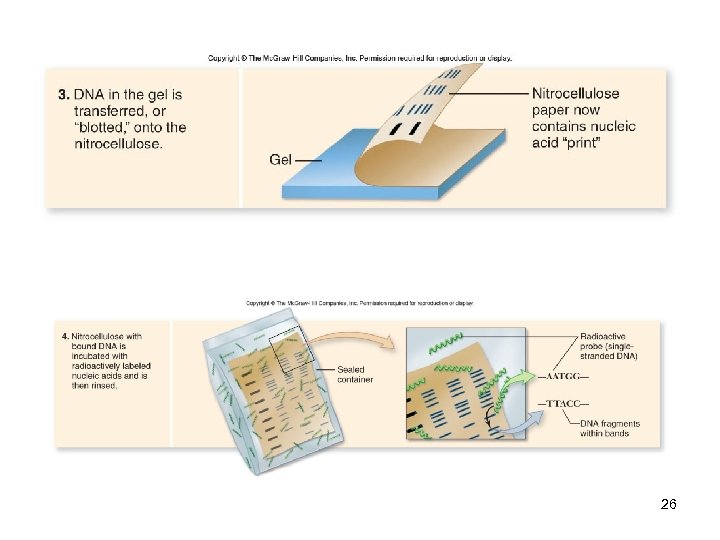
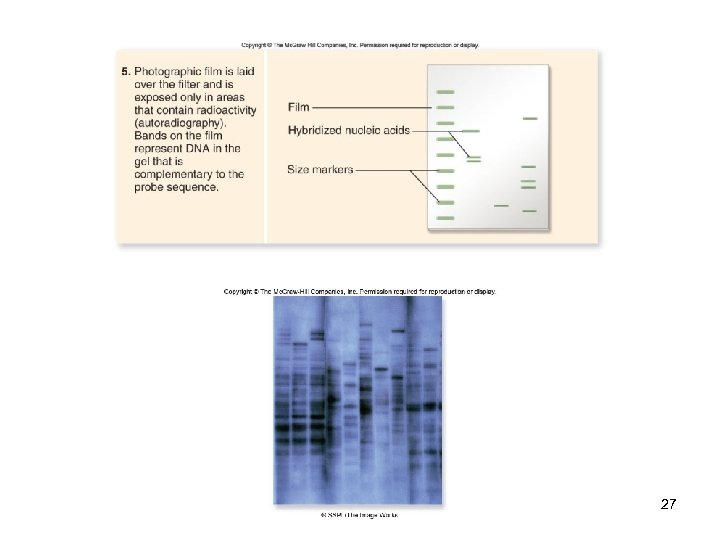
DNA Analysis Southern blotting -A sample DNA is digested by restriction enzymes & separated by gel electrophoresis -Gel is transferred (“blotted”) onto a nitrocellulose filter -Then hybridized with a cloned, radioactively-labeled DNA probe -Complementary sequences are revealed by autoradiography 24
25
26
27
DNA Analysis Northern blotting -m. RNA is electrophoresed and then blotted onto the filter Western blotting -Proteins are electrophoresed and then blotted onto the filter -Detection requires an antibody that can bind to one protein 28
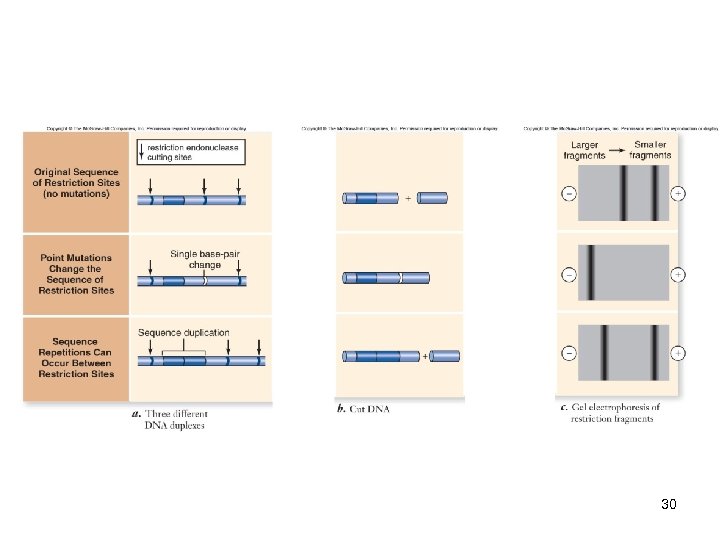
DNA Analysis RFLP analysis -Restriction fragment length polymorphisms (RFLPs) are generated by point mutations or sequence duplications -These fragments are often not identical in different individuals -Can be detected by Southern blotting 29
30
DNA Analysis DNA fingerprinting -An identification technique used to detect differences in the DNA of individuals -Makes use of a variety of molecular procedures, including RFLP analysis -First used in a US criminal trial in 1987 -Tommie Lee Andrews was found guilty of rape 31
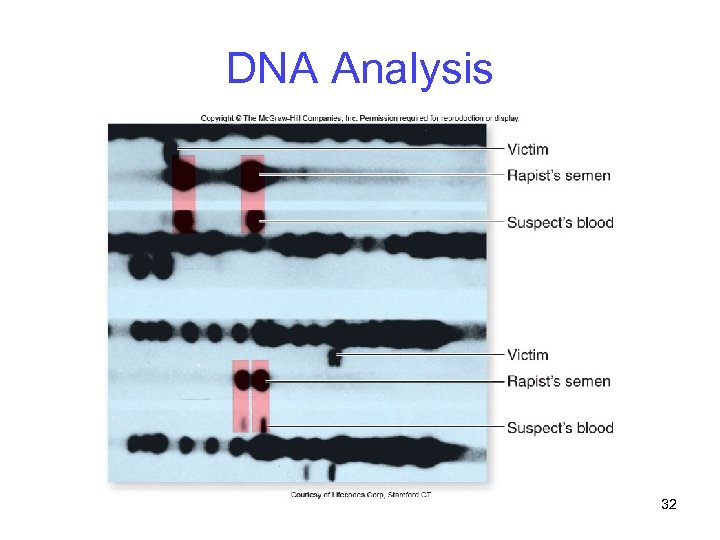
DNA Analysis 32
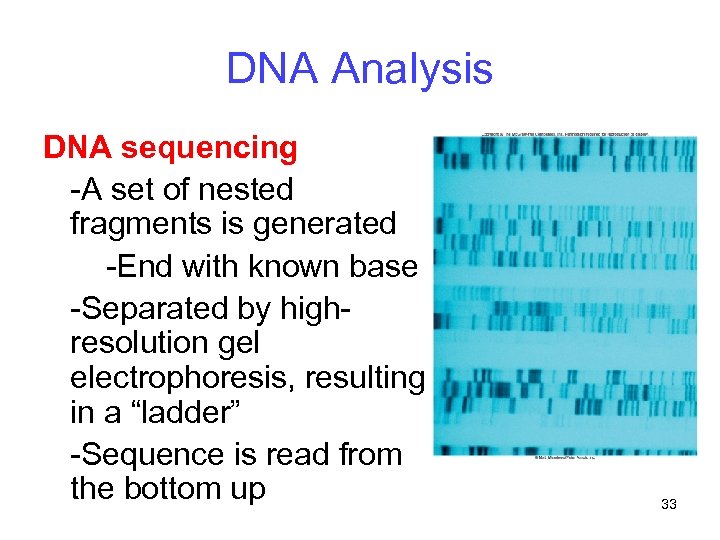
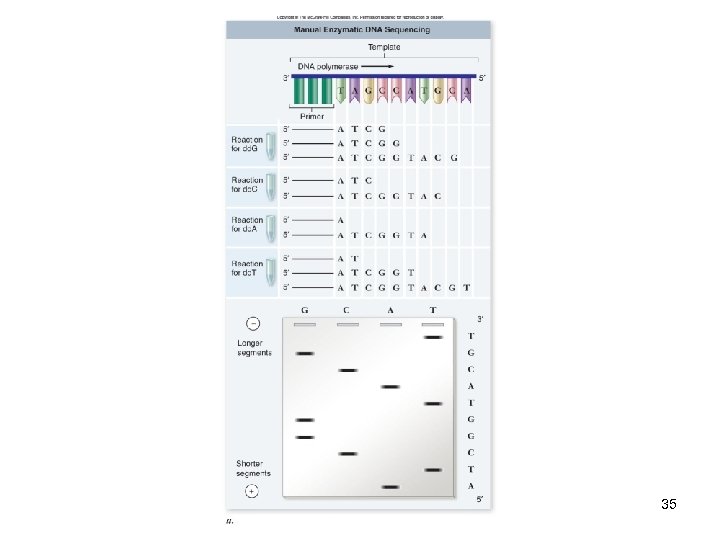
DNA Analysis DNA sequencing -A set of nested fragments is generated -End with known base -Separated by highresolution gel electrophoresis, resulting in a “ladder” -Sequence is read from the bottom up 33
DNA Analysis DNA sequencing -The enzymatic method was developed by Frederick Sanger -Dideoxynucleotides are used as chain terminators in DNA synthesis reactions 34
35
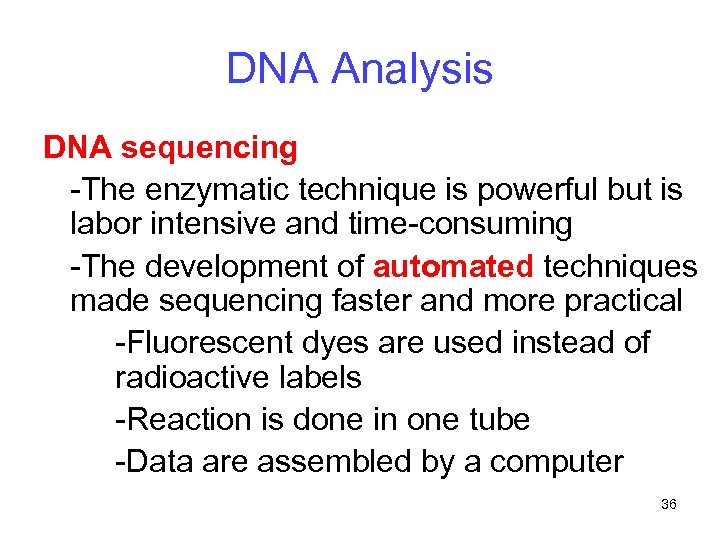
DNA Analysis DNA sequencing -The enzymatic technique is powerful but is labor intensive and time-consuming -The development of automated techniques made sequencing faster and more practical -Fluorescent dyes are used instead of radioactive labels -Reaction is done in one tube -Data are assembled by a computer 36
37
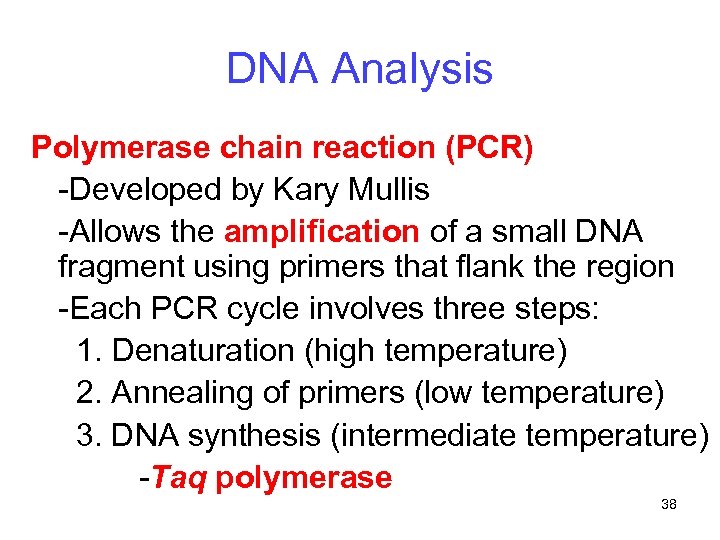
DNA Analysis Polymerase chain reaction (PCR) -Developed by Kary Mullis -Allows the amplification of a small DNA fragment using primers that flank the region -Each PCR cycle involves three steps: 1. Denaturation (high temperature) 2. Annealing of primers (low temperature) 3. DNA synthesis (intermediate temperature) -Taq polymerase 38
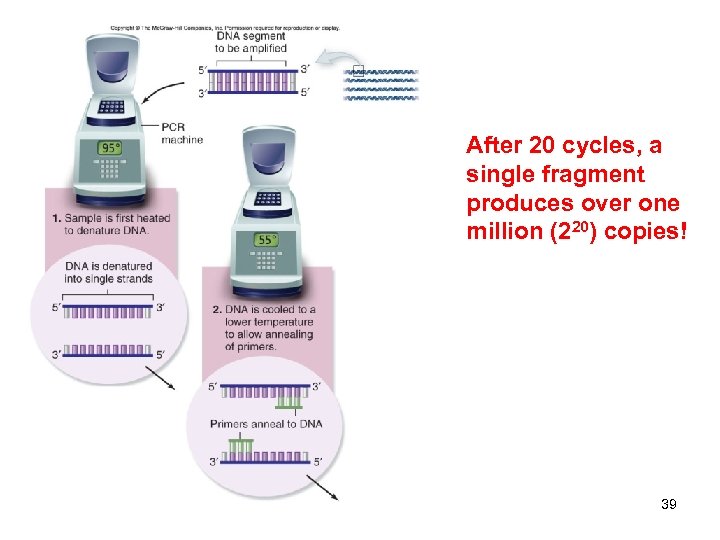
After 20 cycles, a single fragment produces over one million (220) copies! 39
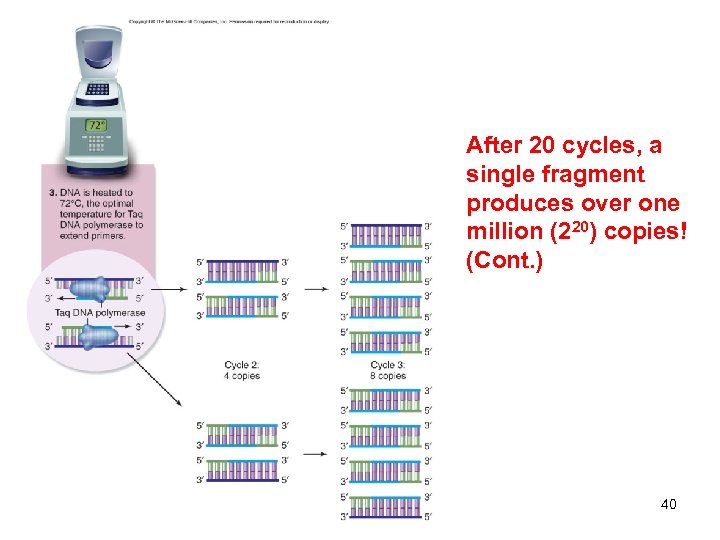
After 20 cycles, a single fragment produces over one million (220) copies! (Cont. ) 40
DNA Analysis Polymerase chain reaction (PCR) -Has revolutionized science and medicine because it allows the investigation of minute samples of DNA -Forensics -Detection of genetic defects in embryos -Analysis of mitochondrial DNA from early human species 41
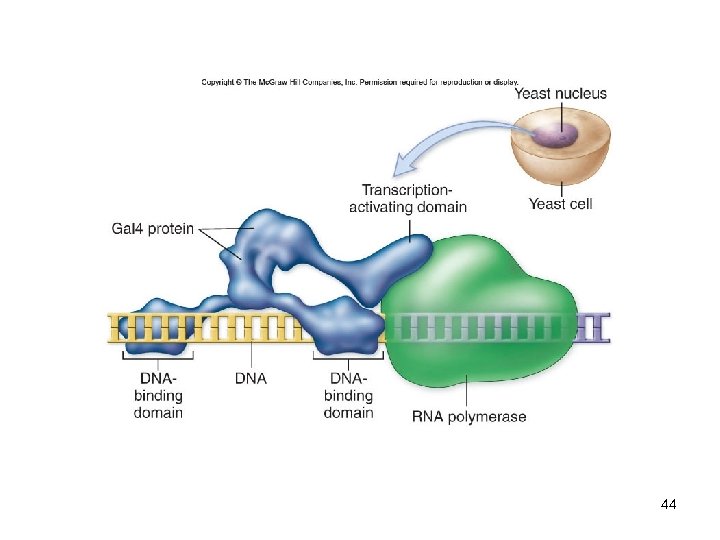
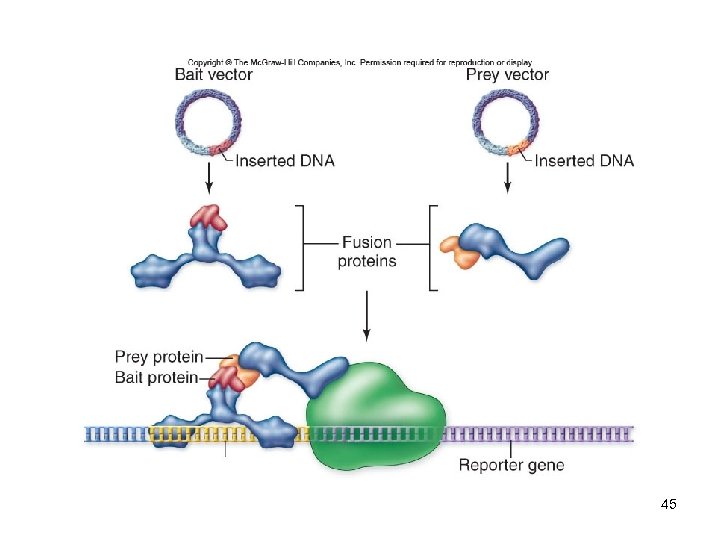
DNA Analysis Yeast two-hybrid system -Used to study protein-protein interactions -Gal 4 is a transcriptional activator with a modular structure -The Gal 4 gene is split into two vectors -Bait vector: has DNA-binding domain -Prey vector: has transcription-activating domain -Neither of these alone can activate 42 transcription
DNA Analysis Yeast two-hybrid system -When other genes are inserted into these vectors, they produce fusion proteins -Contain part of Gal 4 and the protein of interest -If the proteins being tested interact, Gal 4 function will be restored -A reporter gene will be expressed -Detected by an enzyme assay 43
44
45
Genetic Engineering Has generated excitement and controversy Expression vectors contain the sequences necessary to express inserted DNA in a specific cell type Transgenic animals contain genes that have been inserted without the use of conventional breeding 46
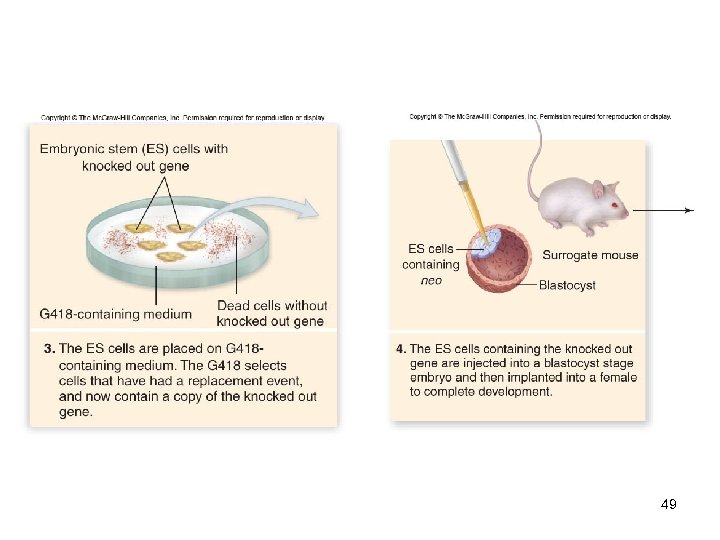
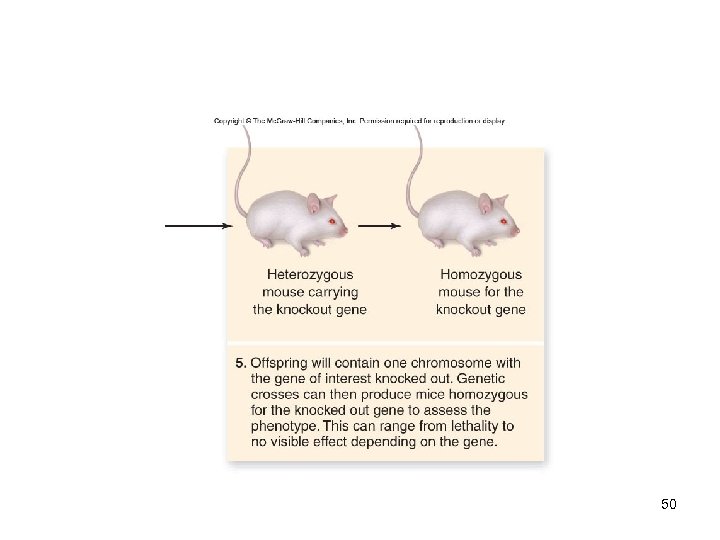
Genetic Engineering In vitro mutagenesis -Ability to create mutations at any site in a cloned gene -Has been used to produce knockout mice, in which a known gene is inactivated -The effect of loss of this function is then assessed on the entire organism -An example of reverse genetics 47
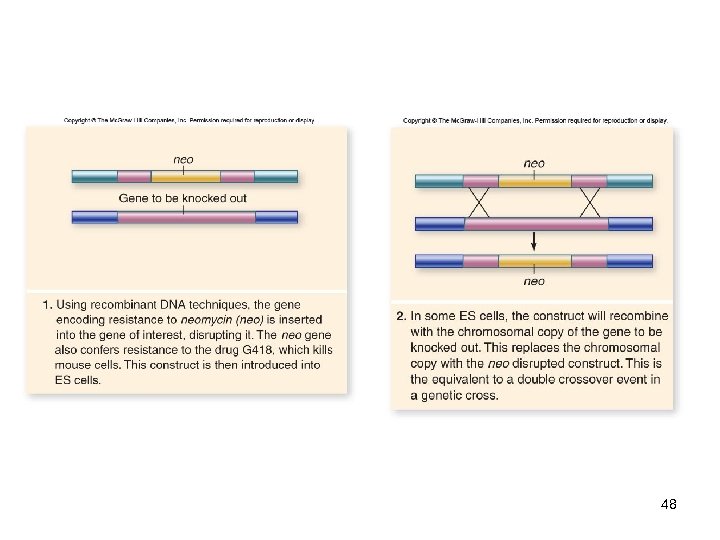
48
49
50
Medical Applications Human proteins -Medically important proteins can be produced in bacteria -Human insulin -Interferon -Atrial peptides -Tissue plasminogen activator -Human growth hormone 51

Medical Applications 52
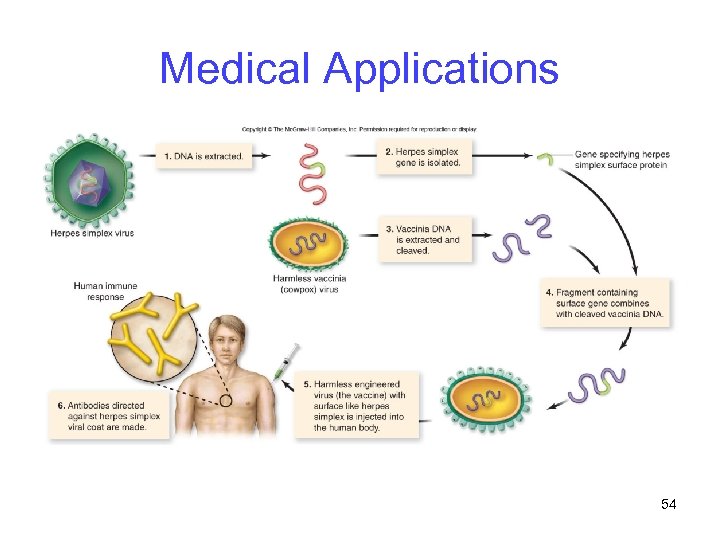
Medical Applications Vaccines -Subunit vaccines: Genes encoding a part of the protein coat are spliced into a fragment of the vaccinia (cowpox) genome -DNA vaccines: Depend on the cellular immune response (not antibodies) 53
Medical Applications 54

Medical Applications Gene therapy -Adding a functional copy of a gene to correct a hereditary disorder -Severe combined immunodeficiency disease (SCID) illustrates both the potential and the problems -Successful at first, but then patients developed a rare leukemia 55
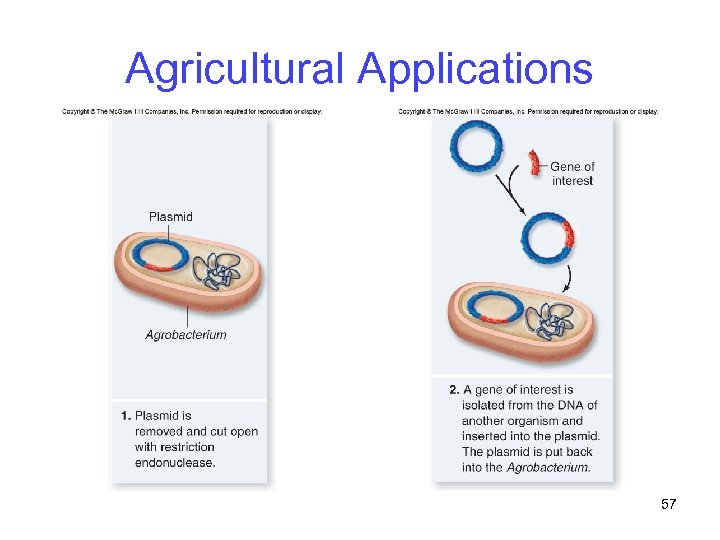
Agricultural Applications Ti (tumor-inducing) plasmid is the most used vector for plant genetic engineering -Obtained from Agrobacterium tumefaciens, which normally infects broadleaf plants -However, bacterium does not infect cereals such as corn, rice and wheat 56
Agricultural Applications 57
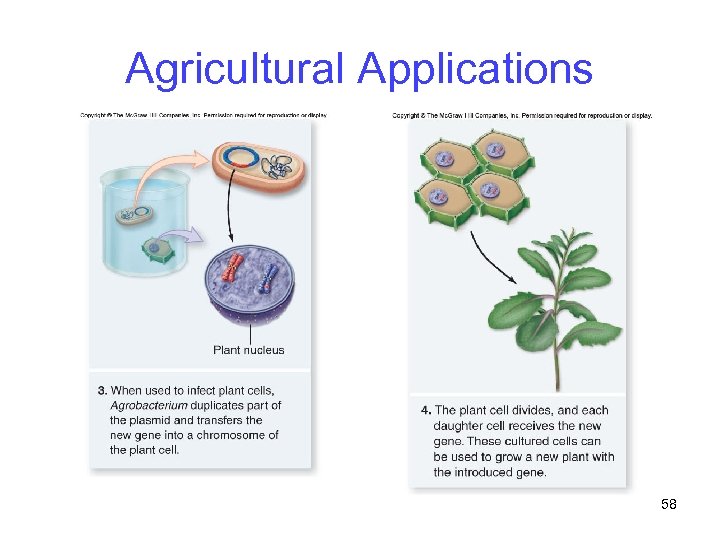
Agricultural Applications 58

Agricultural Applications Gene guns -Uses bombardment with tiny gold particles coated with DNA -Possible for any species -However, the copy number of inserted genes cannot be controlled 59

Agricultural Applications Herbicide resistance -Broadleaf plants have been engineered to be resistant to the herbicide glyphosate -This allows for no-till planting 60
Agricultural Applications Pest resistance -Insecticidal proteins have been transferred into crop plants to make them pest-resistant -Bt toxin from Bacillus thuringiensis Golden rice -Rice that has been genetically modified to produce b-carotene (provitamin A) -Converted in the body to vitamin A 61
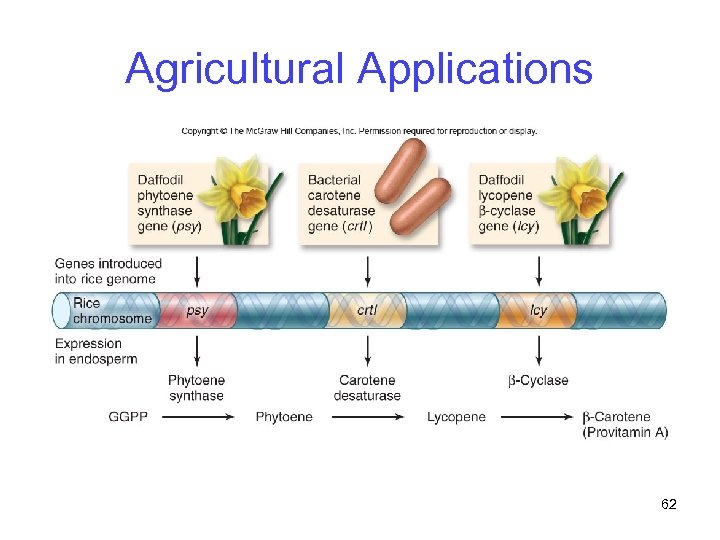
Agricultural Applications 62

Agricultural Applications Adoption of genetically modified (GM) crops has been resisted in some areas because of questions about: -Crop safety for human consumption -Movement of genes into wild relatives -Loss of biodiversity 63
Agricultural Applications Biopharming -Transgenic plants are used to produce pharmaceuticals -Human serum albumin -Recombinant subunit vaccines -Against Norwalk and rabies viruses -Recombinant monoclonal antibodies -Against tooth decay-causing bacteria 64

Agricultural Applications Transgenic animal technology has not been as successful as that in plants -One interesting example is the Enviro. Pig -Engineered to carry the gene for the enzyme phytase -Breaks down phosphorus in feed -Reduces excretion of harmful phosphates in the environment 65

Agricultural Applications 66
5ec6efe7feef7c0235880acf7b0b5583.ppt