856f5f50114e3001e61788d2c32d0a11.ppt
- Количество слайдов: 11

Biotech to Big Pharma Personal Experience Heather Davis September 27, 2011 Ottawa, Canada 1
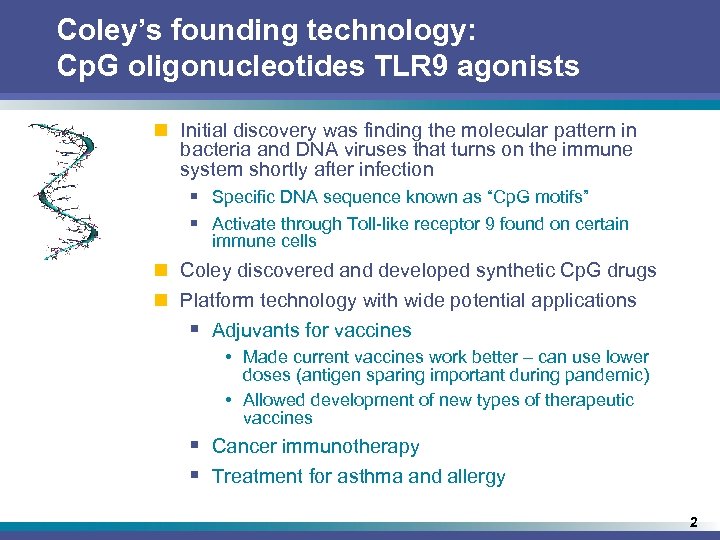
Coley’s founding technology: Cp. G oligonucleotides TLR 9 agonists n Initial discovery was finding the molecular pattern in bacteria and DNA viruses that turns on the immune system shortly after infection § Specific DNA sequence known as “Cp. G motifs” § Activate through Toll-like receptor 9 found on certain immune cells n Coley discovered and developed synthetic Cp. G drugs n Platform technology with wide potential applications § Adjuvants for vaccines • Made current vaccines work better – can use lower doses (antigen sparing important during pandemic) • Allowed development of new types of therapeutic vaccines § Cancer immunotherapy § Treatment for asthma and allergy 2
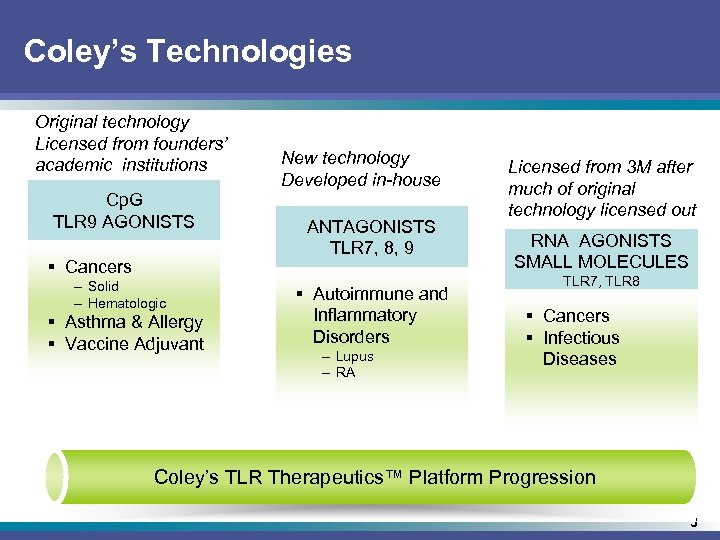
Coley’s Technologies Original technology Licensed from founders’ academic institutions Cp. G TLR 9 AGONISTS § Cancers – Solid – Hematologic § Asthma & Allergy § Vaccine Adjuvant New technology Developed in-house ANTAGONISTS TLR 7, 8, 9 § Autoimmune and Inflammatory Disorders – Lupus – RA Licensed from 3 M after much of original technology licensed out RNA AGONISTS SMALL MOLECULES TLR 7, TLR 8 § Cancers § Infectious Diseases Coley’s TLR Therapeutics™ Platform Progression 3
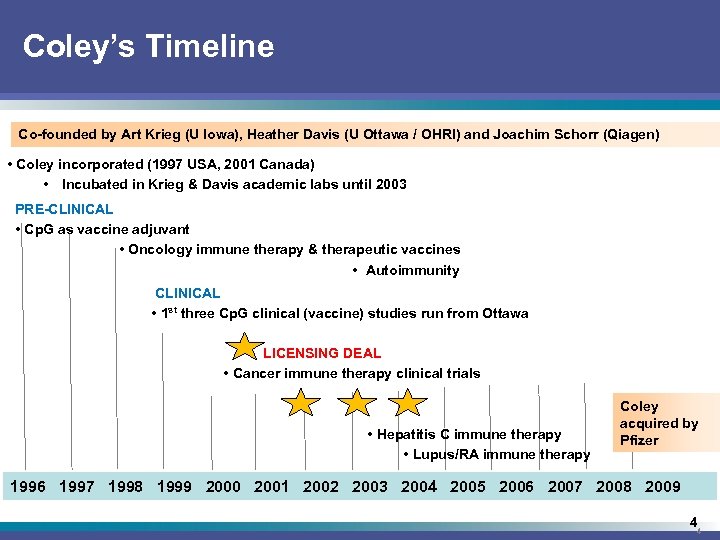
Coley’s Timeline Co-founded by Art Krieg (U Iowa), Heather Davis (U Ottawa / OHRI) and Joachim Schorr (Qiagen) • Coley incorporated (1997 USA, 2001 Canada) • Incubated in Krieg & Davis academic labs until 2003 PRE-CLINICAL • Cp. G as vaccine adjuvant • Oncology immune therapy & therapeutic vaccines • Autoimmunity CLINICAL • 1 st three Cp. G clinical (vaccine) studies run from Ottawa LICENSING DEAL • Cancer immune therapy clinical trials • Hepatitis C immune therapy • Lupus/RA immune therapy Coley acquired by Pfizer 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 4 4

Licensing Deals & Outcomes PARTNER GSK Novartis Merck Other Sanofi aventis Pfizer Indication Total Deal Milestones Received by 2007 ID Vaccines $62 M Took to Phase 1 – no clinical activity since Cancer Vaccines In Phase 3 testing $44 M Vaccines $35 M Took to Phase 1 – cancelled license Vaccines No clinical testing $33 M $11. 5 M $9 M $1. 8 M $4 M Other vaccines $21 Emergent – completed $35 M 1, ongoing activities. M Phase Asthma, allergic 1 – cancelled license Took to Phase $265 M rhinitis $14 M Cancer therapy Phase 3 trial failed $65 M $515 M 5
How to succeed in biotech n Founder characteristics n Secure financing n Recruit senior management n Valuable technology, protected by IP n Luck & timing 6

Founder characteristics n Hard working, driven n Adaptable § Able to wear many hats § Successful transition from academia to corporate world requires thinking differently n Hard working 7

Securing financing n Coley had 1 year funding from founder n Initial venture funding was Swiss / German investors who had previously invested in corporate founder n Subsequent rounds introduced US investors n No Canadian VC’s ever participated § Too late § Expected too much control for their investment 8

Recruit senior management n Founders rarely make good senior managers n Coley benefitted for 3 years by using senior management of another company n Coley later hired own management team § Huge advantage having head office in Boston area • Candidates with senior drug development experience are relatively rare in Canad § Critical hires since each represents a single point of failure § Drug development complex and only learned through experience • Senior executives coming from Big Pharma sometimes couldn’t scale down to biotech appropriate activities 9

Valuable technology n Technology must actually work § Positive clinical data trumps all § Selling technology on mouse data possible, but typically brings low value or back-loaded deals n Platform technologies have greater chance to succeed n Need to know when to let go § Unrealistic for biotech company to expect to develop pharmaceutical product to market n IP protection critical § Needs to be broad in scope and geography § IP protection costs escalate rapidly after provisional filing – difficult for academic unit to sustain until alternate financing secured 10
Wild cards n. Luck n. Timing 11
856f5f50114e3001e61788d2c32d0a11.ppt