d81da2a3ee89ed083a3ead5807ed203d.ppt
- Количество слайдов: 60

Biosurveillance and Bioterrorism: Ronald M. Atlas President American Society for Microbiology Graduate Dean, Professor of Biology and Professor of Public Health, and Co-Director Center for Deterrence of Biowarfare and Bioterrorism University of Louisville, Louisville KY

The Threat of Bioterrorism Before September 11 th--Predictions of Bioterrorism We were. . . “ at the brink of a new age—what some experts call catastrophic terrorism. . . I do not believe it is a question of whether a lone terrorist or terrorist group will use infectious disease agents to kill unsuspecting citizens; I’m convinced it’s really just a question of when and where. ” Michael Osterholm, Former State Epidemiologist for Minnesota After September 11 th--Reality of Bioterrorism Attack with Anthrax through the mail Fear grips the Nation Scientists become suspects Science is needed

Anthrax Attack 2001 Unknowns Who sent the letters? l l Unknown--individual yet to be apprehended Believed to be from US--sent by someone with access to US biodefense program information Where were the letters sent from l Certainly NJ--initially thought to be Trenton but now thought that it may have been Princeton based upon detection of spores in mailbox Where did the anthrax come from l l Ames strain originated in Texas--never in Iowa as originally thought Forensics indicate grown in last 2 years and clearly same as the strain at USAMRIID

Anthrax Attack 2001 Unknowns How many spores are required to cause inhalational anthrax l l Originally thought to be 8000 -10000 spores based upon animal studies Fatal Connecticut case of 94 year old womain indicates far fewer spores--means that secondary contamination of mail can present serious health risk Best prophylactic treatment l l Vaccine for military Doxycycline or Cirpofloxacin for those exposed-time period uncertain

Anthrax Attack 2001 Unknowns Appropriate method for safeguarding the mail l l Irradiation for high risk mail--can cause toxic thermal decomposition products Gloves reduce risk of cutaneous anthrax for mail handlers How to communicate to the public l l l Need to communicate uncertainties Need to communicate real risk levels Need to offer authoritative advice even when there is uncertainty--problem in DC with postal workers where CDC and HHS said what you do is up to you-we don’t know if you should be vaccinated or how long you should continue taking antibiotics

What is the next threat Impossible to tell which leaves us trying to defend against many possible biothreat agents Greatest risk placed on agents of mass destruction l l Anthrax, smallpox, tularemia, plague, botulinum toxin, hemorrhagic fevers Aerosol dissemination Is smallpox really the greatest threat? l l l Do North Korea, Iran, and Iraq have smallpox? Are the Russian stocks secure? Should Russia and US eliminate remaining stocks? If smallpox is the greatest threat because it has been eradicated and vaccination was stopped, should we cease efforts to eliminate other diseases like measles and polio?
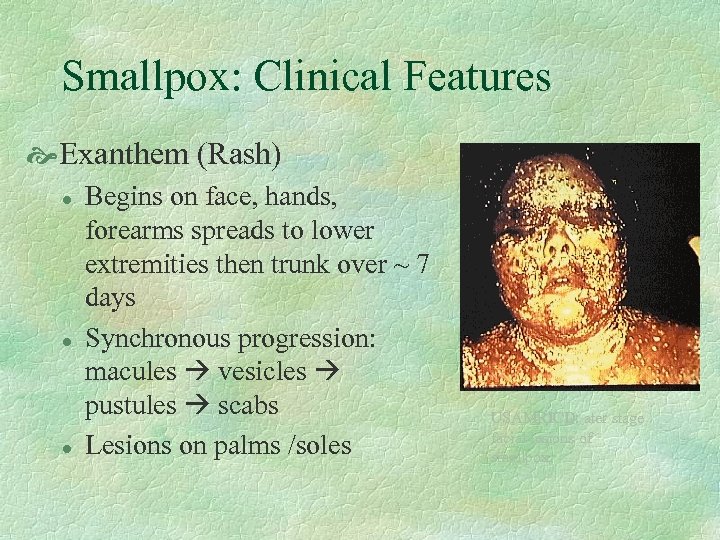
Smallpox: Clinical Features Exanthem (Rash) l l l Begins on face, hands, forearms spreads to lower extremities then trunk over ~ 7 days Synchronous progression: macules vesicles pustules scabs Lesions on palms /soles USAMRICD: ater stage facial lesions of smallpox
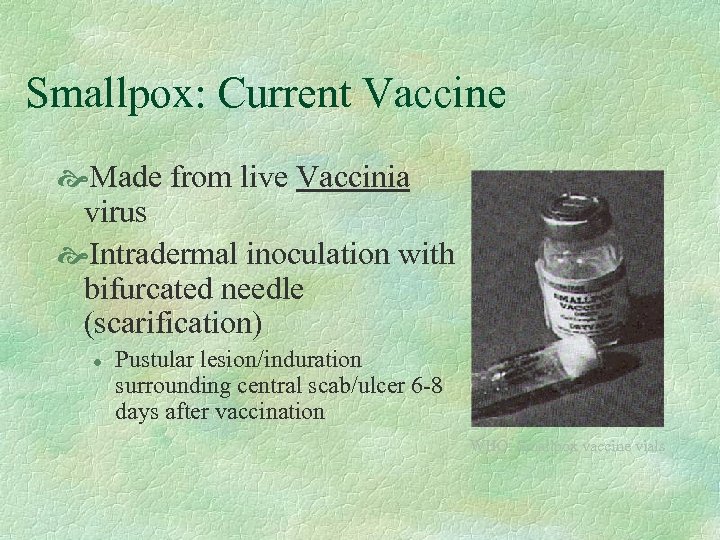
Smallpox: Current Vaccine Made from live Vaccinia virus Intradermal inoculation with bifurcated needle (scarification) l Pustular lesion/induration surrounding central scab/ulcer 6 -8 days after vaccination WHO: Smallpox vaccine vials
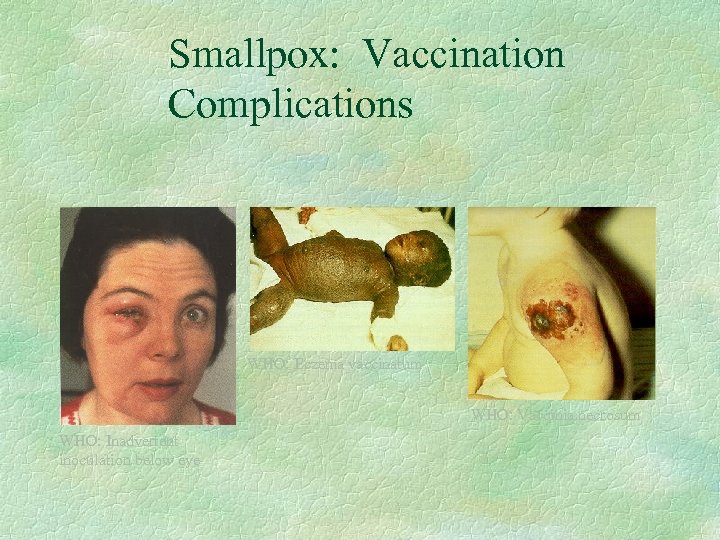
Smallpox: Vaccination Complications WHO: Eczema vaccinatum WHO: Vaccinia necrosum WHO: Inadvertent inoculation below eye

Smallpox Issues Catastrophic public health consequences l l l Mass casualties which overwhelm medical systems High morbidity and mortality Contagious Vaccination Cost-benefit analysis uncertain l 400 -1000 deaths likely in US from vaccination Quarantine l

How should we respond? Build detectors and sensors but there is a problem with sensitivity--must be overly sensitive to avoid missing an attack but that leads to false alarms and panic Build public health response capacity Stockpile drugs and vaccines but what to place in the stockpile and how to deploy remains an issue What should we do about smallpox vaccination? l l l Given high rate of adverse reactions, should we institute mandatory vaccination--estimates are that it would result in 4001, 000 deaths in US--at what point is the threat high enough? How can we ensure efficacy of a new safer vaccine? Current strategy is to produce enough vaccine within a year for all Americans--but will ring vaccination work after an attack? Current strategy is now based upon “mass vaccination”

Need more Research NIAID research plan l l Basic Research into microbes with bioterrorism potential, and the specific and non-specific host defense mechanisms against these agents Applied/Translational Research with predetermined milestones and the ultimate production of new/improved diagnostics, vaccines, and therapies NIH Research Priorities l l l l Microbial biology Host factors Genomics Therapeutics Vaccines Diagnostics Expanded research resources (BL 3, BL 4)

NIH Bioterrorism Research Funding TOTAL 2001 TOTAL 2002 TOTAL 2003 $25 M $275 M $1, 748 M Basic research and development $440. 6 M Drug/vaccine discovery and development $591. 9 M Clinical research $194. 3 M Research facilities intramural $371. 1 M Research facilities extramural $150. 0 M TOTAL $1, 747. 9 M

National Academies of Science: Research needs to combat terrorism Restructure government organizational structure Develop NIH/Industry translational research partnerships Revamp FDA licensing process Establish Agricultural CDC Develop Vaccines and broad spectrum antimicrobics Investigate Microbial forensics Study Microbial Pathogenesis
CDC Approach Increase funding to States for Public Health l Increase education and response capability Enhance surveillance l l Build Health Alert Network Build Laboratory response network

Clinical Laboratories as Sentinels for Bioterrorism ER’s and their labs are key sentinels Laboratory personnel require training l l l Methods to rule out non-BT agents Mechanism to forward the balance Safety for laboratory personnel Variability in states’ training Uniform procedures ideal

LRNB Level A Labs (protocols available for anthrax at CDC and ASM web sites) Initial responsibility for the early detection of intentional dissemination of biological agents Public health and hospital laboratories with low-level biosafety facilities Will rule out organisms rather than actually identify biological weapons Use clinical data and standard microbiological tests to decide which specimens and isolates should be forwarded to higher level biocontainment labs Staff trained in the safe collection, packaging, labeling, and shipping of samples that might contain dangerous pathogens to the next level laboratories in the network

LRNB Level B Labs Core capacity for agent isolation and presumptive testing of suspect specimens State and local public health agency labs Test for specific agents Forward organisms or specimens to higher biocontainment labs Minimize false positives Protect Level C labs from overload Will maintain capacity to perform confirmatory testing and characterize drug susceptibility

LRNB Level C Labs Advanced capacity for rapid identification Located at state health agencies, academic research centers, or federal facilities, Perform advanced and specialized testing Capacity to perform toxicity testing Employ advanced diagnostic technologies Participate in the evaluation of new tests and reagents Determine assays to be transferred to Level B

LRNB Level D Labs Highest level containment and diagnosis of rare and dangerous agents Specialized federal laboratories with unique experience in diagnosis of rare diseases (e. g. smallpox and Ebola) Develop or evaluate new tests/methods Maintains a strain bank of biological agents Maintain the highest biocontainment facilities Conduct all tests performed in Level A, B, and C labs, plus confirmatory testing and characterization Capacity to detect genetically engineered agents

Medical Preparedness Efforts to enhance medical preparedness towards a bioterrorist attack: s s developing new vaccines & medicinals stockpiling of existing pharmaceuticals Local and national response plans are being developed to cope with the potentially devastating impact of a bioterrorist attack.

National Health Response Network Drugs are stockpiled at various locations for dispatch to locations where bioterrorism events may occur Ciprofloxacin in stockpiles for protection against anthrax Doses of available smallpox vaccine being increased System tested in simulations--some difficulties in local distribution

CDC Biological Threat Categories The Centers for Disease Control and Prevention (CDC) has divided biological agents that are the critical biothreat agents into categories based upon their risks for causing mass casualties in the event of a bioterrorist attack.

CDC Biological Threat Category A The highest priority agents that pose a risk to national security Easily disseminated or transmitted person-toperson Cause high mortality Potential for major public health impact Might cause public panic & social disruption Require public health preparedness

CDC Biological Threat Category A Agents l l l l l Variola major (smallpox) Bacillus anthracis (anthrax) Yersinia pestis (plague) Clostridium botulinum toxin (botulism) Francisella tularensis (tularaemia) Filoviruses Ebola hemorrhagic fever Marburg hemorrhagic fever Lassa (Lassa fever) Junin (Argentine hemorrhagic fever)

CDC Biological Threat Category B l Moderately easy to disseminate l Cause moderate morbidity and low mortality l l Require specific enhancements of CDC’s diagnostic capacity Enhanced disease surveillance

CDC Biological Threat Category B Agents l Coxiella burnetti (Q fever) l Brucella species (brucellosis) l Burkholderia mallei (glanders) l Alphaviruses l Venezuelan encephalomyelitis l Eastern and western equine encephalomyelitis l Ricin toxin from Ricinus communis (castor beans) l Epsilon toxin of Clostridium perfringens l Staphylococcus enterotoxin B

CDC Biological Threat Category B Agents (Subset List) Food- or water-borne pathogens include but are not limited to: l Salmonella species l Shigella dysenteriae l Escherichia coli O 157: H 7 l Vibrio cholerae l Cryptosporidium parvum.

Food and Water as Targets Water difficult l Already assume water contaminated and we treat and test regularly Food easy l Salmonella attack in Dalles Oregon • Contamination of salad bars • Over 750 illnesses • Political election the target

CDC Biological Threat Category C Includes emerging pathogens that could be engineered for mass dissemination in the future due to: l Availability l Ease of production and dissemination l Potential for high morbidity and mortality l Major health impact Preparedness for List C agents requires ongoing research to improve disease detection, diagnosis, treatment, and prevention.

CDC Biological Threat Category C Agents l l l Nipah virus Hantaviruses Tick-borne hemorrhagic fever viruses Tick-borne encephalitis viruses Yellow fever Multidrug-resistant tuberculosis

Suggested Policy Mechanisms to Reduce Future Biological Weapons Threats Tighten restrictions on access to dangerous pathogens Impose restrictions on the conduct and publication of “contentious research, ” i. e. fundamental biological or biomedical investigations that produce organisms or knowledge that could have immediate weapons implications Restrict access and dissemination of “relevant information” Controlling Biological Warfare Threats: Resolving Potential Tensions Among the Research Community, Industry, and the National Security Community. Gerald L. Epstein. Critical Reviews in Microbiology, 27 (2001)

Restrictions on Access to Select Agents Possession of potentially dangerous biological agents should be regulated more tightly l Some individuals should not be given access to dangerous pathogens Physical security at institutions that maintain cultures of potentially dangerous biological agents needs to be reexamined to provide not only legal but also physical barriers to help prevent unauthorized individuals from obtaining such agents l Are locks enough? l Should armed guards secure laboratories with select agents?

CDC Laboratory Registration/ Select Agent Transfer Program These regulations place shipping and handling requirements on laboratory facilities that transfer or receive select agents capable of causing substantial harm to human health. They are designed to ensure that select agents are not shipped to parties who are not equipped to handle them appropriately or who lack proper authorization for their requests. Currently regulates shipment of 36 select agents and their disease related genes Requires adherence to CDC biosafety manual In effect since April 1997

USA Patriot Act Imposes restrictions on possession of select agents l l l Restricts aliens from countries designated as supporting terrorism from possessing select agents within the United States Restricts individuals who are not permitted to purchase handguns, e. g. some individuals with a history of mental illness or a criminal record, from possessing select agents No provision for exemptions under any circumstances Does not require registration for possession of select agents Requires that requests by law enforcement be kept confidential so as not to alert would-be terrorists. In effect since October 26, 2001

Definition of a Restricted Person · is under indictment for a crime punishable by imprisonment for a term exceeding one year; · has been convicted in any court of a crime punishable by imprisonment for a term exceeding one year; · is a fugitive from justice; · is an unlawful user of any controlled substance; · is an alien illegally or unlawfully in the US; · has been adjudicated as a mental defective or has been committed to any mental institution; · is an alien who is a national of a country as to which the Secretary of State has made a determination (that remains in effect) that such country has repeatedly provided support for acts of international terrorism; or · has been discharged from the Armed Services of the United States under dishonorable conditions.

Patriot Act and Legitimate Research The USA Patriot Act also makes it an offense for a person to knowingly possess any biological agent, toxin or delivery system of a type or in a quantity that, under the circumstances, is not reasonably justified by prophylactic, protective, bona fide research or other peaceful purpose. Senator Patrick Leahy warned during passage of the Patriot Act, this provision could have unanticipated ramifications depending upon how one defined “bona fide” or “reasonably justified. ” U Conn case raises questions

Regulation of Possession of Select Agents On December 12, 2001 H. R. 3448, the Public Health Security and Bioterrorism Response Act of 2001, suported by Representative W. J. "Billy" Tauzin (R-LA), was passed by the House of Representatives, under suspension of the rules. On December 20, 2001 the Senate passed by unanimous consent the Bioterrorism Preparedness Act, S. 1765, which is supported by Senators William Frist (R-TN) and Edward Kennedy (D-MA). On June 12, 2002 signed into law the Bioterrorism Preparedness Act

Regulation of Possession of Select Agents The provisions of the bioterrorism preparedness act: l l l Requires registration for possession (first step notification by Sept. 10, 2002) tracks the acquisition, transfer and possession of certain biological agents and toxins requires safeguards and security regulations to be followed collects information for law enforcement; establishes a process for alerting authorities about unauthorized attempts to acquire select agents

Biopreparedness Act · Requires registered facilities to submit the names of individuals with access to select agents to the Attorney General, who will use criminal, immigration, and national security databases available to the federal government to ensure that such individuals identified as restricted are denied access to select agents and toxins · Regulations remain to be issued · How will we notify the Attorney General? · What specific information will we need to submit? · What will be the timeframe for notification? · Will current research have to stop? · Exempts medical uses but not necessarily investigational products

National Security and Openness of Scientific Research Are new mechanisms needed to govern scientific research so as to lessen the probability of the development of advanced biological weapons? l If so what should be done? The research and national security communities have different objectives, cultures, and norms, and are likely to weigh the costs and benefits of proposed policy measures differently l What should the National Academy and scientific societies like the American Society for Microbiology do to foster the critical dialog among these communities? Controlling Biological Warfare Threats: Resolving Potential Tensions Among the Research Community, Industry, and the National Security Community. Gerald L. Epstein. Critical Reviews in Microbiology, 27 (2001)

The potential for misuse of scientific information is pitting national security concerns against the traditional openness of biomedical research. The anthrax attacks that followed the horror of September 11 have made scientists and physicians suspects as well as saviors. The fear that information from that research may fall into the wrong hands is causing great anxiety within the scientific community and uncertainties among the public and policy makers as to how to balance national security with traditional openness of science.

Concern Over Scientific Information ASM made information available for the education of the scientific community and to inform the public l “The principle right now is one of openness in science--if someone wants to publish a legitimate research paper we’re not going to be the censor. ” Ronald Atlas-President elect ASM Position of openness of science draws scorn “We have to get away from the ethos that knowledge is good, knowledge should be publicly available, that information will liberate us. . . Information will kill us in the techno-terrorist age, and I think it's nuts to put that stuff on Web sites. ” Arthur Caplan--U. Penn. Bioethicist Eric Lichtblau Response to Terror: Rising Fears That We Do Know Can Hurt Us, Los Angeles Times November 18, 2001

Even at the height of the Cold War era it was recognized that “greater security would be achieved by the open pursuit of scientific knowledge than by curtailing the free exchange of scientific information. ” l National Academy of Sciences 1982 Corson Report “It is the policy of this Administration that, to the maximum extent possible, the products of fundamental research remain unrestricted. It is also the policy of this Administration that, where the national security requires control, the mechanism for control of information generated during federally-funded fundamental research in science, technology and engineering at colleges, universities and laboratories is classification. ” l National Security Decision Directive #189. National Policy on the Transfer of Scientific, Technical and Engineering Information. September 21, 1985.

ASM Opinion on Secrecy “Terrorism feeds on fear, and fear feeds on ignorance. The best defense against anthrax or any other infectious disease is information – information in a form that can be used by scientists and by members of the public to guide rational and effective actions to ensure public safety. Placing major new barriers in the path of the flow of information between scientists and the public is more likely to contribute to terrorism than to prevent it. ” Abigail Salyers, Past President American Society for Microbiology

The US is trying to balance openness with national security concerns but is struggling with how to achieve the right balance. “The key to maintaining U. S. technological preeminence is to encourage open and collaborative basic research. The linkage between the free exchange of ideas and scientific innovation, prosperity, and U. S. national security is undeniable…the policy on the transfer of scientific, technical, and engineering information set forth in NSDD 189 shall remain in effect, and we will ensure that this policy is followed. ” l Condoleezza Rice, Special Assistant to President Bush for National Security Affairs affirmed the importance of openness of fundamental research in a letter of November, 2001

Federal departments and agencies ordered to take steps to protect information regarding weapons of mass destruction as well as other information that could compromise national security l Memorandum from Andrew H. Card, Jr. , Assistant to the President and Chief of Staff, for the heads of executive departments. March 19, 2002. Departments ordered to take steps to protect sensitive but unclassified information that might reasonably be expected to assist in the development or use of weapons of mass destruction. l Memorandum from Laura Kimberly, Acting Director Information Security Oversight Office, National Archives and Records Administration, and Richard Huff and Daniel Metcalfe, Codirectors of Information and Privacy, Department of Justice. March 19, 2002.
Concern about sensitive biological information and the threat of recombinant DNA technology was sparked by the publication of experiments in which IL-4 genes were inserted into mousepox viruses, resulting in near total suppression of the immune response. l Jackson RJ. et al. 2001. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J. Virology 75: 1205 -10. The IL-4 mousepox study was done in Australia, beyond the reach of US government regulations. It was, however, potentially subject to restraint, raising the question of ethical responsibility within the scientific community.

Report demonstrating that artificially synthesized polio virus genome produced infective pathogenic virus l Cello, J. , A. V. Paul, and E. Wimmer. 2002. Chemical synthesis of poliovirus c. DNA: Generation of infectious virus in the absence of natural template. Science express. July 2002/ Page 1/ 10. 1126/science. 1072266. Executive Branch called upon to “…examine all policies, including national security directives, relevant to the classification or publication of federally funded research to ensure that, although the free exchange of information is encouraged, information that could be useful in the development of chemical, biological, or nuclear weapons is not made accessible to terrorists or countries of proliferation concern. ” l House Resolution 514. Introduced by Congressman Dave Weldon

“And this we do also: we have consultations, which of the inventions and experiences which we have discovered shall be published, and which not; and take all an oath of secrecy for the concealing of those which we think fit to keep secret; though some of those we do reveal sometime to the State, and some not. ” l Sir Francis Bacon 1626. The New Atlantis “…our Nation’s progress depends on the free flow of information. Nevertheless, throughout our history, the national interest has required that certain information be maintained in confidence in order to protect our citizens, our democratic institutions, and our participation within the community of nations. Protecting information critical to our Nation’s security remains a priority. ” l President Clinton Executive order 12958. Classified National Security Information. April 17, 1995.

Problem is that there is no clear definition of what constitutes "sensitive information" in the Life Sciences. “the concept [of sensitive but unclassified information] is so squishy [ill defined] and fraught with danger that the only sensible thing for the research community to do is to demand [classification]. ” l William Wulf, President of the National Academy of Engineering, Leo, A. 2002. Science and secrets. Technology Review, June 20. Many academic institutions, like MIT, reject classified research. A recent report recommended that MIT ban classified research on its main campus to protect its educational mission although faculty could conduct such research on MITs Lincoln campus. l In the Public Interest. Report of the ad hoc faculty committee on access to and disclosure of scientific information. Massachusetts Institute of Technology, June 12, 2002

US decisions have international ramifications “National security requires scientific excellence. Scientific excellence requires openness. Openness is inherently international. ” l Neil Lane. Scientific Advisor to President Clinton If government moves toward restraining the flow of information across national boundaries there will be an inevitable clash with the academic research community that is increasingly seeking international collaborations and partnerships. l Skolnikoff, E. B. 2002. Protecting university research amid national -security fears. Chronicle of Higher Education May 10. Given the potential dual use nature of biodefense activities, silence could raise suspicions of US research intent and lead to illicit proliferation activities by others.

Thus we are left with a series of perplexing questions that are at the heart of the debate between national security and the openness of biomedical research and publication. l l l Should scientists be constrained regarding which questions they can ask? Should journals reject papers containing potentially sensitive information? Should secrecy clearances be required for attendees at biodefense research meetings? Should there be mandatory government review before publishing information, even from unclassified studies and those not funded by government? And, perhaps the most difficult questions of all, exactly what is sensitive information, and who is empowered to decide what is potentially dangerous?

It will not be simple to achieve consensus between the national security and scientific communities on questions like: l l l Should more research be declared classified? Should there be review boards to consider the national security implications of all publications and presentations of research results? Should we impose restrictions on the conduct and publication of “contentious research, ” i. e. , fundamental biological or biomedical investigations that produce organisms or knowledge that could have immediate weapons implications? Must we restrict access and dissemination of such information? How can one define what is dangerous and how can we design a system that contains that danger while allowing legitimate biomedical research to proceed in a manner acceptable to society?

The closest experience that the biological research community has had with such contentious issues occurred in the 1970 s with the advent of recombinant DNA technology. Then, the scientific community paused to examine the consequences of the newly discovered power to alter genetics and gathered in public at Asilomar. “The issue is not whether areas of ‘forbidden knowledge’ should be defined in which research is prohibited. Rather, it is about defining boundaries for ‘constrained knowledge’ whereby freedom of research enquiry is not impeded, but access to certain forms of research data would be limited to those with bona fide credentials… Asilomar is a model for how science can independently regulate its own inquiries. This ethos must now be reawakened. ” l Poste, G. 2002. Biotechnology and terrorism. Prospect Magazine.

But, can we use our experience with the debate over recombinant DNA technology as a model to confront concerns about the potential misuse of biotechnology? Can an open debate be held regarding the concerns about bioterrorism and recombinant DNA technology without compromising national security interests? The controversy is likely to continue until we have a national debate and reach consensus on how to balance traditional openness of science with national security in the new age of bioterrorism. We must recognize that the advancement of the science of recombinant DNA technology and the complex national security issues raised by the threat of bioterrorism make the task more complex than the problems faced at Asilomar.

The scientific community should come together to establish the norms for information communication in the age of bioterrorism. It is up to us to define the standards and to establish the framework to ensure that critical information is withheld from terrorists while permitting the continued advancement of biomedical research and the protection of public health. This process needs to begin by defining what is sensitive and then move to considering how best to protect that information—going beyond classification to ethically responsible citizenship. We cannot do this alone. The scientific and national security communities must establish a dialog and the outcome must be acceptable to the public. And like Asilomar, the public must be part of the process throughout.

ASM Resolution on Bioethics The Council Policy Committee of the American Society for Microbiology affirms the longstanding position of the Society that microbiologists will work for the proper and beneficent application of science and will call to the attention of the public or the appropriate authorities misuses of microbiology or of information derived from microbiology. ASM members are obligated to discourage any use of microbiology contrary to the welfare of humankind, including the use of microbes as biological weapons. Bioterrorism violates the fundamental principles expressed in the Code of Ethics of the Society and is abhorrent to the ASM and its members.

ASM Publication Board Statement “The ASM recognizes that there are valid concerns regarding the publication of information in scientific journals that could be put to inappropriate use. The ASM hopes to participate in the public debate on these issues. Until a national consensus is reached, the rare manuscript that might raise such issues will be reviewed by the ASM Publications Board prior to the Society proceeding to publication. " The editors of the ASM journals are trying to be responsible stewards of scientific information and communication by carefully balancing national security with the value of advancing science for the benefit of humanity. This is a policy of responsible citizenship--not one of censorship

Concluding Remarks Infectious diseases and bioterrorism present major threats to national and global security By enhancing global epidemiological surveillance systems, by developing advanced diagnostics, and by discovering new and better vaccines, antibacterials and antivirals we will have the tools needed to comba both natural outbreaks of infectious disease and intentional acts of bioterrorism We need to support increased investment and research efforts aimed at eliminating the threats of bioterrorism and of epidemics that can strike anywhere in the world
d81da2a3ee89ed083a3ead5807ed203d.ppt