Biopolymeric systems of targeted delivery of taxanes for























1_main_presentation.ppt
- Размер: 3.8 Mегабайта
- Количество слайдов: 24
Описание презентации Biopolymeric systems of targeted delivery of taxanes for по слайдам
 Biopolymeric systems of targeted delivery of taxanes for anticancer therapy. Saint-Petersburg State Chemical-Pharmaceutical Academy Ph. D student Golyshev A. A. Supervisor Ph. D, Skorik Y. A.
Biopolymeric systems of targeted delivery of taxanes for anticancer therapy. Saint-Petersburg State Chemical-Pharmaceutical Academy Ph. D student Golyshev A. A. Supervisor Ph. D, Skorik Y. A.
 My supervisor and me Anton Golyshev (27. 12. 1989) MS in Chemistry 2012 Chemistry Department of the Saint-Petersburg State University (Organic Chemistry) School : Academic gymnasium of Saint-Petersburg state university anton_golishev@mail. ru Yury Skorik ( 11. 10. 1973) Ph. D in Chemistry 1998 Urals State University (Ekaterinburg, Russian Federation) MS in Chemistry 1995 Urals State University (Ekaterinburg, Russian Federation) yury _ skorik @ mail. ru(www. skorik. pro)
My supervisor and me Anton Golyshev (27. 12. 1989) MS in Chemistry 2012 Chemistry Department of the Saint-Petersburg State University (Organic Chemistry) School : Academic gymnasium of Saint-Petersburg state university anton_golishev@mail. ru Yury Skorik ( 11. 10. 1973) Ph. D in Chemistry 1998 Urals State University (Ekaterinburg, Russian Federation) MS in Chemistry 1995 Urals State University (Ekaterinburg, Russian Federation) yury _ skorik @ mail. ru(www. skorik. pro)
 3 My home 6700 km 10 h flight Russian Federation (Russia) India Area 17 mln. km 2 3, 3 mln. km 2 Population 144 mln. 1200 mln. Population density 8. 4/km 2 379. 6/km 2 Towns with more than 1 mln. people
3 My home 6700 km 10 h flight Russian Federation (Russia) India Area 17 mln. km 2 3, 3 mln. km 2 Population 144 mln. 1200 mln. Population density 8. 4/km 2 379. 6/km 2 Towns with more than 1 mln. people
 4 Education systems Russia (from USSR) Russia (modern) India Ph. D student 3 -5 3 -5 University 5 (specialist) 2 (master) 4 (bachelor) School 2 10 10 Kindergarten maximum 5 2 Start minimum 2 years old minimum 3 years old
4 Education systems Russia (from USSR) Russia (modern) India Ph. D student 3 -5 3 -5 University 5 (specialist) 2 (master) 4 (bachelor) School 2 10 10 Kindergarten maximum 5 2 Start minimum 2 years old minimum 3 years old
 5 Academic gymnasium of Saint-Petersburg state university (since 1963). Students 500 AG, SPb. SU Main building of the Saint-Petersburg State University (since 1730) Students 30. 000˃ Staff 13. 000 ˃
5 Academic gymnasium of Saint-Petersburg state university (since 1963). Students 500 AG, SPb. SU Main building of the Saint-Petersburg State University (since 1730) Students 30. 000˃ Staff 13. 000 ˃
 6 Chemical Faculty of SPb. SU Students 2000 Research Areas : Analytical Methods Biomedical Chemistry Chemical sensors Colloid nanosystems Material science, nanotechnology & nanomaterials Organic Synthesis Organometallic & Coordination Chemistry Simulation & Modeling of Nanostructures
6 Chemical Faculty of SPb. SU Students 2000 Research Areas : Analytical Methods Biomedical Chemistry Chemical sensors Colloid nanosystems Material science, nanotechnology & nanomaterials Organic Synthesis Organometallic & Coordination Chemistry Simulation & Modeling of Nanostructures
 7 « Characteristycal chromatographic and electrophoretic steroid hormones profiles as an additional diagnostic criteria of endocrinal pathologies » University diploma work Methods : -HPLS (high performance liquid chromatography) -HPTLC (high performance thin layer chromatography) -capillary electrophoresis
7 « Characteristycal chromatographic and electrophoretic steroid hormones profiles as an additional diagnostic criteria of endocrinal pathologies » University diploma work Methods : -HPLS (high performance liquid chromatography) -HPTLC (high performance thin layer chromatography) -capillary electrophoresis
 Steroid hormones profile of healthy patient 8 Micellar electrokinetic chromatography Steroid hormones profile of a patient with Icing-Cushing disease
Steroid hormones profile of healthy patient 8 Micellar electrokinetic chromatography Steroid hormones profile of a patient with Icing-Cushing disease
 ps – diseased patients ns – healthy patience. MC 1 — MC 2 MC 3 — MC 4 9 Mathematical modeling of samples analysis
ps – diseased patients ns – healthy patience. MC 1 — MC 2 MC 3 — MC 4 9 Mathematical modeling of samples analysis
 10 Ph. D student
10 Ph. D student
 11 Institute of macromolecular compounds (since 1948) Saint-Petersburg State Chemical-Pharmaceuti cal Academy (since 1919) Students
11 Institute of macromolecular compounds (since 1948) Saint-Petersburg State Chemical-Pharmaceuti cal Academy (since 1919) Students
 12 Taxanes Táxus baccáta High anticancer activity Widespread drug Water insoluble High general toxicity. Docetaxel (DTX) : R 1 =-COOC(CH 3 ) 3 ; R 2 =H Paclitaxel (PTX) : R 1 =-COCC 6 H 5 ; R 2 = — COCH
12 Taxanes Táxus baccáta High anticancer activity Widespread drug Water insoluble High general toxicity. Docetaxel (DTX) : R 1 =-COOC(CH 3 ) 3 ; R 2 =H Paclitaxel (PTX) : R 1 =-COCC 6 H 5 ; R 2 = — COCH
 13 Chitosan Crab chitosan : -80% of particles have a size 0, 125 -0, 25 mm -molecular mass 80 k. Da (according to viscosimetry) -degree of deacetylation 93% (according to 1 H-NMR, elemental analysis).
13 Chitosan Crab chitosan : -80% of particles have a size 0, 125 -0, 25 mm -molecular mass 80 k. Da (according to viscosimetry) -degree of deacetylation 93% (according to 1 H-NMR, elemental analysis).
 Noncovalent bonding (self assembled nanoparticles) Synthesis strategy 14 Covalent bonding (conjugates)
Noncovalent bonding (self assembled nanoparticles) Synthesis strategy 14 Covalent bonding (conjugates)
 15 Main synthetic idea For both strategies we should synthesize separately 1) water-soluble chitosan derivatives 2) water-insoluble chitosan derivatives (containing model substitution group for self assembled nanoparticles and conjugated PTX or DTX for conjugates) 3) combine two approaches in one compound
15 Main synthetic idea For both strategies we should synthesize separately 1) water-soluble chitosan derivatives 2) water-insoluble chitosan derivatives (containing model substitution group for self assembled nanoparticles and conjugated PTX or DTX for conjugates) 3) combine two approaches in one compound
 16 Water-soluble compounds -nontoxic (tricarbon acid cycle) -easy to synthesize in different reaction conditions -GC is almost unknown substance succinic anhydride (SA) glutaric anhydride (GA)
16 Water-soluble compounds -nontoxic (tricarbon acid cycle) -easy to synthesize in different reaction conditions -GC is almost unknown substance succinic anhydride (SA) glutaric anhydride (GA)
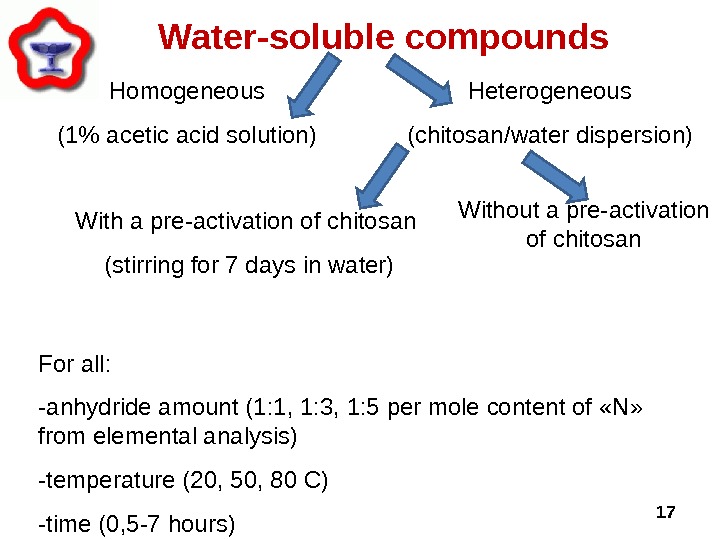
 17 Water-soluble compounds For all : -anhydride amount (1 : 1 , 1: 3 , 1: 5 per mole content of « N » from elemental analysis) -temperature (20, 50, 80 C) -time (0, 5 -7 hours) Heterogeneous (chitosan/water dispersion)Homogeneous (1% acetic acid solution) With a pre-activation of chitosan (stirring for 7 days in water) Without a pre-activation of chitosan
17 Water-soluble compounds For all : -anhydride amount (1 : 1 , 1: 3 , 1: 5 per mole content of « N » from elemental analysis) -temperature (20, 50, 80 C) -time (0, 5 -7 hours) Heterogeneous (chitosan/water dispersion)Homogeneous (1% acetic acid solution) With a pre-activation of chitosan (stirring for 7 days in water) Without a pre-activation of chitosan
 182) DS of chitosan increases with increasing of amount of anhydride in both heterogeneous and homogeneous conditions. 3) Heterogeneous synthesis provides a product with higher DS (about 20% higher) as compared with homogeneous synthesis. But this synthesizes gives not so equally samples then homogeneous synthesizes. 4) DS decreases with increasing of temperature. We suppose that this effect related with different temperature dependence of the reaction of primary interest and of the side reaction (hydrolysis of anhydride). 5) Pre-activation of chitosan was not as effective as expected — the DS is roughly the same in both cases. Results of water-soluble compounds synthesis 1) GC with DS 14 -79% (per all « N » mole content) and SC with DS 11 -72% was synthesized.
182) DS of chitosan increases with increasing of amount of anhydride in both heterogeneous and homogeneous conditions. 3) Heterogeneous synthesis provides a product with higher DS (about 20% higher) as compared with homogeneous synthesis. But this synthesizes gives not so equally samples then homogeneous synthesizes. 4) DS decreases with increasing of temperature. We suppose that this effect related with different temperature dependence of the reaction of primary interest and of the side reaction (hydrolysis of anhydride). 5) Pre-activation of chitosan was not as effective as expected — the DS is roughly the same in both cases. Results of water-soluble compounds synthesis 1) GC with DS 14 -79% (per all « N » mole content) and SC with DS 11 -72% was synthesized.
 19 Water-insoluble compounds -cholesterol is a nontoxic substance -using the same anhydrides
19 Water-insoluble compounds -cholesterol is a nontoxic substance -using the same anhydrides
 20 Water-insoluble compounds For all : -anhydride amount (1 : 1 , 1: 3 , 1: 5 , 1 : 10 mole ratio) -time (NMR measurements 1 -170 hours) -DTX+SA -DTX+GA -PTX+SA -PTX+GA-Cholesterol + SA -Cholesterol + G
20 Water-insoluble compounds For all : -anhydride amount (1 : 1 , 1: 3 , 1: 5 , 1 : 10 mole ratio) -time (NMR measurements 1 -170 hours) -DTX+SA -DTX+GA -PTX+SA -PTX+GA-Cholesterol + SA -Cholesterol + G
 211) NMR-kinetic measurements for all cases 2) Reaction conditions (anhydride amount, time) to achieve 100% substitution for all substances 3) Monosubstitution for DTX+SC and DTX+GC Results of water-insoluble compounds synthesis Docetaxel (DTX) : R 1 =-COOC(CH 3 ) 3 ; R 2 =H Paclitaxel (PTX) : R 1 =-COCC 6 H 5 ; R 2 = — COCH
211) NMR-kinetic measurements for all cases 2) Reaction conditions (anhydride amount, time) to achieve 100% substitution for all substances 3) Monosubstitution for DTX+SC and DTX+GC Results of water-insoluble compounds synthesis Docetaxel (DTX) : R 1 =-COOC(CH 3 ) 3 ; R 2 =H Paclitaxel (PTX) : R 1 =-COCC 6 H 5 ; R 2 = — COCH
 22 Combination of two parts
22 Combination of two parts
 23 — Preparation of carrier (SC/GC nanoparticles), size and zeta potential measurements — Loading of taxanes (PTX/DTX), size and zeta potential measurements — Drug encapsulation, loading and release analysis from nanoparticles — Blood compatibility studies (Hemolysis assay) TASK 2: IN VITRO CELL CULTURE STUDIES ( 3 months ) — In vitro toxicity studies two cancer cell lines — Cellular uptake studies using microscopic techniques — Flowcytometric assays TASK 3: MANUSCRIPT PREPARATION TASK 1: PREPARATION &CHARACTERIZATIONS OF NANOPARTICLES ( 3 months )Work Plan
23 — Preparation of carrier (SC/GC nanoparticles), size and zeta potential measurements — Loading of taxanes (PTX/DTX), size and zeta potential measurements — Drug encapsulation, loading and release analysis from nanoparticles — Blood compatibility studies (Hemolysis assay) TASK 2: IN VITRO CELL CULTURE STUDIES ( 3 months ) — In vitro toxicity studies two cancer cell lines — Cellular uptake studies using microscopic techniques — Flowcytometric assays TASK 3: MANUSCRIPT PREPARATION TASK 1: PREPARATION &CHARACTERIZATIONS OF NANOPARTICLES ( 3 months )Work Plan
 24 Thank you for your attention!
24 Thank you for your attention!

