d6313c7339b2096556f42059bf46e126.ppt
- Количество слайдов: 83
Biomedical technologies for blood cell measurements Introduction to the terminology, types of measurements, capabilities of flow cytometry, uses & applications • Comparison between flow cytometry and fluorescence microscopy • Scatter • Fluorescence • Sensitivity, precision of measurements, statistics, populations • Speed, combinatorial measurements (multiparameter) J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
• • • What can Flow Cytometry Do? Enumerate particles in suspension Determine “biologicals” from “non-biologicals” Separate “live” from “dead” particles Evaluate 105 to 5 x 106 particles/min Measure particle-scatter as well as innate fluorescence or 2 o fluorescence • Sort single particles for subsequent analysis J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 2
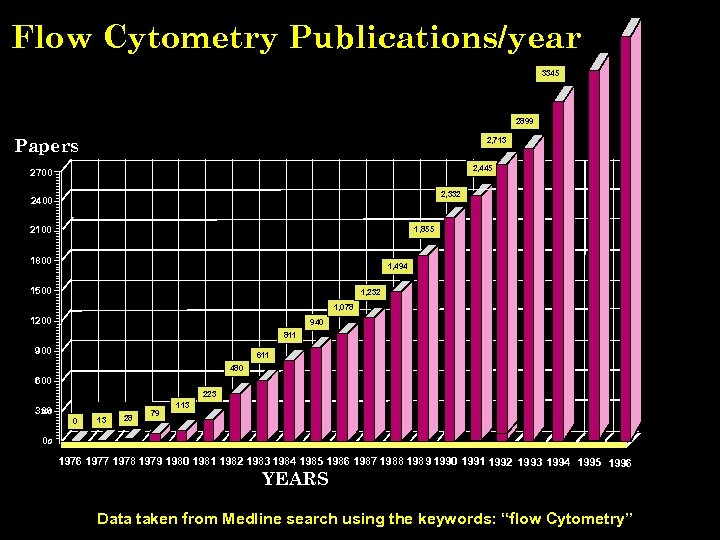
Flow Cytometry Publications/year 3345 2899 Papers 2, 713 2, 445 2700 2, 332 2400 2100 1, 855 1800 1, 494 1500 1, 232 1, 078 1200 940 811 900 611 480 600 223 300 0 13 28 79 113 00 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 YEARS Data taken from Medline search using the keywords: “flow Cytometry” J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 3
Papanicolaou 1941 - originally studies the reproductive system of primates during the estrous cycle and observed changes in cells exfoliated from the female genital tract during the cycle - mixed a series of stains to identify changes he observed - Developed for using quantitative cytology and morphology for the exfoliative cytologic diagnosis of cervical carcinoma in humans - developed sets of critical stains and interpretations J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 4
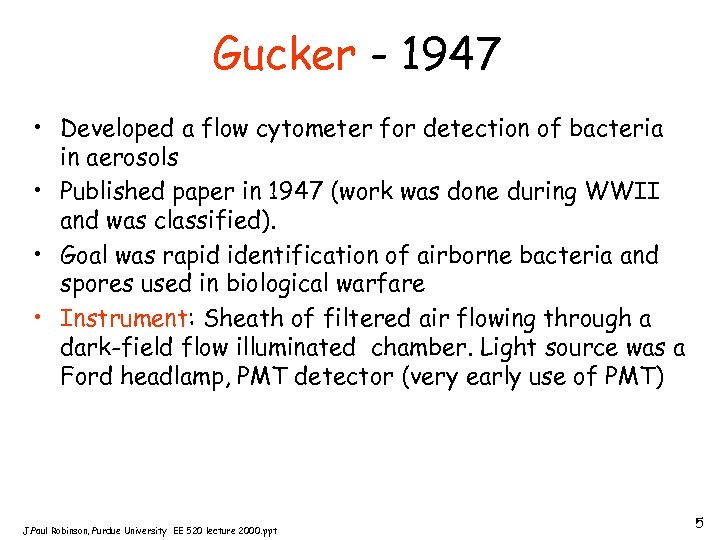
Gucker - 1947 • Developed a flow cytometer for detection of bacteria in aerosols • Published paper in 1947 (work was done during WWII and was classified). • Goal was rapid identification of airborne bacteria and spores used in biological warfare • Instrument: Sheath of filtered air flowing through a dark-field flow illuminated chamber. Light source was a Ford headlamp, PMT detector (very early use of PMT) J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 5
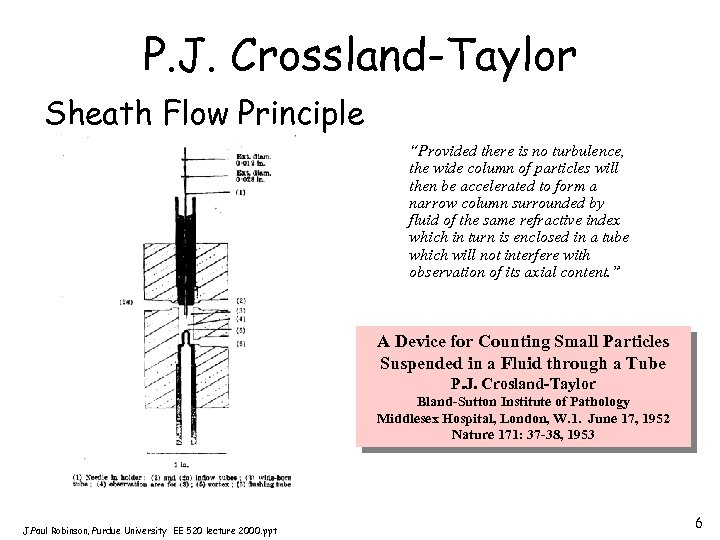
P. J. Crossland-Taylor Sheath Flow Principle “Provided there is no turbulence, the wide column of particles will then be accelerated to form a narrow column surrounded by fluid of the same refractive index which in turn is enclosed in a tube which will not interfere with observation of its axial content. ” A Device for Counting Small Particles Suspended in a Fluid through a Tube P. J. Crosland-Taylor Bland-Sutton Institute of Pathology Middlesex Hospital, London, W. 1. June 17, 1952 Nature 171: 37 -38, 1953 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 6
Wallace Coulter - Coulter orifice - 1956 (as early as 1948) - measured changes in electrical conductance as cells suspended in saline passed through a small orifice • Cells are relatively poor conductors • Blood is a suspension of cells in plasma which is a relatively good conductor • Previously it was known that the cellular fraction of blood could be estimated from the conductance of blood • As the ratio of cells to plasma increases the conductance of blood decreases J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 7
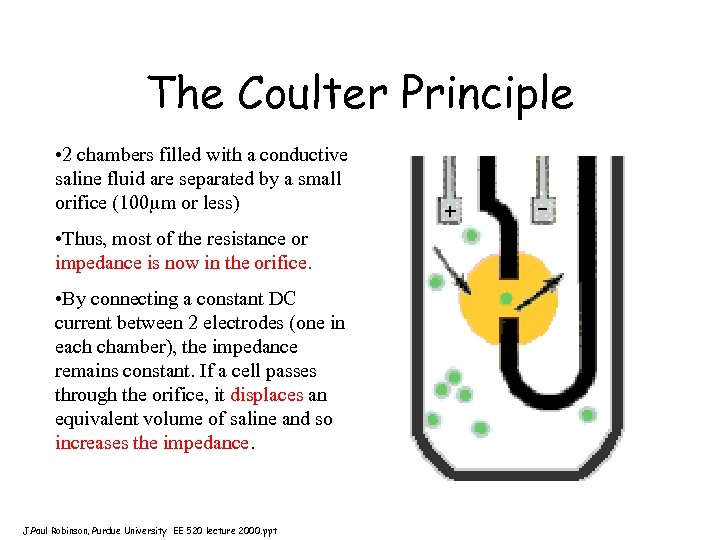
The Coulter Principle • 2 chambers filled with a conductive saline fluid are separated by a small orifice (100 m or less) • Thus, most of the resistance or impedance is now in the orifice. • By connecting a constant DC current between 2 electrodes (one in each chamber), the impedance remains constant. If a cell passes through the orifice, it displaces an equivalent volume of saline and so increases the impedance. J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
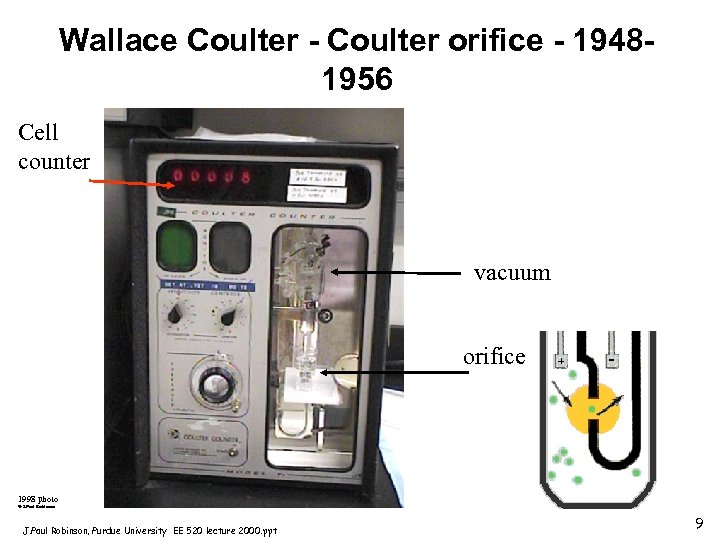
Wallace Coulter - Coulter orifice - 19481956 Cell counter vacuum orifice 1998 photo © J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 9
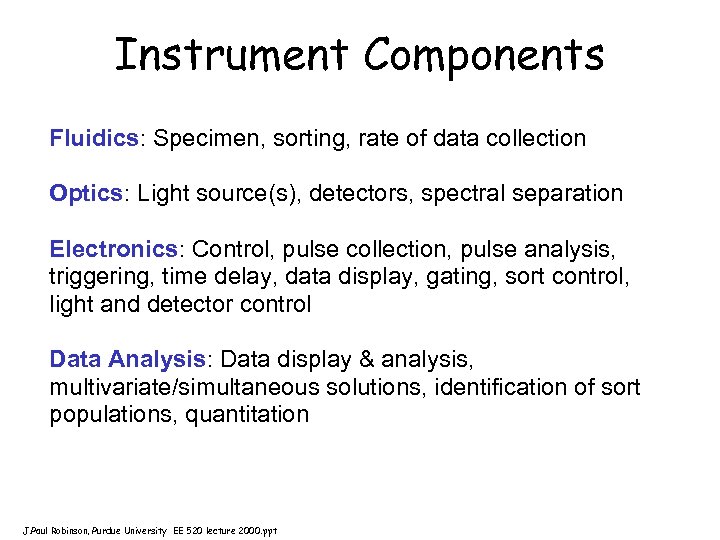
Instrument Components Fluidics: Specimen, sorting, rate of data collection Optics: Light source(s), detectors, spectral separation Electronics: Control, pulse collection, pulse analysis, triggering, time delay, data display, gating, sort control, light and detector control Data Analysis: Data display & analysis, multivariate/simultaneous solutions, identification of sort populations, quantitation J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
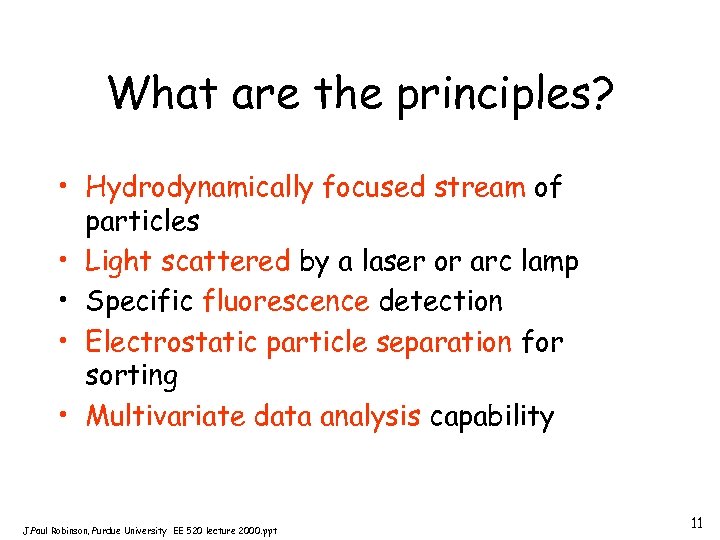
What are the principles? • Hydrodynamically focused stream of particles • Light scattered by a laser or arc lamp • Specific fluorescence detection • Electrostatic particle separation for sorting • Multivariate data analysis capability J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 11
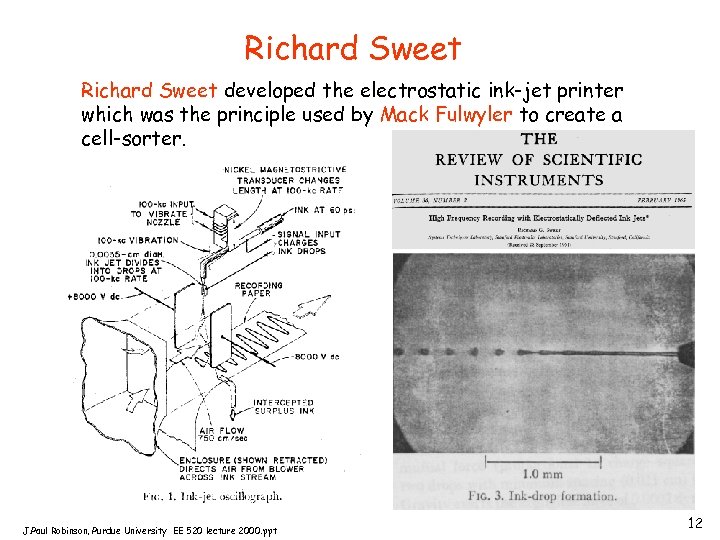
Richard Sweet developed the electrostatic ink-jet printer which was the principle used by Mack Fulwyler to create a cell-sorter. J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 12
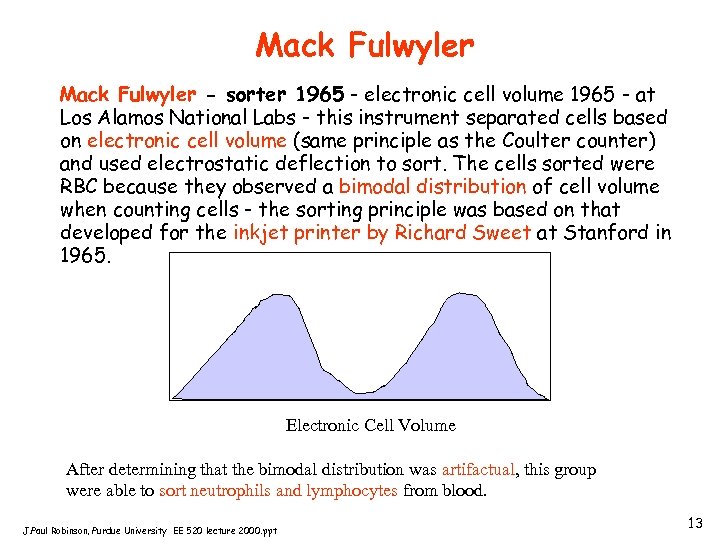
Mack Fulwyler - sorter 1965 - electronic cell volume 1965 - at Los Alamos National Labs - this instrument separated cells based on electronic cell volume (same principle as the Coulter counter) and used electrostatic deflection to sort. The cells sorted were RBC because they observed a bimodal distribution of cell volume when counting cells - the sorting principle was based on that developed for the inkjet printer by Richard Sweet at Stanford in 1965. Electronic Cell Volume After determining that the bimodal distribution was artifactual, this group were able to sort neutrophils and lymphocytes from blood. J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 13
The mysterous red cell problem J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 14

J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
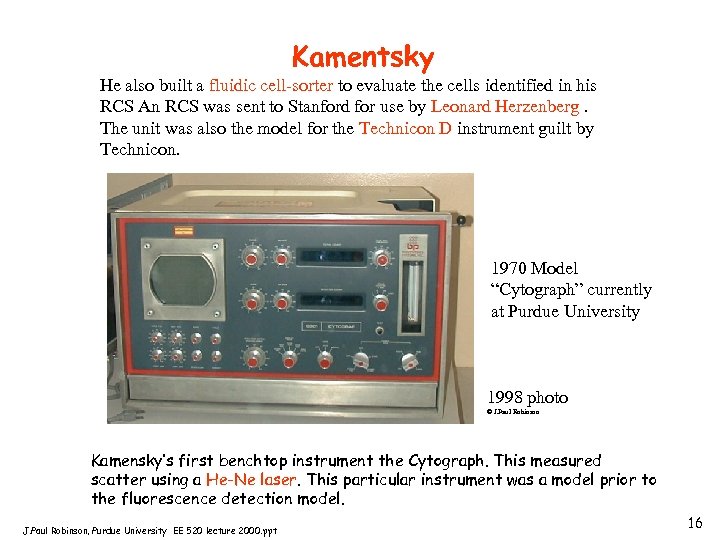
Kamentsky He also built a fluidic cell-sorter to evaluate the cells identified in his RCS An RCS was sent to Stanford for use by Leonard Herzenberg. The unit was also the model for the Technicon D instrument guilt by Technicon. 1970 Model “Cytograph” currently at Purdue University 1998 photo © J. Paul Robinson Kamensky’s first benchtop instrument the Cytograph. This measured scatter using a He-Ne laser. This particular instrument was a model prior to the fluorescence detection model. J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 16

J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 17

J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 18

J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 19

Hydrodynamics and Fluid Systems • Cells are always in suspension • The usual fluid for cells is saline • The sheath fluid can be saline or water • The sheath must be saline for sorting • Samples are driven either by syringes or by pressure systems J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 20
Fluidics • Need to have cells in suspension flow in single file through an illuminated volume • In most instruments, accomplished by injecting sample into a sheath fluid as it passes through a small (50 -300 µm) orifice • When conditions are right, sample fluid flows in a central core that does not mix with the sheath fluid • This is termed Laminar flow (Sheath Flow Principle) • The introduction of a large volume into a small volume in such a way that it becomes “focused” along an axis is called Hydrodynamic Focusing [RFM] J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 21
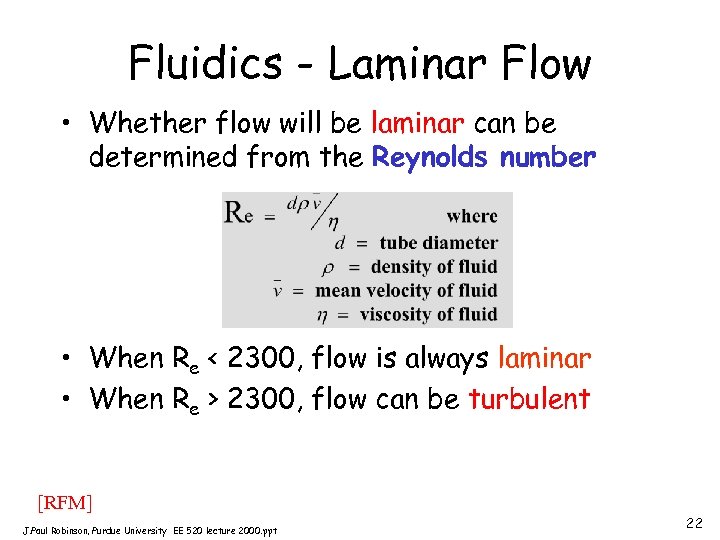
Fluidics - Laminar Flow • Whether flow will be laminar can be determined from the Reynolds number • When Re < 2300, flow is always laminar • When Re > 2300, flow can be turbulent [RFM] J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 22
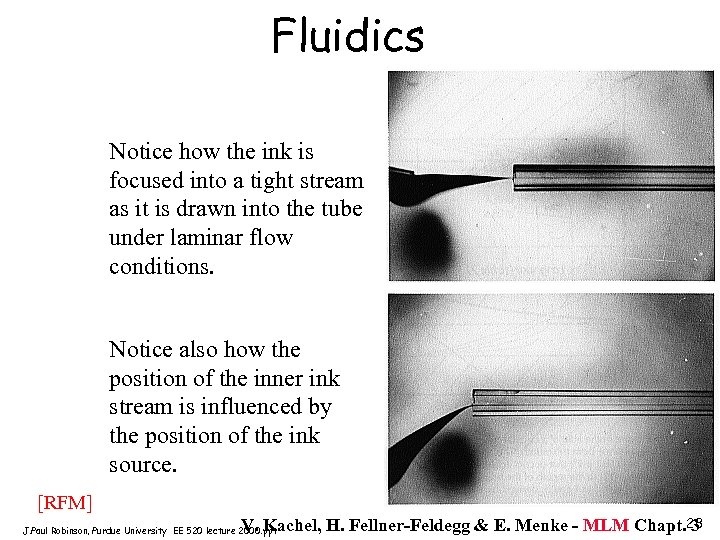
Fluidics Notice how the ink is focused into a tight stream as it is drawn into the tube under laminar flow conditions. Notice also how the position of the inner ink stream is influenced by the position of the ink source. [RFM] V. Kachel, H. Fellner-Feldegg & E. Menke - MLM Chapt. 23 3 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
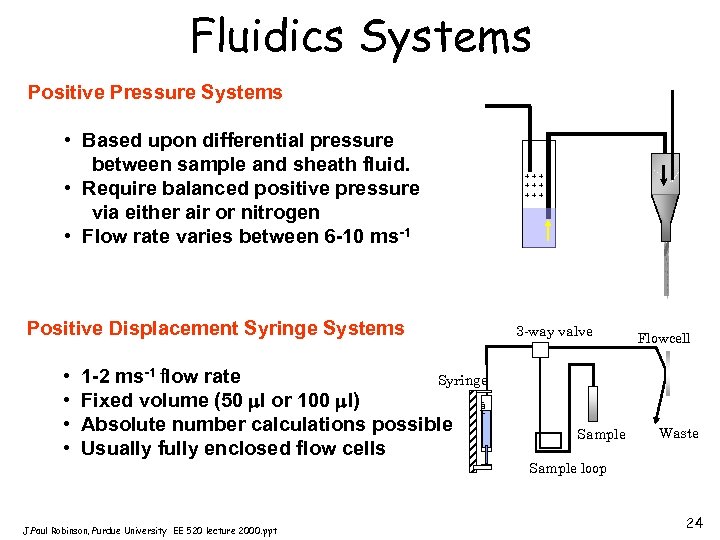
Fluidics Systems Positive Pressure Systems • Based upon differential pressure between sample and sheath fluid. • Require balanced positive pressure via either air or nitrogen • Flow rate varies between 6 -10 ms-1 +++ +++ Positive Displacement Syringe Systems 1 -2 ms-1 flow rate Syringe Fixed volume (50 l or 100 l) Absolute number calculations possible Usually fully enclosed flow cells J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt Flowcell 100 l • • 3 -way valve Sample Waste Sample loop 24
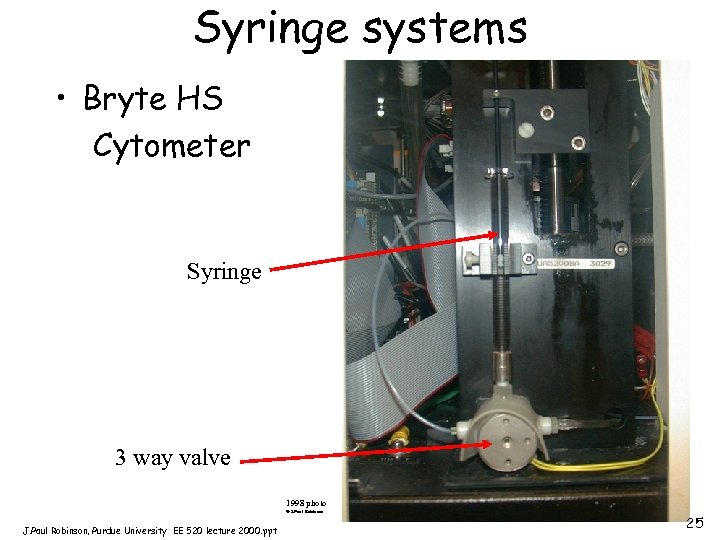
Syringe systems • Bryte HS Cytometer Syringe 3 way valve 1998 photo © J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 25
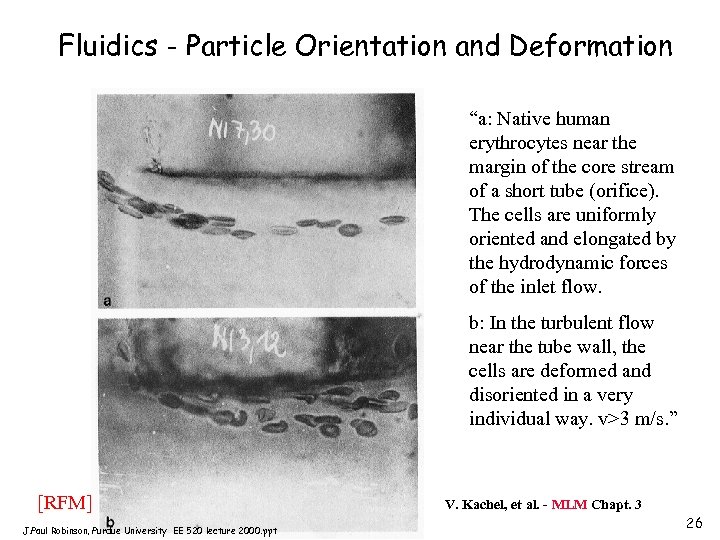
Fluidics - Particle Orientation and Deformation “a: Native human erythrocytes near the margin of the core stream of a short tube (orifice). The cells are uniformly oriented and elongated by the hydrodynamic forces of the inlet flow. b: In the turbulent flow near the tube wall, the cells are deformed and disoriented in a very individual way. v>3 m/s. ” [RFM] J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt V. Kachel, et al. - MLM Chapt. 3 26
Closed flow cells Laser direction 1998 photo © J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 27
![Fluidics - Flow Chambers Flow through cuvette (sense in quartz) [RFM] J. Paul Robinson, Fluidics - Flow Chambers Flow through cuvette (sense in quartz) [RFM] J. Paul Robinson,](https://present5.com/presentation/d6313c7339b2096556f42059bf46e126/image-28.jpg)
Fluidics - Flow Chambers Flow through cuvette (sense in quartz) [RFM] J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 28 H. B. Steen - MLM Chapt. 2
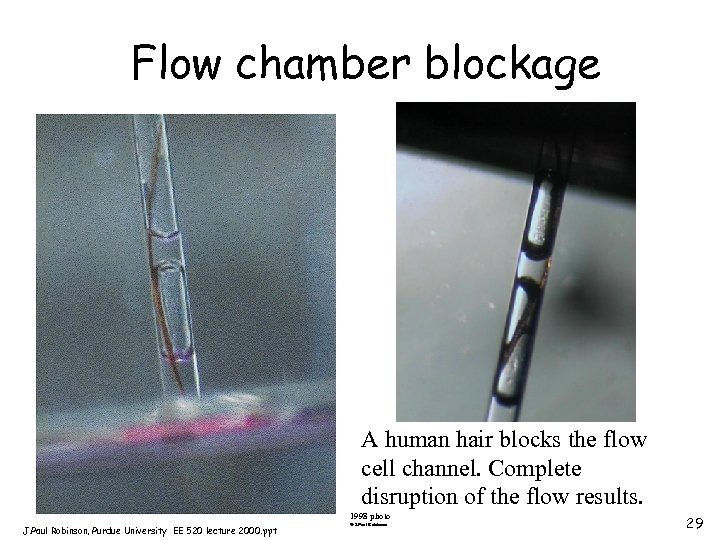
Flow chamber blockage A human hair blocks the flow cell channel. Complete disruption of the flow results. 1998 photo J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt © J. Paul Robinson 29

The Elements of Flow Sorting • • • Sample Preparation Hardware Setup Droplet formation Timing Coincidence - Purity and Efficiency Sterile Sorting Concepts J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 30
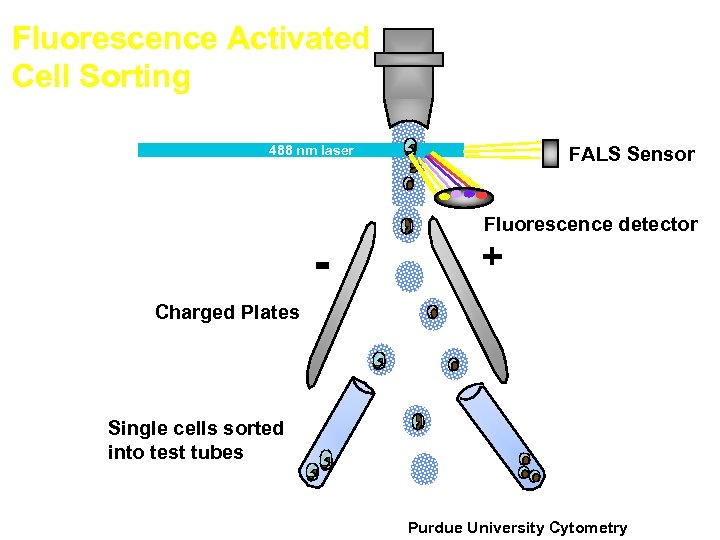
Fluorescence Activated Cell Sorting FALS Sensor 488 nm laser - Fluorescence detector + Charged Plates Single cells sorted into test tubes J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt Purdue University Cytometry 31
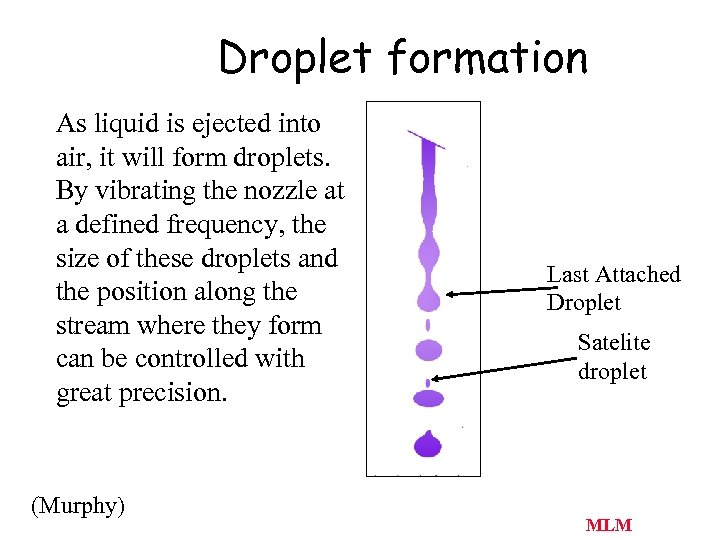
Droplet formation As liquid is ejected into air, it will form droplets. By vibrating the nozzle at a defined frequency, the size of these droplets and the position along the stream where they form can be controlled with great precision. (Murphy) J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt Last Attached Droplet Satelite droplet T. Lindmo, D. C. Peters & R. G Sweet - MLM Chapt. 32 8
Droplet break off Video 2. mpg Video of the droplet formation in a sort stream from a Cytomation instrument. Source: Purdue CDROM vol 4, 1998 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 33
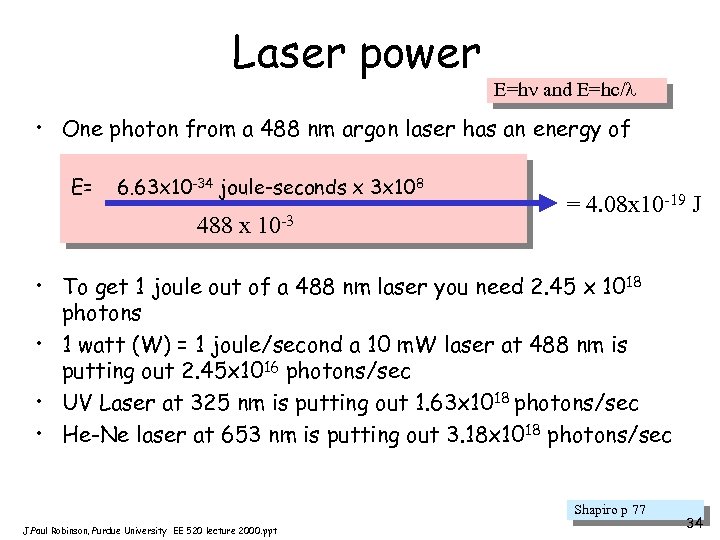
Laser power E=h and E=hc/ • One photon from a 488 nm argon laser has an energy of E= 6. 63 x 10 -34 joule-seconds x 3 x 108 488 x 10 -3 = 4. 08 x 10 -19 J • To get 1 joule out of a 488 nm laser you need 2. 45 x 10 18 photons • 1 watt (W) = 1 joule/second a 10 m. W laser at 488 nm is putting out 2. 45 x 1016 photons/sec • UV Laser at 325 nm is putting out 1. 63 x 1018 photons/sec • He-Ne laser at 653 nm is putting out 3. 18 x 1018 photons/sec Shapiro p 77 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 34
Light Scatter • Materials scatter light at wavelengths at which they do not absorb • If we consider the visible spectrum to be 350 -850 nm then small particles (< 1/10 ) scatter rather than absorb light • For small particles (molecular up to sub micron) the Rayleigh scatter intensity at 0 o and 180 o are about the same • For larger particles (i. e. size from 1/4 to tens of wavelengths) larger amounts of scatter occur in the forward not the side scatter direction - this is called Mie Scatter (after Gustav Mie) - this is how we come up with forward scatter be related to size Shapiro p 79 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 35
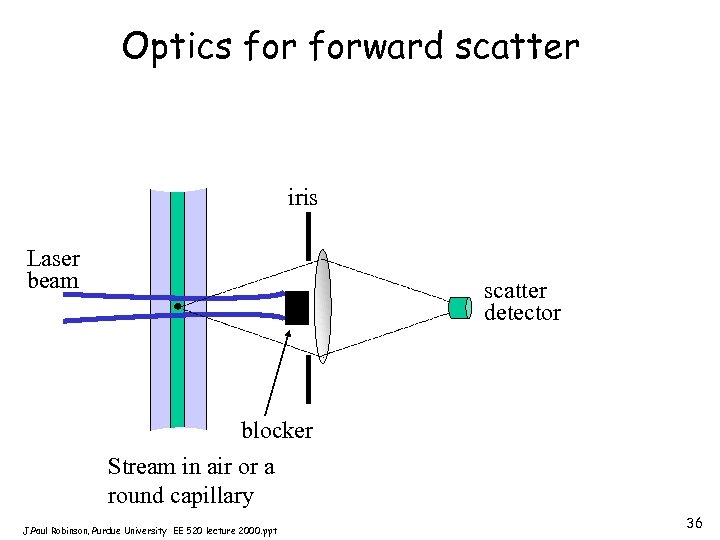
Optics forward scatter iris Laser beam scatter detector blocker Stream in air or a round capillary J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 36
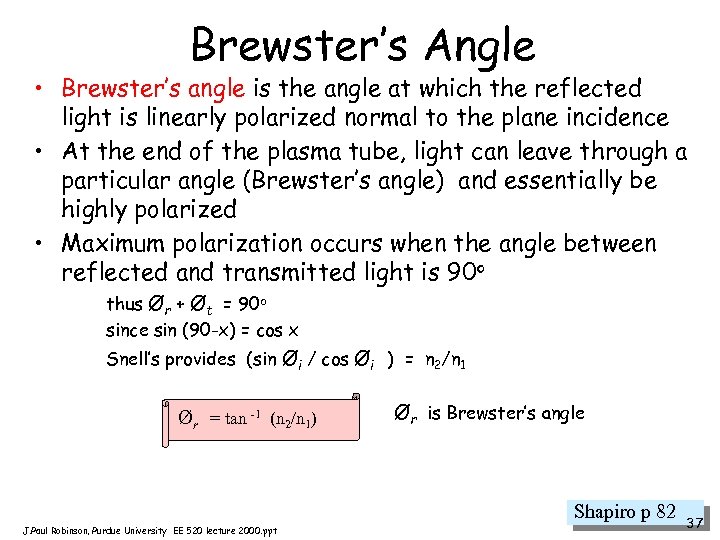
Brewster’s Angle • Brewster’s angle is the angle at which the reflected light is linearly polarized normal to the plane incidence • At the end of the plasma tube, light can leave through a particular angle (Brewster’s angle) and essentially be highly polarized • Maximum polarization occurs when the angle between reflected and transmitted light is 90 o thus Ør + Øt = 90 o since sin (90 -x) = cos x Snell’s provides (sin Øi / cos Øi ) = n 2/n 1 Ør = tan -1 (n 2/n 1) Ør is Brewster’s angle Shapiro p 82 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 37
Brewster’s Angle 1998 photo © J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 38
Fluorescence • Excitation Spectrum – Intensity of emission as a function of exciting wavelength • Chromophores are components of molecules which absorb light • They are generally aromatic rings • The wavelength of absorption is related to the size of the chromophores • Smaller chromophores, higher energy (shorter wavelength) J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 39
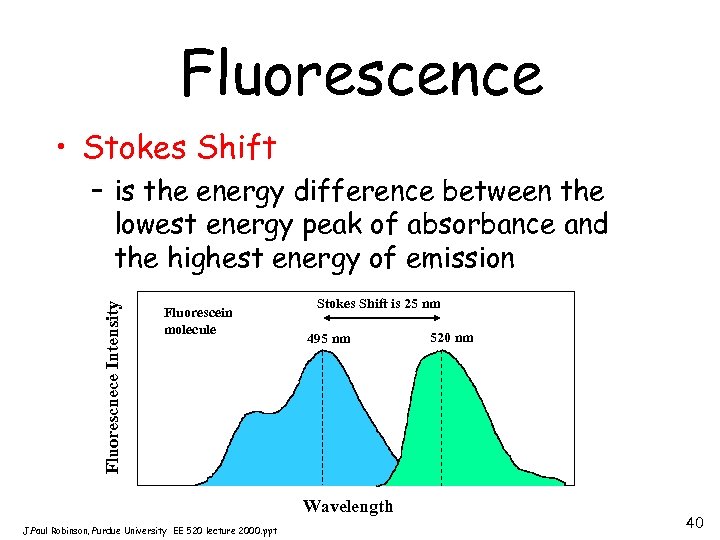
Fluorescence • Stokes Shift Fluorescnece Intensity – is the energy difference between the lowest energy peak of absorbance and the highest energy of emission Fluorescein molecule Stokes Shift is 25 nm 495 nm Wavelength J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 520 nm 40
Properties of Fluorescent Molecules n n n n n Large extinction coefficient at the region of excitation High quantum yield Optimal excitation wavelength Photostability Excited-state lifetime Minimal perturbation by probe Dye molecules must be close to but below saturation levels for optimum emission Fluorescence emission is longer than the exciting wavelength The energy of the light increases with reduction of wavelength J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 41
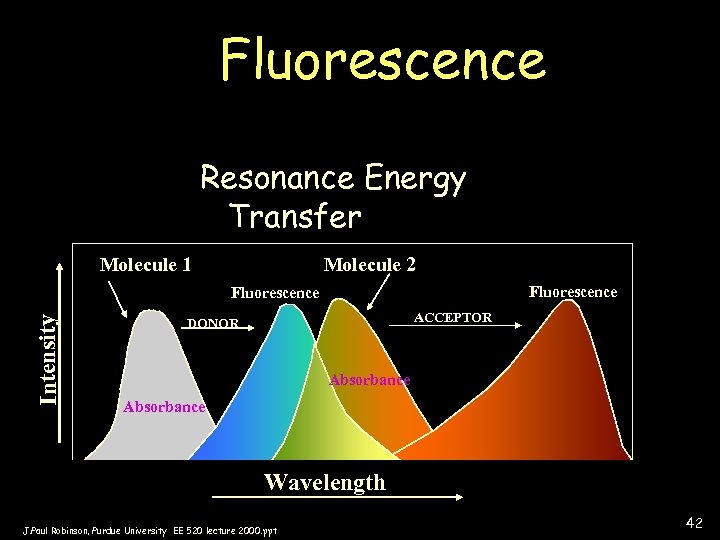
Fluorescence Resonance Energy Transfer Molecule 1 Molecule 2 Fluorescence Intensity Fluorescence ACCEPTOR DONOR Absorbance Wavelength J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 42
Absorption • Basic quantum mechanics requires that molecules absorb energy as quanta (photons) based upon a criteria specific for each molecular structure • Absorption of a photon raises the molecule from ground state to an excited state • Total energy is the sum of all components (electronic, vibrational, rotational, translations, spin orientation energies) (vibrational energies are quite small) • The structure of the molecule dictates the likelyhood of absorption of energy to raise the energy state to an excited one Shapiro p 84 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 43
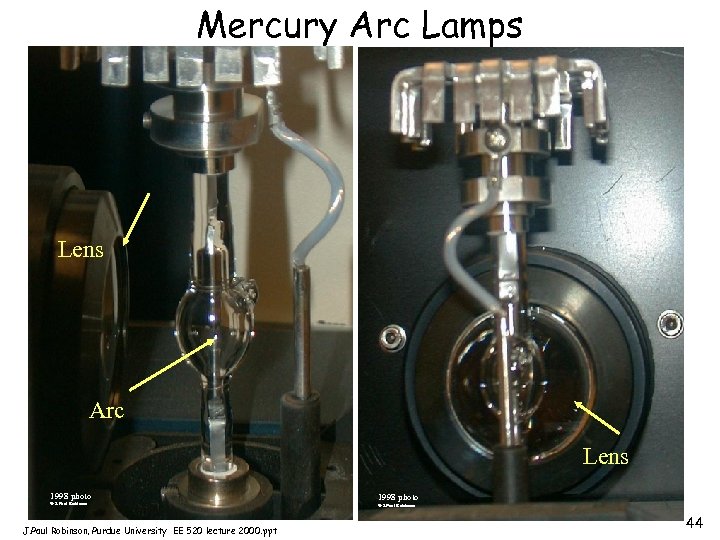
Mercury Arc Lamps Lens Arc Lens 1998 photo © J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 1998 photo © J. Paul Robinson 44
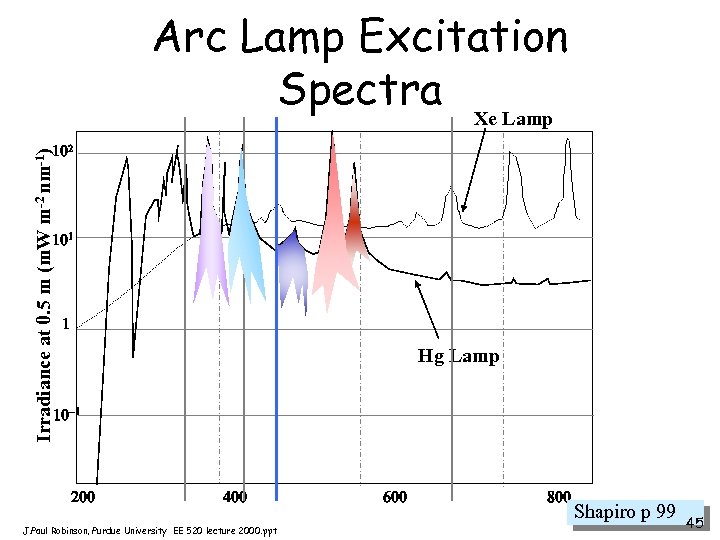
Arc Lamp Excitation Spectra Xe Lamp Irradiance at 0. 5 m (m. W m-2 nm-1) Hg Lamp J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt Shapiro p 99 45

Laser Power & Noise Light Amplification by Stimulated Emission of Radiation • Laser light is coherent and monochromatic (same frequency and wavelength) • This means the emitted radiation is in phase with and propagating in the same direction as the stimulating radiation • ION lasers use electromagnetic energy to produce and confine the ionized gas plasma which serves as the lasing medium. • Lasers can be continuous wave (CW) or pulsed (where flashlamps provide the pulse) • Laser efficiency is variable - argon ion lasers are about 0. 01% efficient (1 W needs 10 KW power) Shapiro p 106 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 46
Lasers J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 47

Goals of Light Collection • • • Maximum signal, minimum noise Maximum area of collection Inexpensive system if possible Easy alignment Reduced heat generation Reduced power requirement J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 48
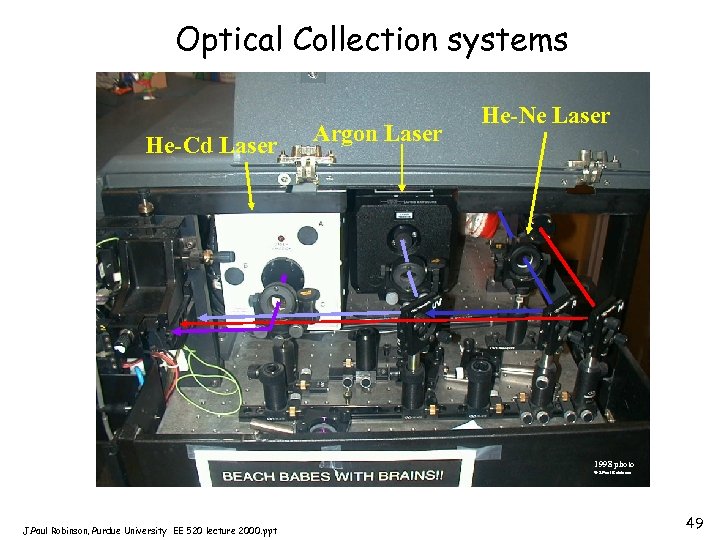
Optical Collection systems He-Cd Laser Argon Laser He-Ne Laser 1998 photo © J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 49
Interference in Thin Films • Small amounts of incident light are reflected at the interface between two material of different RI • Thickness of the material will alter the constructive or destructive interference patterns - increasing or decreasing certain wavelengths • Optical filters can thus be created that “interfere” with the normal transmission of light Shapiro p 82 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 50
Interference and Diffraction: Gratings • Diffraction essentially describes a departure from theoretical geometric optics • Thus a sharp objet casts an alternating shadow of light and dark “patterns” because of interference • Diffraction is the component that limits resolution Shapiro p 83 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 51
Interference filters • They are composed of transparent glass or quartz substrate on which multiple thin layers of dielectric material, sometimes separated by spacer layers. • Permit great selectivity. J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 52
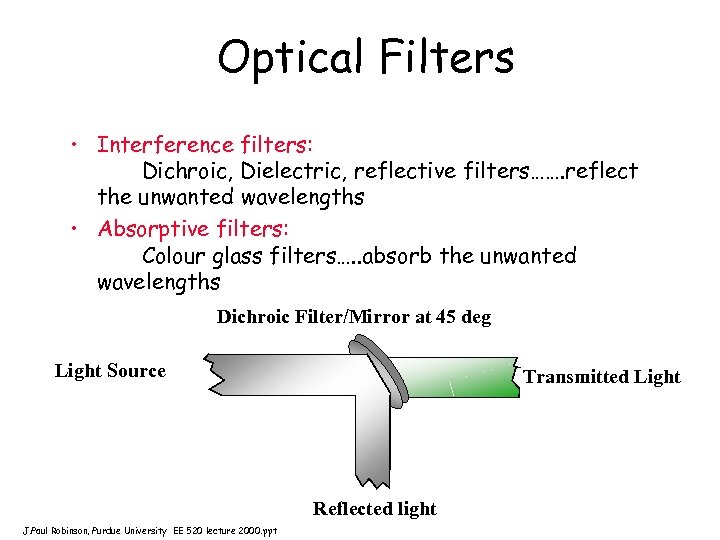
Optical Filters • Interference filters: Dichroic, Dielectric, reflective filters……. reflect the unwanted wavelengths • Absorptive filters: Colour glass filters…. . absorb the unwanted wavelengths Dichroic Filter/Mirror at 45 deg Light Source Transmitted Light Reflected light J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
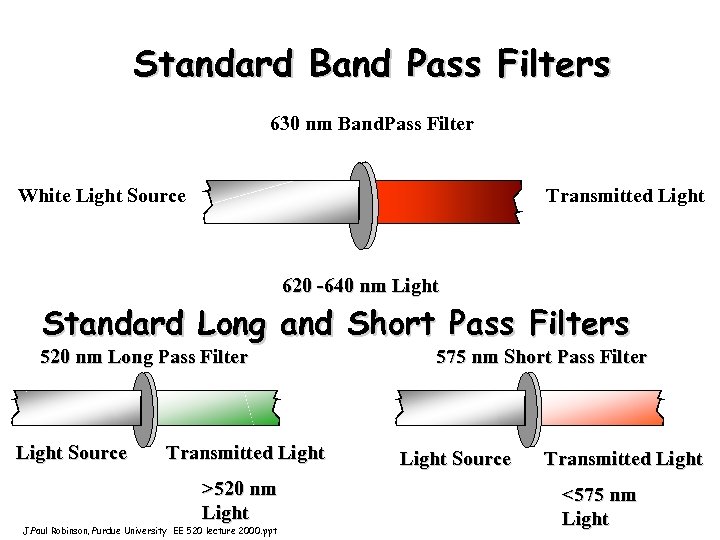
Standard Band Pass Filters 630 nm Band. Pass Filter White Light Source Transmitted Light 620 -640 nm Light Standard Long and Short Pass Filters 520 nm Long Pass Filter Light Source Transmitted Light >520 nm Light J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 575 nm Short Pass Filter Light Source Transmitted Light <575 nm Light
Transmission determination • Constructive and destructive interference occurs between reflections from various layers • Transmission determined by : – thickness of the dielectric layers – number of these layers – angle of incidence light on the filters J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 55
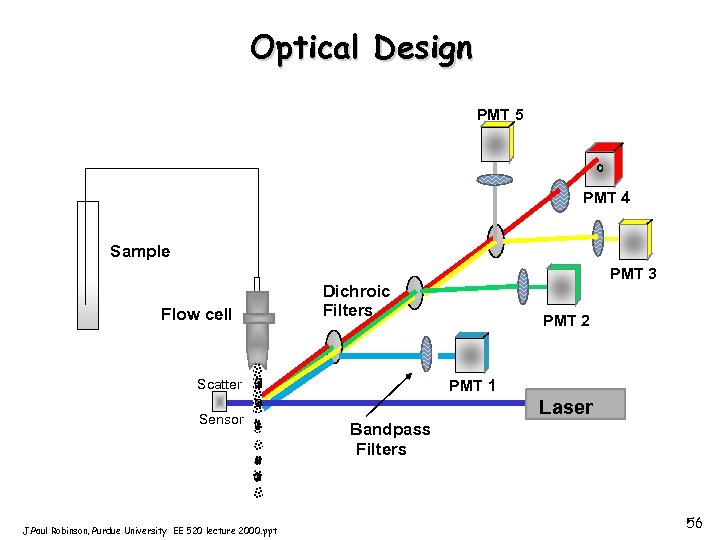
Optical Design PMT 5 PMT 4 Sample Flow cell Dichroic Filters Scatter Sensor J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt PMT 3 PMT 2 PMT 1 Laser Bandpass Filters 56
PMT • Produce current at their anodes when photons impinge upon their light-sensitive cathodes • Require external powersource • Their gain is as high as 107 electrons out per photon in • Noise can be generated from thermionic emission of electrons this is called “dark current” • If very low levels of signal are available, PMTs are often cooled to reduce heat effects • Spectral response of PMTs is determined by the composition of the photocathode • Bi-alkali PMTs have peak sensitivity at 400 nm • Multialkali PMTs extend to 750 nm • Gallium Arsenide (Ga. As) cathodes operate from 300 -850 nm (very costly and have lower gain) J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 57
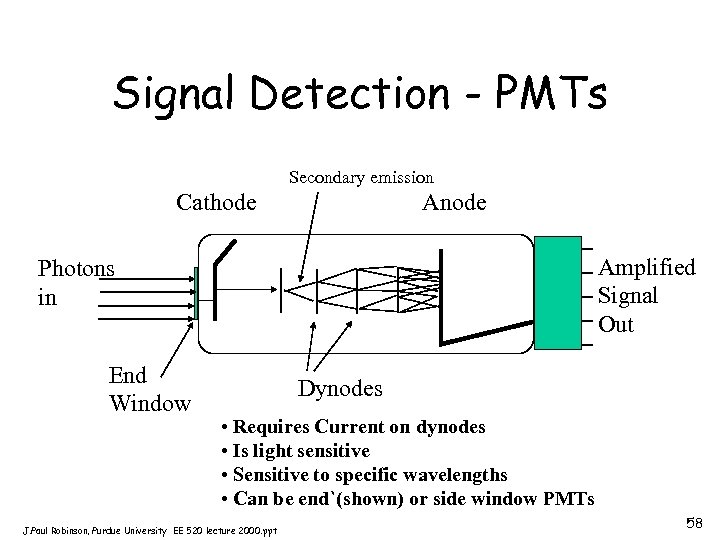
Signal Detection - PMTs Secondary emission Cathode Anode Amplified Signal Out Photons in End Window Dynodes • Requires Current on dynodes • Is light sensitive • Sensitive to specific wavelengths • Can be end`(shown) or side window PMTs J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 58
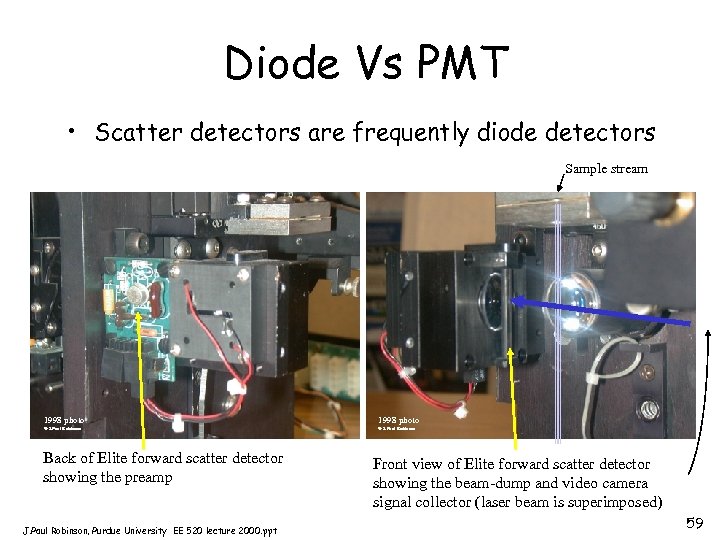
Diode Vs PMT • Scatter detectors are frequently diode detectors Sample stream 1998 photo © J. Paul Robinson Back of Elite forward scatter detector showing the preamp J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt Front view of Elite forward scatter detector showing the beam-dump and video camera signal collector (laser beam is superimposed) 59
Review of Electronics • Reactance like resistance provides an impediment to the flow of current, but unlike resistance is dependent on the frequency of the current • If a DC current is applied to a capacitor a transient current flows but stops when the potential difference between the conductors equals the potential of the source • The capacitance measured in Farads (F) is equal to the amount of charge on either electrode in Coulombs divided by the potential difference between the electrodes in volts - 1 Farad = 1 coulomb/volt • DC current will not flow “through” a capacitor - AC current will and the higher the frequency the better the conduction • In a circuit that contains both inductance and capacitance, one cancels the other out • The combined effect of resistance, inductive reactance and capacitive reactance is referred to as impedance (Z) of the circuit 60 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
Linear and Log circuits • • Linear circuits Logarithmic circuits Dynamic range Fluorescence compensation J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 61
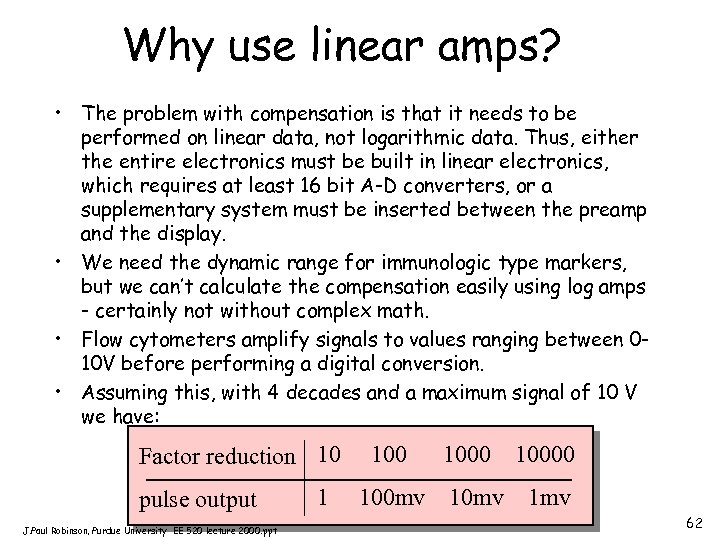
Why use linear amps? • The problem with compensation is that it needs to be performed on linear data, not logarithmic data. Thus, either the entire electronics must be built in linear electronics, which requires at least 16 bit A-D converters, or a supplementary system must be inserted between the preamp and the display. • We need the dynamic range for immunologic type markers, but we can’t calculate the compensation easily using log amps - certainly not without complex math. • Flow cytometers amplify signals to values ranging between 010 V before performing a digital conversion. • Assuming this, with 4 decades and a maximum signal of 10 V we have: Factor reduction 10 pulse output J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 1 100 mv 1000 10 mv 10000 1 mv 62
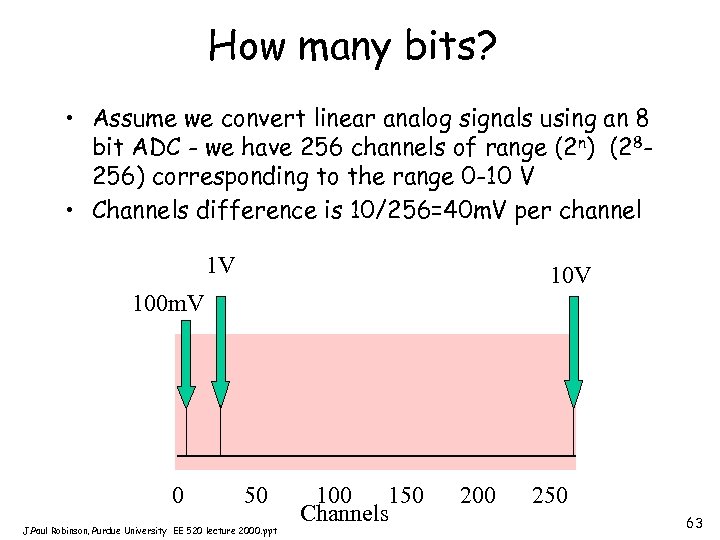
How many bits? • Assume we convert linear analog signals using an 8 bit ADC - we have 256 channels of range (2 n) (28256) corresponding to the range 0 -10 V • Channels difference is 10/256=40 m. V per channel 1 V 100 m. V 0 50 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 100 150 Channels 200 250 63
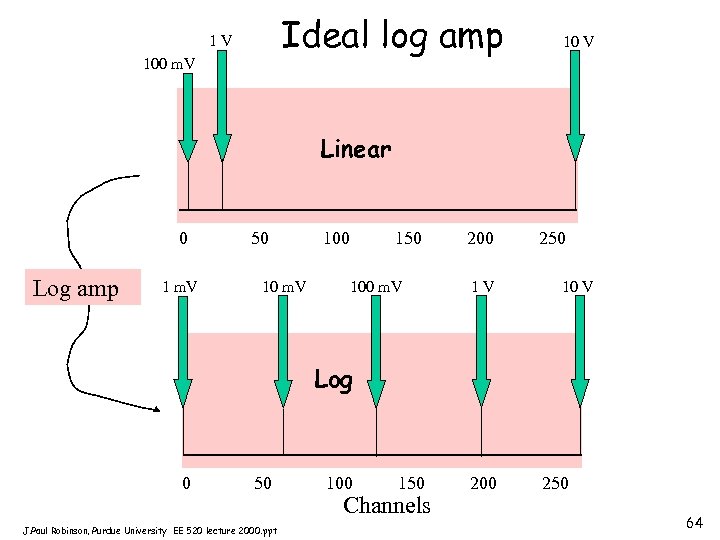
Ideal log amp 1 V 100 m. V 10 V Linear 0 Log amp 1 m. V 50 10 m. V 100 150 100 m. V 200 1 V 250 10 V Log 0 50 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 100 150 Channels 200 250 64
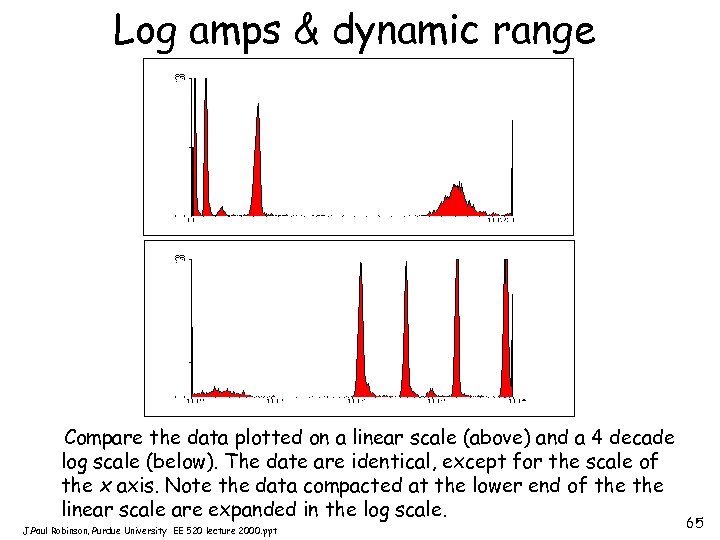
Log amps & dynamic range Compare the data plotted on a linear scale (above) and a 4 decade log scale (below). The date are identical, except for the scale of the x axis. Note the data compacted at the lower end of the linear scale are expanded in the log scale. J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 65
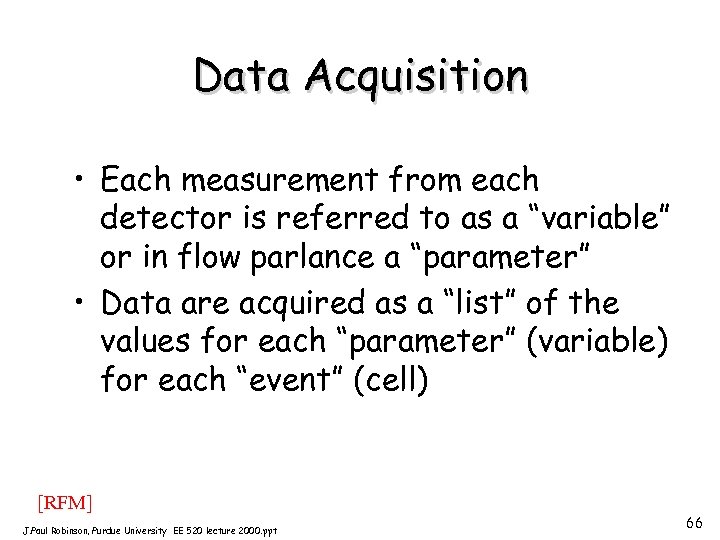
Data Acquisition • Each measurement from each detector is referred to as a “variable” or in flow parlance a “parameter” • Data are acquired as a “list” of the values for each “parameter” (variable) for each “event” (cell) [RFM] J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 66
![Data Acquisition - Listmode [RFM] J. Paul Robinson, Purdue University EE 520 lecture 2000. Data Acquisition - Listmode [RFM] J. Paul Robinson, Purdue University EE 520 lecture 2000.](https://present5.com/presentation/d6313c7339b2096556f42059bf46e126/image-67.jpg)
Data Acquisition - Listmode [RFM] J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 67

Data Presentation Formats • Histogram • Dot plot • Contour plot • 3 D plots • Dot plot with projection • Overviews (multiple histograms) J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
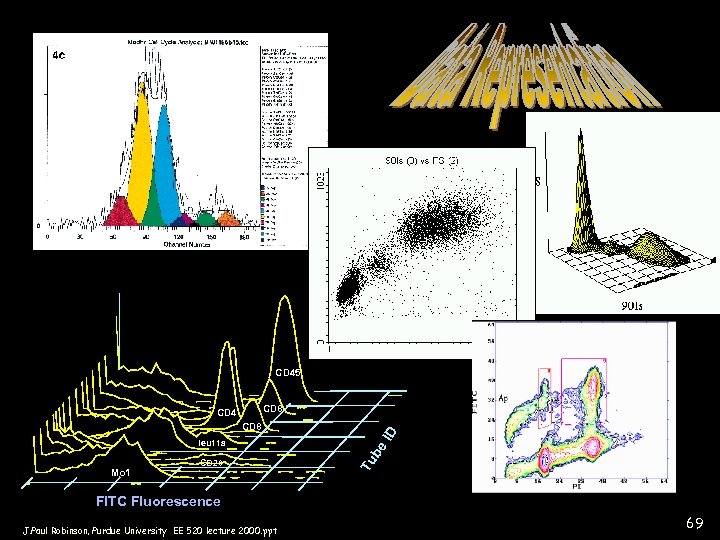
CD 45 leu 11 a Mo 1 CD 20 Tu be CD 8 ID CD 8 CD 4 FITC Fluorescence J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 69
Data Analysis • • • Frequency Distributions Gaussian distribution Normal distributions Statistics Skewness and Kurtosis J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 70
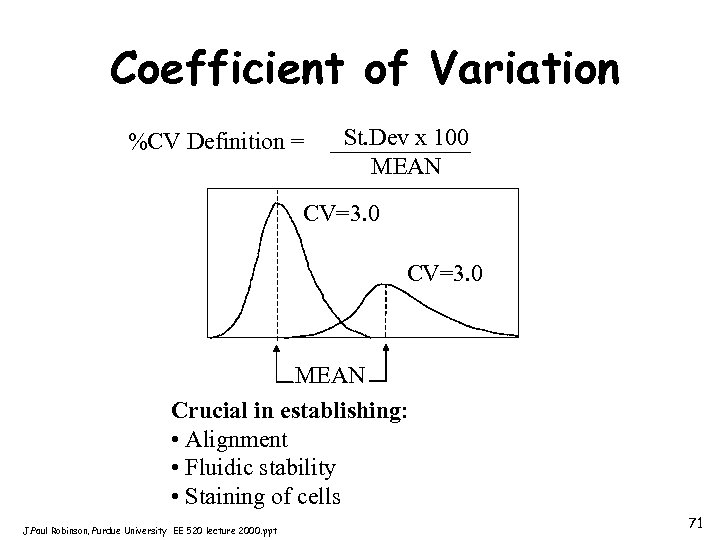
Coefficient of Variation %CV Definition = St. Dev x 100 MEAN CV=3. 0 MEAN Crucial in establishing: • Alignment • Fluidic stability • Staining of cells J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 71
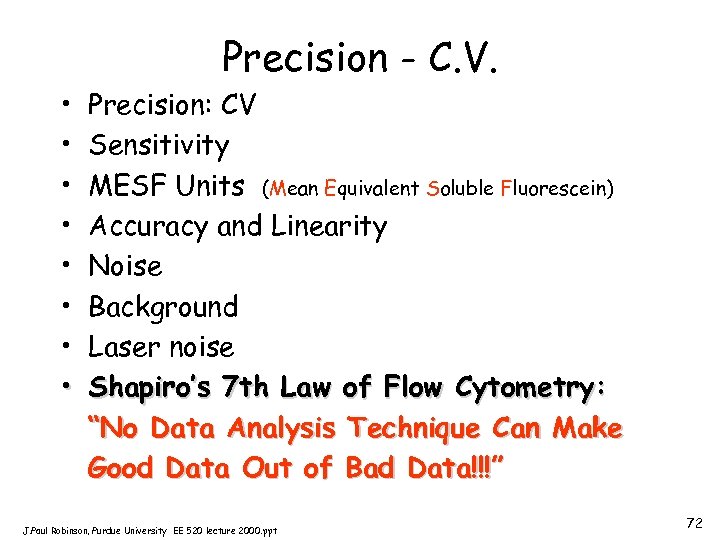
Precision - C. V. • • Precision: CV Sensitivity MESF Units (Mean Equivalent Soluble Fluorescein) Accuracy and Linearity Noise Background Laser noise Shapiro’s 7 th Law of Flow Cytometry: “No Data Analysis Technique Can Make Good Data Out of Bad Data!!!” J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 72
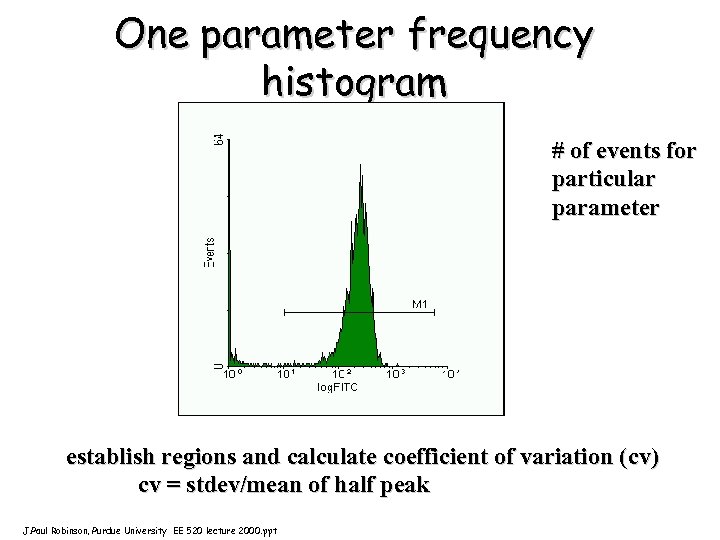
One parameter frequency histogram # of events for particular parameter establish regions and calculate coefficient of variation (cv) cv = stdev/mean of half peak J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
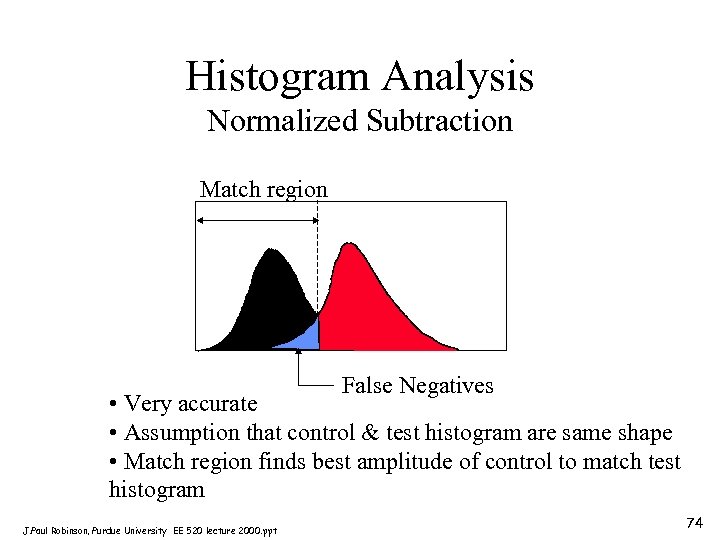
Histogram Analysis Normalized Subtraction Match region False Negatives • Very accurate • Assumption that control & test histogram are same shape • Match region finds best amplitude of control to match test histogram J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 74
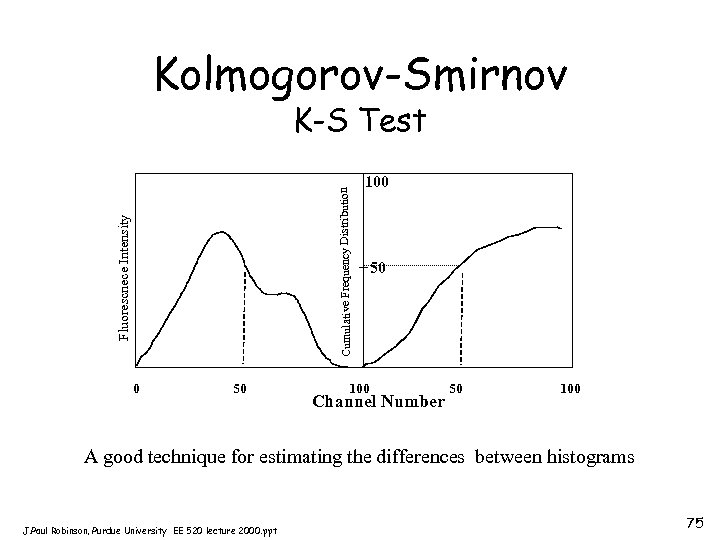
Kolmogorov-Smirnov Fluorescnece Intensity Cumulative Frequency Distribution K-S Test 0 50 100 Channel Number 50 100 A good technique for estimating the differences between histograms J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 75
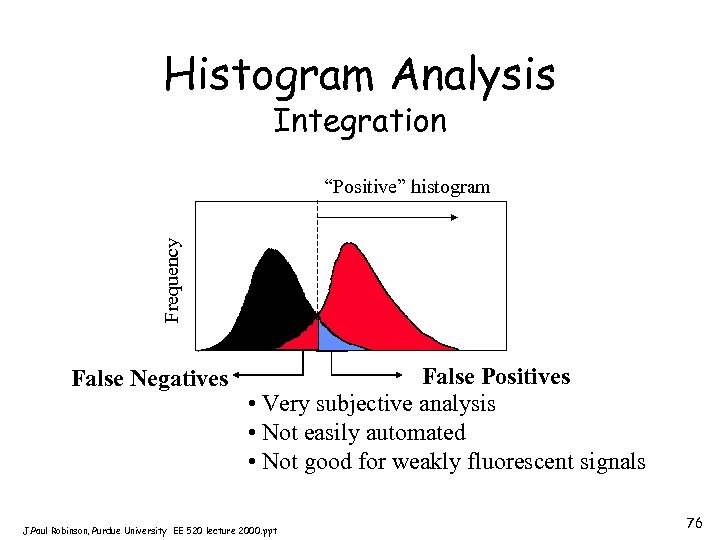
Histogram Analysis Integration Frequency “Positive” histogram False Negatives False Positives • Very subjective analysis • Not easily automated • Not good for weakly fluorescent signals J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 76
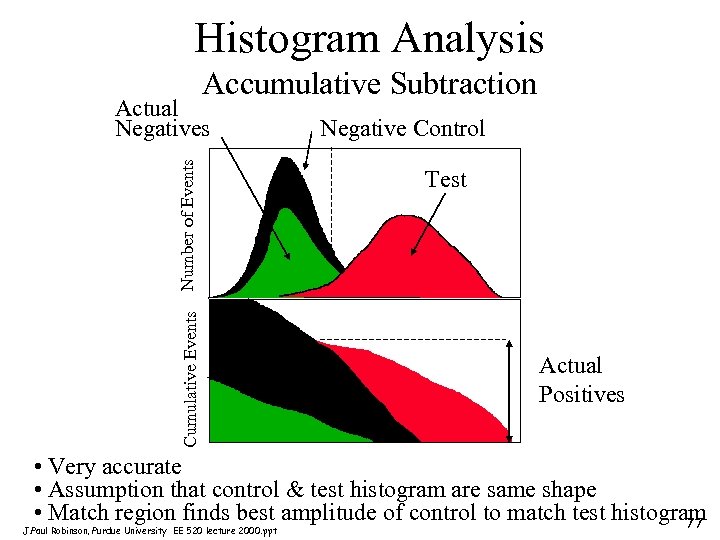
Histogram Analysis Accumulative Subtraction Cumulative Events Number of Events Actual Negatives Negative Control Test Actual Positives • Very accurate • Assumption that control & test histogram are same shape • Match region finds best amplitude of control to match test histogram 77 J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
Histogram Overlays J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt
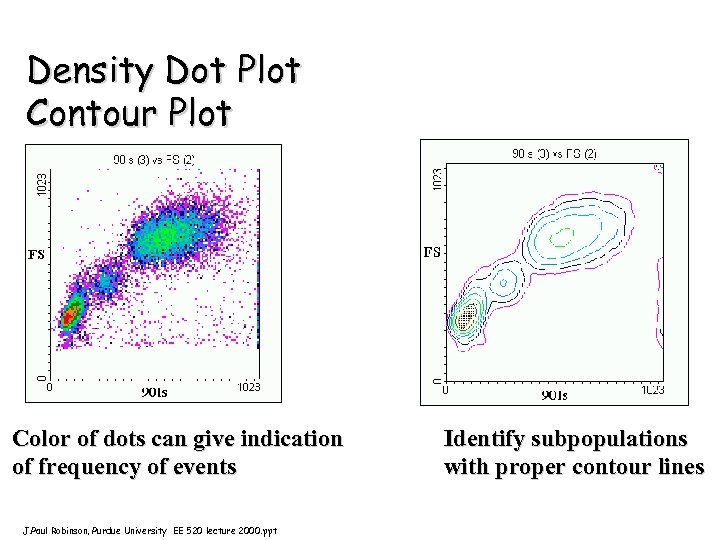
Density Dot Plot Contour Plot Color of dots can give indication of frequency of events J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt Identify subpopulations with proper contour lines
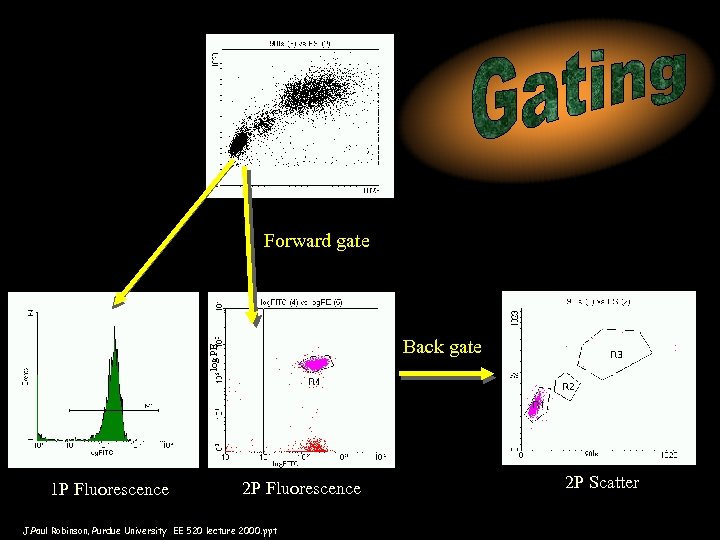
Forward gate log PE Back gate 1 P Fluorescence 2 P Fluorescence J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 2 P Scatter
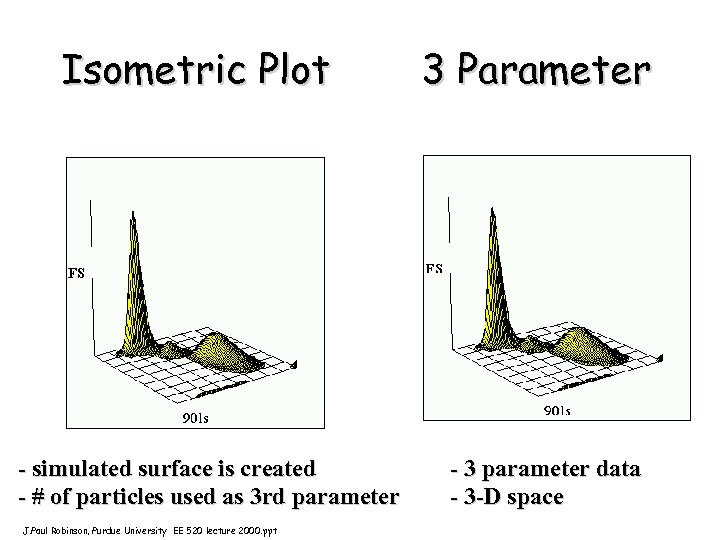
Isometric Plot - simulated surface is created - # of particles used as 3 rd parameter J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 3 Parameter - 3 parameter data - 3 -D space
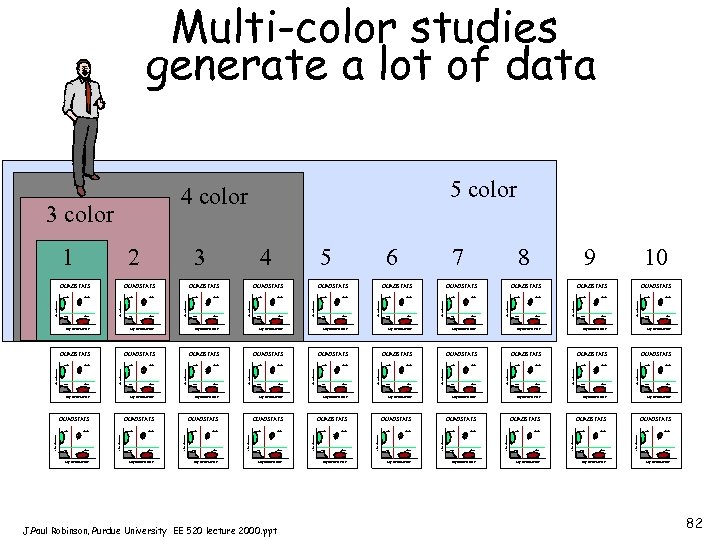
Multi-color studies generate a lot of data +- +- Log Fluorescence QUADSTATS -+ ++ -- +- -+ +- +- -+ Log Fluorescence -+ +- -- +- QUADSTATS ++ -- ++ Log Fluorescence QUADSTATS ++ -- -- QUADSTATS ++ Log Fluorescence +- -+ Log Fluorescence -- QUADSTATS ++ -- +- -+ 10 -+ Log Fluorescence +- -+ -- QUADSTATS ++ 9 Log Fluorescence QUADSTATS ++ -- +- -+ 8 Log Fluorescence +- -+ -- QUADSTATS ++ Log Fluorescence QUADSTATS ++ -- +- -+ 7 Log Fluorescence +- -+ -- QUADSTATS ++ Log Fluorescence QUADSTATS ++ -- +- -+ 6 Log Fluorescence +- -+ -- QUADSTATS ++ Log Fluorescence QUADSTATS ++ -- +- Log Fluorescence QUADSTATS -+ -- -+ 5 Log Fluorescence 4 QUADSTATS ++ Log Fluorescence +- -+ Log Fluorescence -- QUADSTATS ++ Log Fluorescence QUADSTATS 3 Log Fluorescence 2 Log Fluorescence 1 Log Fluorescence 3 color -+ 5 color 4 color ++ -- +- Log Fluorescence Log Fluorescence Log Fluorescence QUADSTATS QUADSTATS QUADSTATS +- Log Fluorescence J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt ++ -- +- Log Fluorescence -+ ++ -- +- Log Fluorescence -+ Log Fluorescence -- -+ Log Fluorescence +- Log Fluorescence ++ Log Fluorescence -- -+ Log Fluorescence ++ Log Fluorescence -+ ++ -- +- Log Fluorescence 82
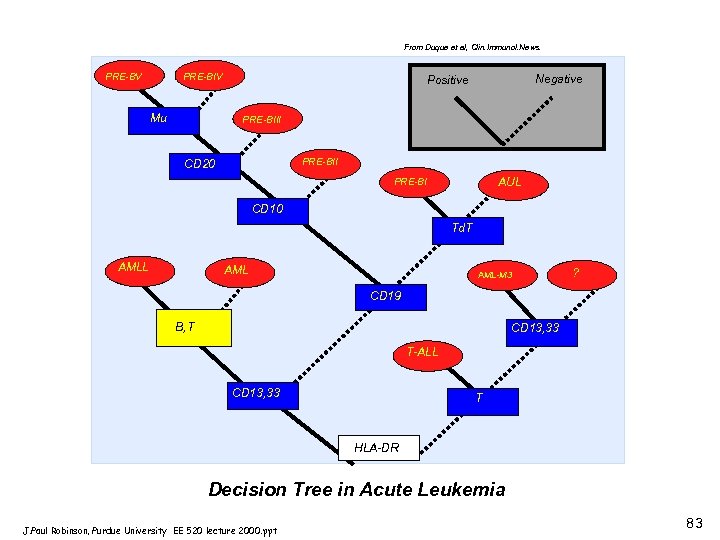
From Duque et al, Clin. Immunol. News. A PRE-BV PRE-BIV Mu Negative Positive PRE-BIII PRE-BII CD 20 AUL PRE-BI CD 10 Td. T AMLL AML-M 3 ? CD 19 B, T CD 13, 33 T-ALL CD 13, 33 T HLA-DR Decision Tree in Acute Leukemia J. Paul Robinson, Purdue University EE 520 lecture 2000. ppt 83
d6313c7339b2096556f42059bf46e126.ppt