eddc516dc0a9abc1b6c8dcc3b6ef6bca.ppt
- Количество слайдов: 31

Biomaterials By Dr. Tejal Ashwin Desai, U. Illinois Chicago Modified, P. H. King, Vanderbilt U.
What is a biomaterial? What are the design constraints?

Biomaterial — A biomaterial is a nonviable material used in a medical device intended to interact with biological systems (Williams 1987) Biocompatibility — The ability of a material to perform with an appropriate host response in a specific application (Williams 1987) Host Response — The response of the host organism (local and systemic) to the implanted material or device.

Keywords ßMetallic/glass/Polymeric/Ceramic/Composite ßFracture/fatigue/creep/corrosion/degredation ßTissue response/healing/biocompatability/host response/carcinogenicity ßHard/soft tissue implants ßVascular/Breast/Urological/Art. Organ ßMucosal contacting …

Material Selection Parameters ßMechanical ßThermal/Electrical Conductivity ßDiffusion ßWater Absorption ßBiostability ßBiocompatibility
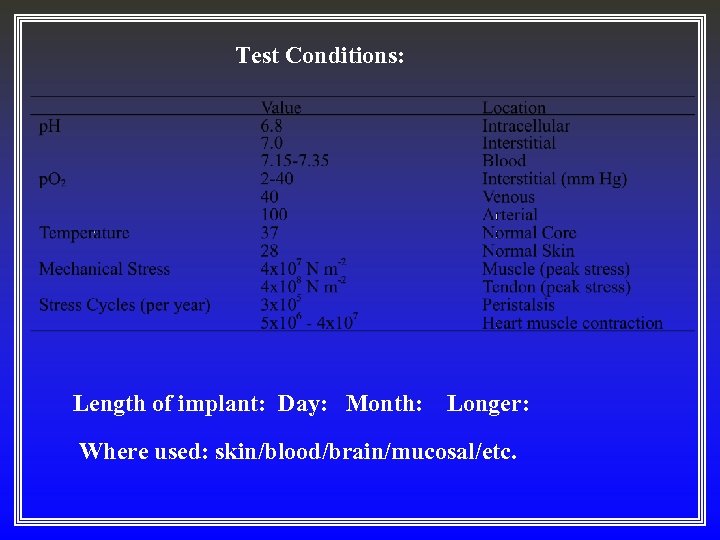
Test Conditions: Length of implant: Day: Month: Longer: Where used: skin/blood/brain/mucosal/etc.

Test Animals ßRabbits – ear, skin, pyrogen ßGuinea Pigs – skin, esp C@ ßMice – genotoxicity ßHorseshoe Crab – endotoxins ßPig – implant ßBacteria - genotoxicity ßTest actual & elutants & extracts… ßPeople – long term

Some Commonly Used Biomaterials Material Silicone rubber Dacron Cellulose Poly(methyl methacrylate) Polyurethanes Hydogels Stainless steel Titanium Alumina Hydroxyapatite Collagen (reprocessed) Applications Catheters, tubing Vascular grafts Dialysis membranes Intraocular lenses, bone cement Catheters, pacemaker leads Opthalmological devices, Drug Delivery Orthopedic devices, stents Orthopedic and dental devices Opthalmologic applications, wound dressings

An Interdisciplinary Field Bioengineers Material Scientists Immunologists Chemists Biologists Surgeons. . .

Journals ßBiomaterials ßJournal of Biomedical Materials Research ßCells and Materials ßJournal of Biomaterials Science ßArtificial Organs ßASAIO Transactions ßTissue Engineering ßAnnals of Biomedical Engineering
A Little History on Biomaterials ßRomans, Chinese, and Aztecs used gold in dentistry over 2000 years ago, Cu not good. ßIvory & wood teeth ßAseptic surgery 1860 (Lister) ßBone plates 1900, joints 1930 ßTurn of the century, synthetic plastics came into use ßWWII, shards of PMMA unintentionally got lodged into eyes of aviators ßParachute cloth used for vascular prosthesis ß 1960 - Polyethylene and stainless steel being used for hip implants
Uses of Biomaterials ßReplace diseased part – dialysis ßAssist in healing – sutures ßImprove function – contacts ßCorrect function – spinal rods ßCorrect cosmetic – nose, ear ßAid dx – probe ßAid tx – catheter ßReplace rotten – amalgam ßReplace dead - skin
Problems/test for w Biomaterials ßAcute toxicity (cytotoxicity) arsenic ßSub chronic/chronic Pb ßSensitization Ni, Cu ßGenotoxicity ßCarcinogenicity ßReproductive &/or developmental Pb ßNeurotoxicity ßImmunotoxicity ßPyrogen, endotoxins

FDA & ISO 10993 ßFDA mandates tests based on length of contact (24 Hr, 1 -30 Days, >30 days) ßSee table for details ßISO 10993 – required for European Union Certification – see flowchart for exemptions ßSee Device Categories & examples ßHarmonization – in process…
First Generation Implants ß “ad hoc” implants ß specified by physicians using common and borrowed materials ß most successes were accidental rather than by design Examples — First Generation Implants • • gold fillings, wooden teeth, PMMA dental prosthesis steel, gold, ivory, etc. , bone plates glass eyes and other body parts dacron and parachute cloth vascular implants
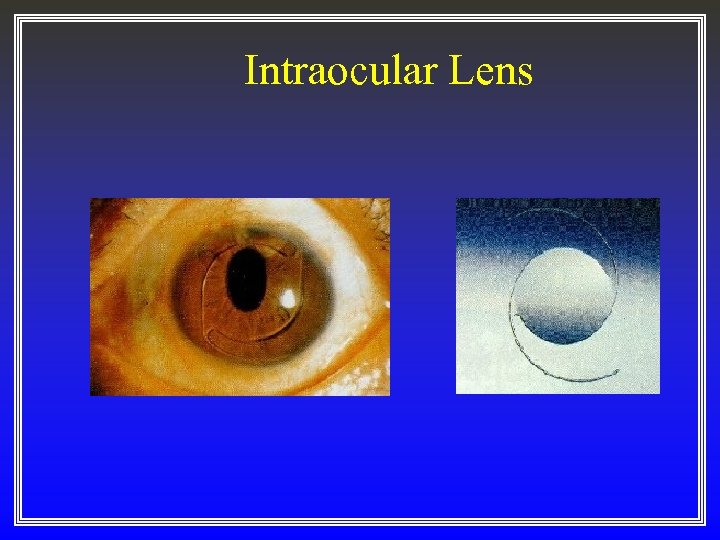
Intraocular Lens
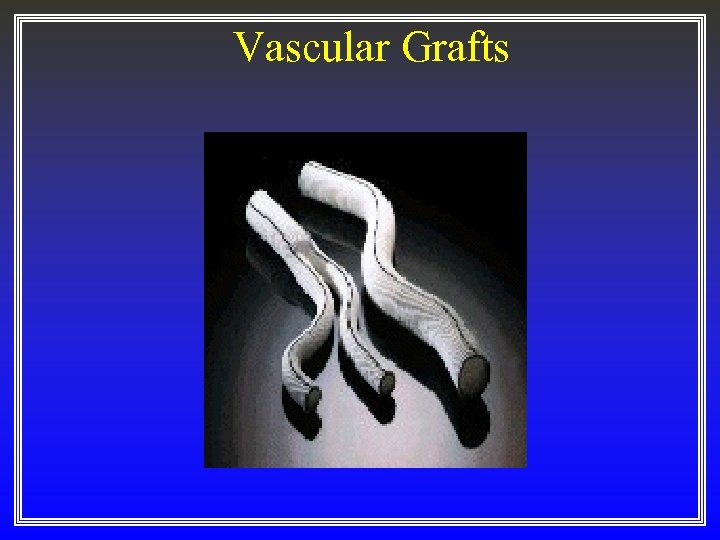
Vascular Grafts
Second generation implants ß ß engineered implants using common and borrowed materials developed through collaborations of physicians and engineers built on first generation experiences used advances in materials science (from other fields) Examples — Second generation implants • • titanium alloy dental and orthopaedic implants cobalt-chromium-molybdinum orthopaedic implants UHMW polyethylene bearing surfaces for total joint replacements heart valves and pacemakers
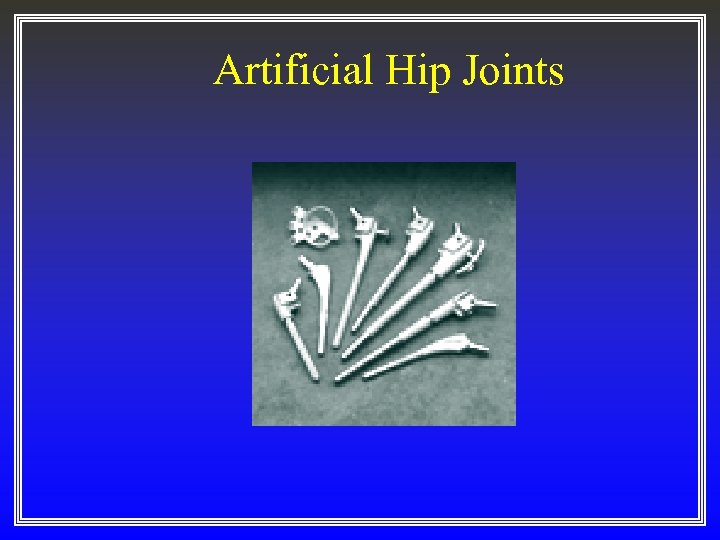
Artificial Hip Joints
Third generation implants ß ß bioengineered implants using bioengineered materials few examples on the market some modified and new polymeric devices many under development Example - Third generation implants • tissue engineered implants designed to regrow rather than replace tissues • Integra Life. Sciences artificial skin • Genzyme cartilage cell procedure • some resorbable bone repair cements • genetically engineered “biological” components (Genetics Institute and Creative Biomolecules BMPs)
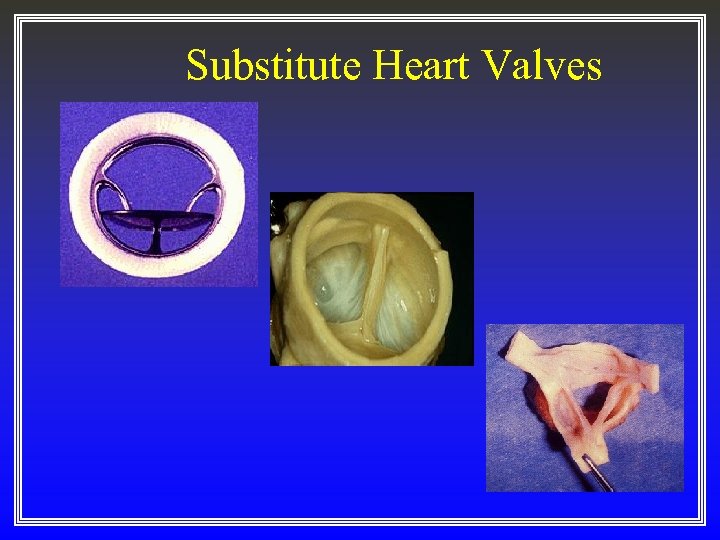
Substitute Heart Valves
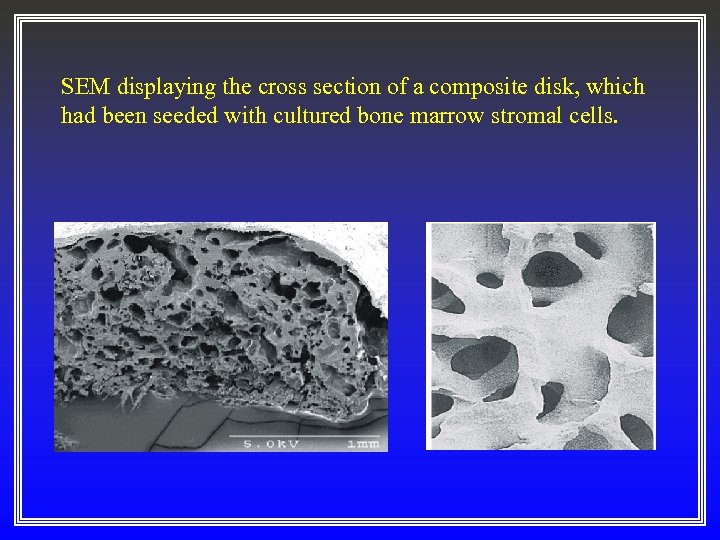
SEM displaying the cross section of a composite disk, which had been seeded with cultured bone marrow stromal cells.
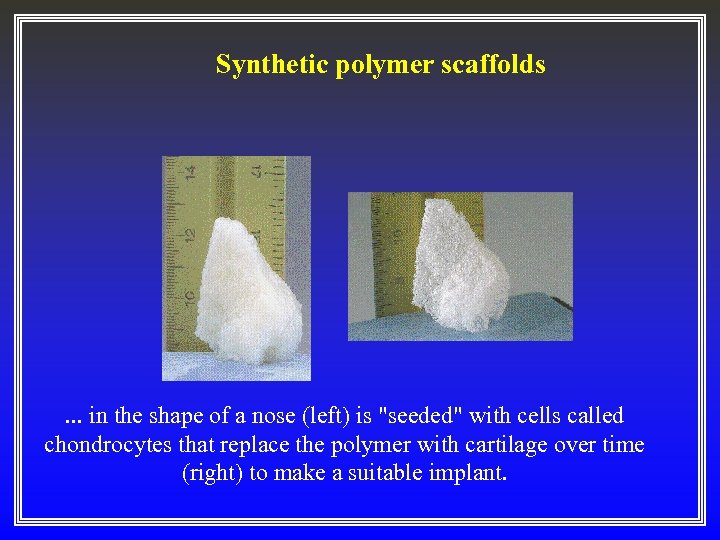
Synthetic polymer scaffolds . . . in the shape of a nose (left) is "seeded" with cells called chondrocytes that replace the polymer with cartilage over time (right) to make a suitable implant.
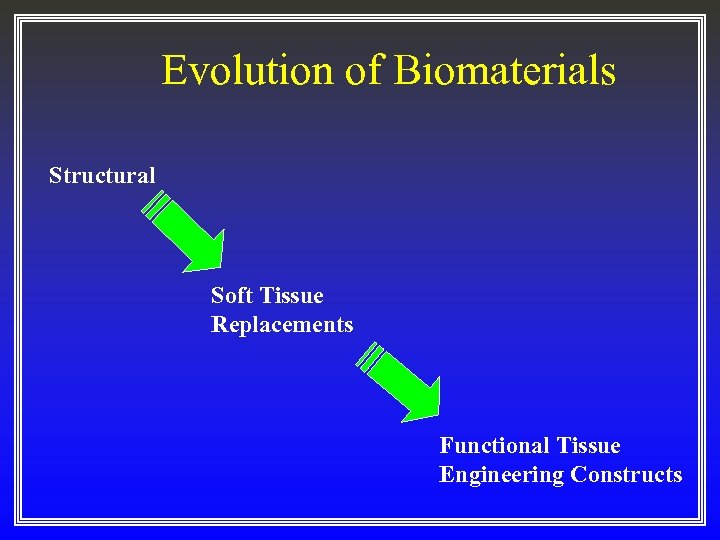
Evolution of Biomaterials Structural Soft Tissue Replacements Functional Tissue Engineering Constructs
Advances in Biomaterials Technology ßCell matrices for 3 -D growth and tissue reconstruction ßBiosensors, Biomimetic , and smart devices ßControlled Drug Delivery/ Targeted delivery ßBiohybrid organs and Cell immunoisolation ßNew biomaterials - bioactive, biodegradable, inorganic ßNew processing techniques
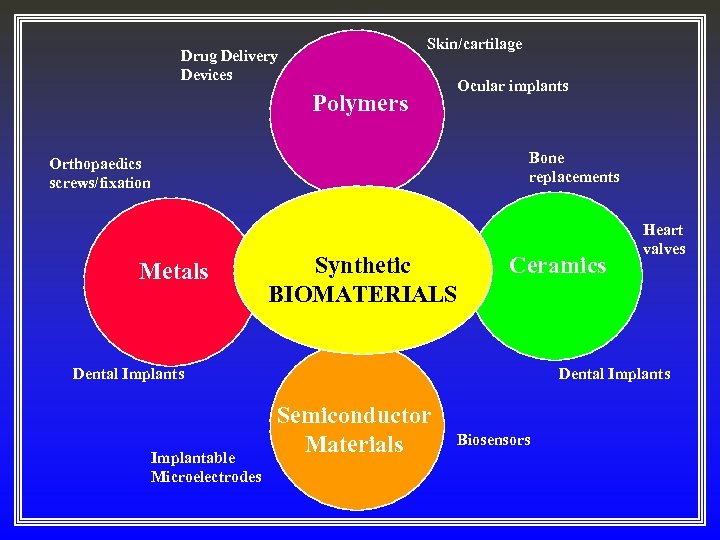
Skin/cartilage Drug Delivery Devices Polymers Ocular implants Bone replacements Orthopaedics screws/fixation Metals Synthetic BIOMATERIALS Ceramics Dental Implants Implantable Microelectrodes Heart valves Dental Implants Semiconductor Materials Biosensors
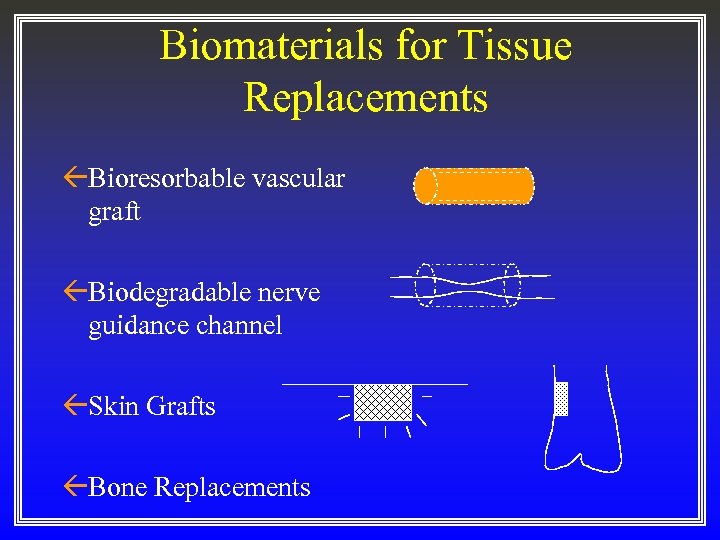
Biomaterials for Tissue Replacements ßBioresorbable vascular graft ßBiodegradable nerve guidance channel ßSkin Grafts ßBone Replacements
Biomaterials - An Emerging Industry ßNext generation of medical implants and therapeutic modalities ßInterface of biotechnology and traditional engineering ßSignificant industrial growth in the next 15 years -- potential of a multi-billion dollar industry
Biomaterials Companies • Bio. Forma Research & Consulting, Inc. , fibrinolytic systems, protein-material interactions • Baxter International develops technologies related to the blood and circulatory system. • Biocompatibles Ltd. develops commercial applications for technology in the field of biocompatibility. • Carmeda makes a biologically active surface that interacts with and supports the bodys own control mechanisms • Collagen Aesthetics Inc. bovine and human placental sourced collagens, recombinant collagens, and PEG-polymers • Endura-Tec Systems Corp. bio-mechanical endurance testing ofstents, grafts, and cardiovascular materials • Howmedica develops and manufactures products in orthopaedics. • MATECH Biomedical Technologies, development of biomaterials by chemical polymerization methods. • Medtronic, Inc. is a medical technology company specializing in implantable and invasive therapies. • Molecular Geodesics Inc. , biomimetic materials for biomedical, industrial, and military applications • Polymer Technology Group is involved in the synthesis, characterization, and manufacture of new polymer products. • Sur. Modics, offers Photo. Link(R) surface modification technology that can be used to immobilize biomolecules • W. L. Gore Medical Products Division, PTFE microstructures configured to exclude or accept tissue ingrowth. • Zimmer, design, manufacture and distribution of orthopaedic implants and related equipment and supplies

What are some of the Challenges? ßTo more closely replicate complex tissue architecture and arrangement in vitro? ßTo better understand extracellular and intracellular modulators of cell function? ßTo develop novel materials and processing techniques that are compatible with biological interfaces ßTo find better strategies for immune acceptance
eddc516dc0a9abc1b6c8dcc3b6ef6bca.ppt