biological 2009.ppt
- Количество слайдов: 41

Biological drugs Alexandra Balbir-Gurman

Inflammation Protein Il-1, IL-6, TNF-α, INF-

Cytokines in Inflammation s. TNFR TNF IL-10 IL-1 Ra IL-1 b Pro-inflammatory Anti-inflammatory

Raising Expectations for IJD Treatment Outcomes Goals of IJD (inflammatory joint diseases) treatment include: n Changing the course of disease progression (slowing or stopping the disease) n No constitutional symptoms n Returning to a normal work schedule n Minimizing the impact on activities of daily living

Treatment regimens • • • NSAID (Nonselective, selective Cox-2) Corticosteroids DMARD – – – – – Methotrexate Azathioprine Salazopyrine Plaquenil Gold Penicillamine Minocycline Leflunomide Cyclophosphamide Chlorambucil MTX Combination MTX NSAID

Reasons for additional therapies in IJD § Delay in onset of clinical response to DMARD § Need of corticosteroid therapy § High discontinuation rate – Lower effectiveness of therapy – High rate of side effects (DMARD) § No effective DMARD for AS

Biologic therapy 1. Anti –tumor necrosis factor agents 2. Anti CD 20 lymphocytes agents 3. Anti IL-1 agents 4. Anti IL-6 receptor Mab
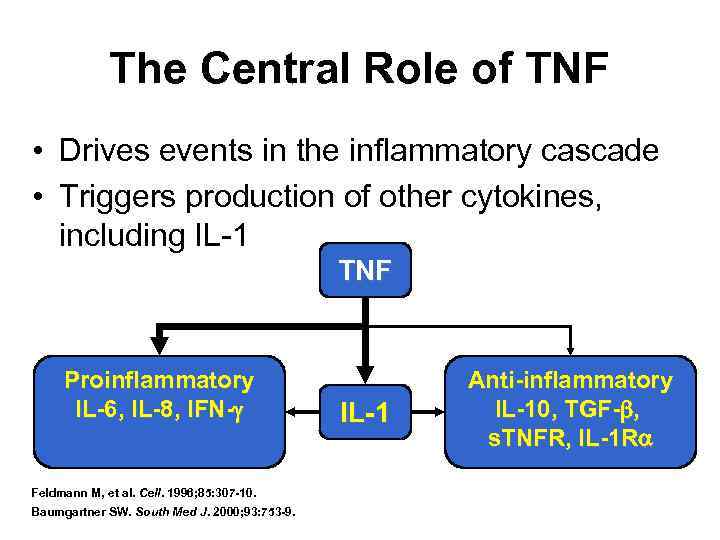
The Central Role of TNF • Drives events in the inflammatory cascade • Triggers production of other cytokines, including IL-1 TNF Proinflammatory IL-6, IL-8, IFN- Feldmann M, et al. Cell. 1996; 85: 307 -10. Baumgartner SW. South Med J. 2000; 93: 753 -9. IL-1 Anti-inflammatory IL-10, TGF- , s. TNFR, IL-1 R
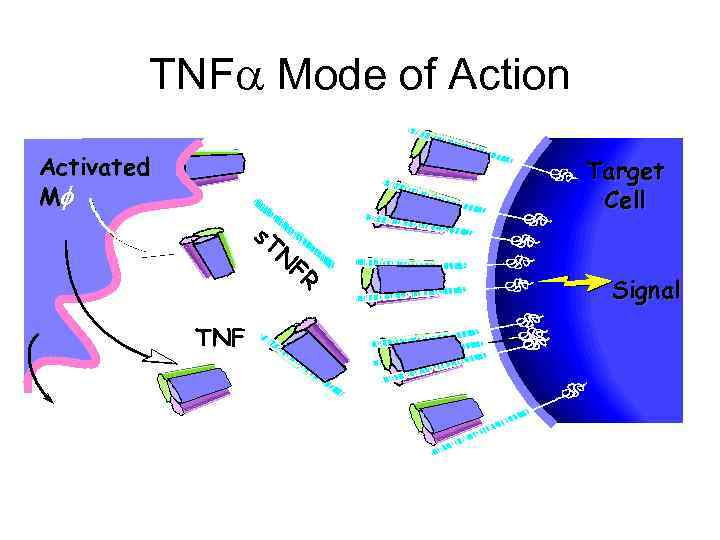
TNF Mode of Action Activated Mf s. T Target Cell N TNF FR Signal

Destructive Effects of TNF osteoclasts synoviocytes chondrocytes Bone Resorption Joint Inflammation Cartilage Degradation Bone Erosion Pain/Joint Swelling Joint Space Narrowing Fox DA. In: Koopman WJ, ed. Arthritis and Allied Conditions: A Textbook of Rheumatology. Vol 1. 14 th ed. Baltimore, Md: Williams & Wilkins; 2001: 1085 -102.
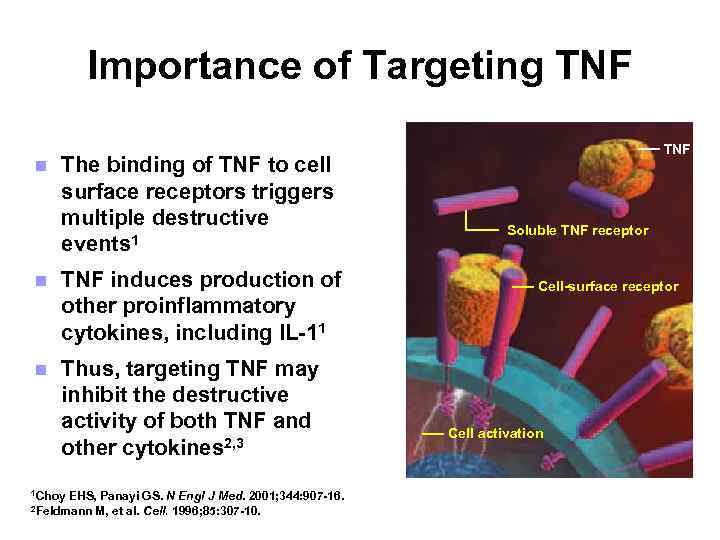
Importance of Targeting TNF n The binding of TNF to cell surface receptors triggers multiple destructive events 1 n TNF induces production of other proinflammatory cytokines, including IL-11 n Thus, targeting TNF may inhibit the destructive activity of both TNF and other cytokines 2, 3 1 Choy EHS, Panayi GS. N Engl J Med. 2001; 344: 907 -16. M, et al. Cell. 1996; 85: 307 -10. 2 Feldmann TNF Soluble TNF receptor Cell-surface receptor Cell activation

Anti-TNF therapies • Infliximab – Remicade –IV 3 mg/kg every 8 weeks • Etanercept – Enbrel – SC 25 mgx 2/week • Adalimumab – Humira – SC 40 mg every 2 weeks

Infliximab Murine Variable Constant (Fc)
Adalimumab Human Variable Constant (Fc)

Etanercept Human p 75 TNF receptor Human antibody fragment
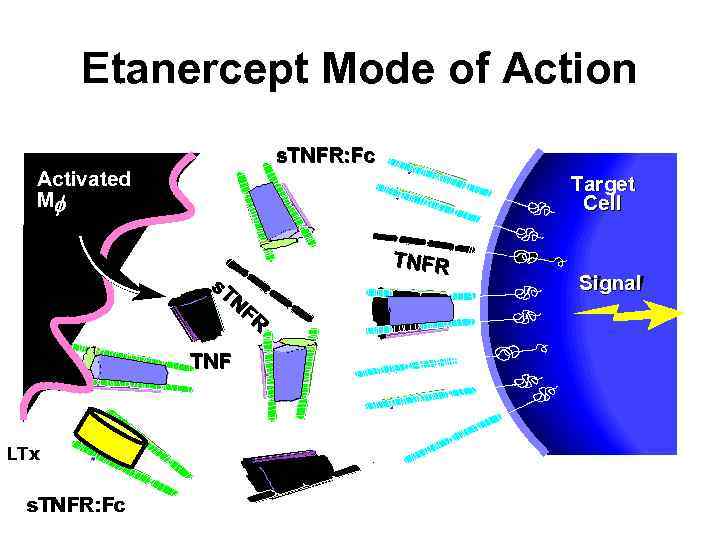
Etanercept Mode of Action s. TNFR: Fc Activated Mf Target Cell s. T NF R TNF LTx s. TNFR: Fc TNFR Signal
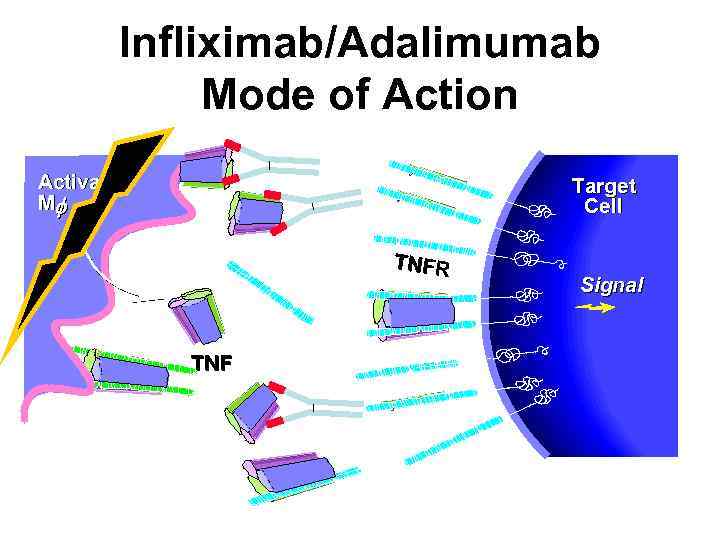
Infliximab/Adalimumab Mode of Action Activated Mf Target Cell TNFR TNF Signal

Efficacy of anti-TNF alpha • Rheumatoid Arthritis : – Combination therapy anti TNF+MTX – ACR 20 – 66 -85% of patients. (TEMPO, ATTRACT, ARMADA, etc trials) – Efficacy sustained over time – 30% remission – Slowing of disease progression – Slowing of joint damage – Restoring of function
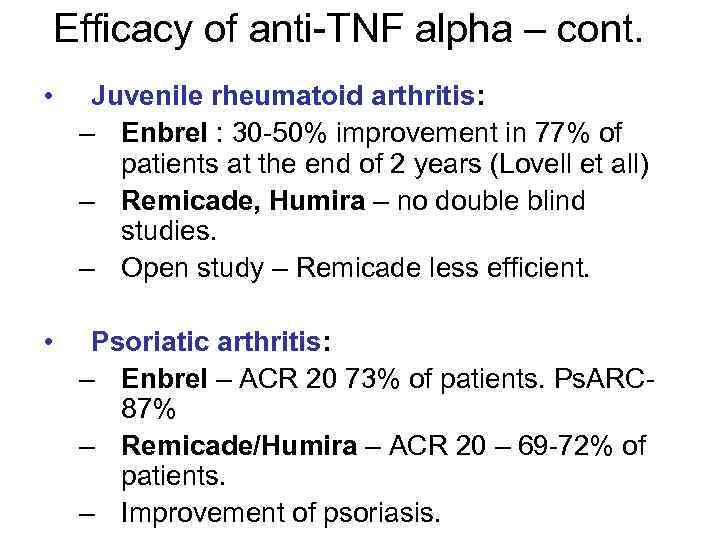
Efficacy of anti-TNF alpha – cont. • Juvenile rheumatoid arthritis: – Enbrel : 30 -50% improvement in 77% of patients at the end of 2 years (Lovell et all) – Remicade, Humira – no double blind studies. – Open study – Remicade less efficient. • Psoriatic arthritis: – Enbrel – ACR 20 73% of patients. Ps. ARC 87% – Remicade/Humira – ACR 20 – 69 -72% of patients. – Improvement of psoriasis.

Efficacy of anti-TNF alpha – cont. • Ankylosing spondylitis: – Enbrel: 20% improvement in 80% of patients. 50% reduction in BASDAI in 57% of patients. (Braun et Brandt) – Remicade: BASDAI 50 response in 53% of patients. – Long term efficacy – BASDAI 50 response in 47% of patients at week 54. (Braun et Brandt)

Safety of anti – TNF alpha 1. Infections: TB • Remicade : 50 pts per 100000 pts treated (242 cases reported) • Enbrel : 17 pts100000 pts treated (38 cases) • Humira: 20 pts/100000 pts treated (31 cases). 2. Infusion reactions/ allergic side effects – Remicade- 10 -20%. 3. Congestive heart failure? 4. Malignancy? 5. Lupus?

Conclusions – cont. • Tremendous improvement in great majority. • A subset with partial or no response. • Most of them react well to 2 nd anti TNF or Mabthera.
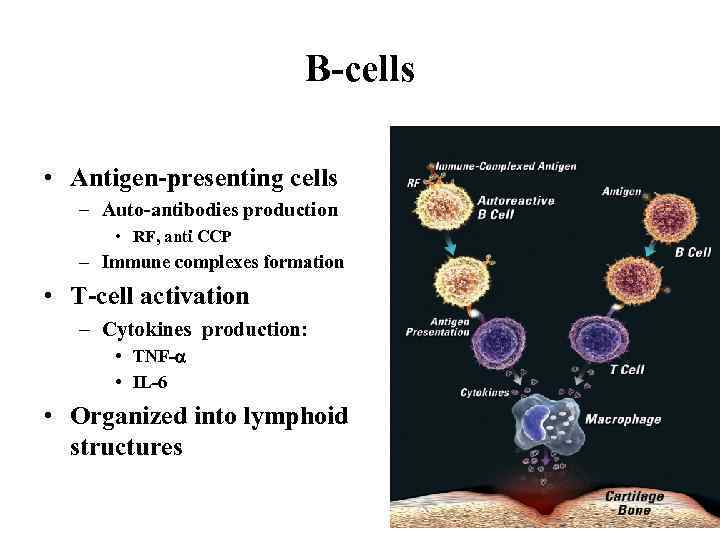
B-cells • Antigen-presenting cells – Auto-antibodies production • RF, anti CCP – Immune complexes formation • T-cell activation – Cytokines production: • TNF- • IL-6 • Organized into lymphoid structures

Rheumatoid arthritis (RA) and synovia • RA and synovial pannus – Macrophage-like (20%) and fibroblast-like cells (30%) – CD 4+ T-helpers (Th 2) (40%) – B-cells and plasma cells (5%) • RF++, anti CCP++ • Immune complexes CD 20+

B-cells have a central role in autoantibodies and IC formation, T-cell activation and progression of inflammation Is it a place for B-cell targeting in rheumatoid inflammation? ? ? (like in lymphomas? ? )

B-cell depletion by targeting CD 20+ lymphocytes – Rituximab (Mab. Thera)
Conception • • • Synovial pannus – tumor-like tissue Targeting B-cell depletion (CD 20+) To induce remission To keep remission Halting of joint damage • 2 IV infusions of 1000 mg Rituximab (Mab. Thera) in 2 weeks

Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis • 161 patients with active RA despite MTX were randomized to receive: – oral MTX (> or =10 mg per week) (control) – RTX (1000 mg on days 1 and 15) plus CP (750 mg on days 3 and 17) – RTX (1000 mg on days 1 and 15) + MTX 24 w Edwards JC et al. N Engl J Med. 2004 48 weeks

REFLEX study: RTX in TNF- failures Treatment Placebo (n=209) Rituximab (n=308) Previous DMARDsa (mean, n) 2. 5 2. 6 Baseline MTX dose (mean, n) 16. 7 16. 4 Baseline glucocorticoid use (%) 61 65 Inadequate efficacy of TNF inhibitors (%) 90 92 Previous TNF inhibitors (mean, n) 1. 5 1 previous TNF inhibitor (%) 60 60 2 previous TNF inhibitors (%) 31 31 3 previous TNF inhibitors (%) 9 9 Cohen SB et al, Arthritis Rheum. 2006

REFLEX study: EULAR responses at (24) 48 weeks Patients (%) p<0. 0001 Cohen SB et al. Arthr Rheum 2006
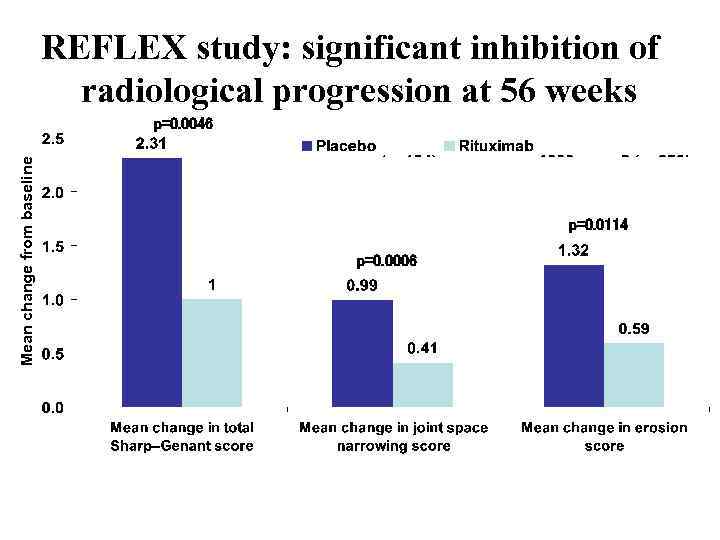
REFLEX study: significant inhibition of radiological progression at 56 weeks Mean change from baseline p=0. 0046 p=0. 0114 p=0. 0006
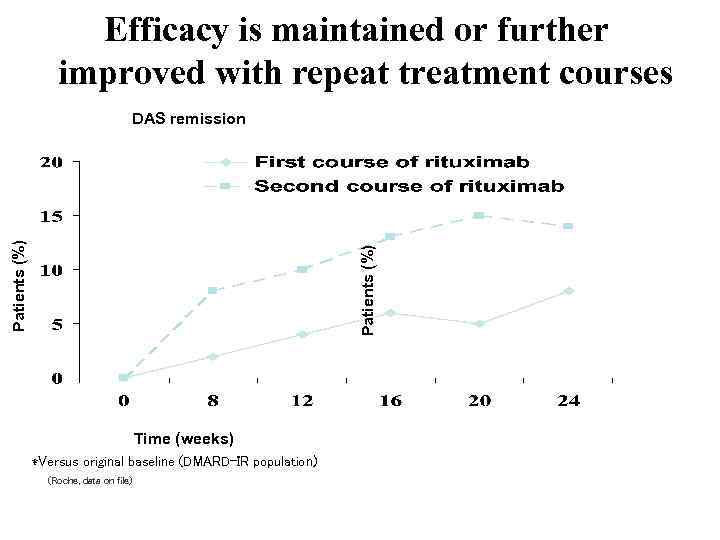
Efficacy is maintained or further improved with repeat treatment courses Patients (%) DAS remission Time (weeks) *Versus original baseline (DMARD-IR population) (Roche, data on file)

Adverse events • Infusion reactions: pruritus, rhinitis, throat irritation, urticaria, pyrexia, angioedema, shock anaphylactic • Infections: URTI • HBV reactivation • Ig reduction • PML • High cost

IL-6

IL-6 Ab

Monthly IV

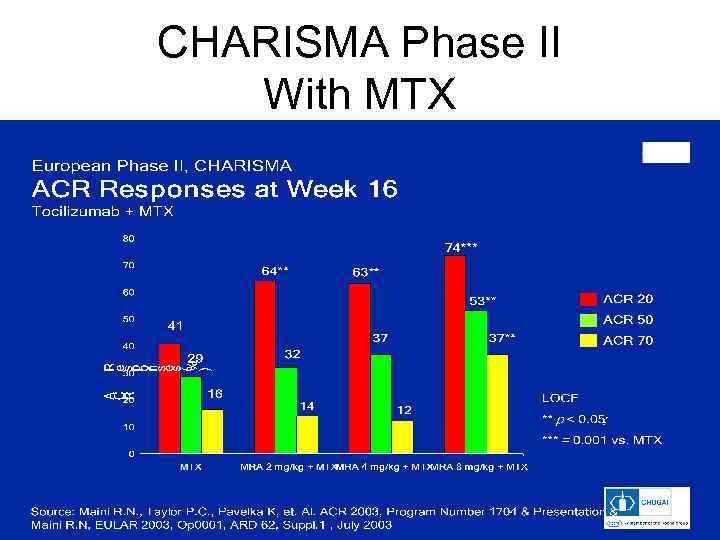
CHARISMA Phase II With MTX
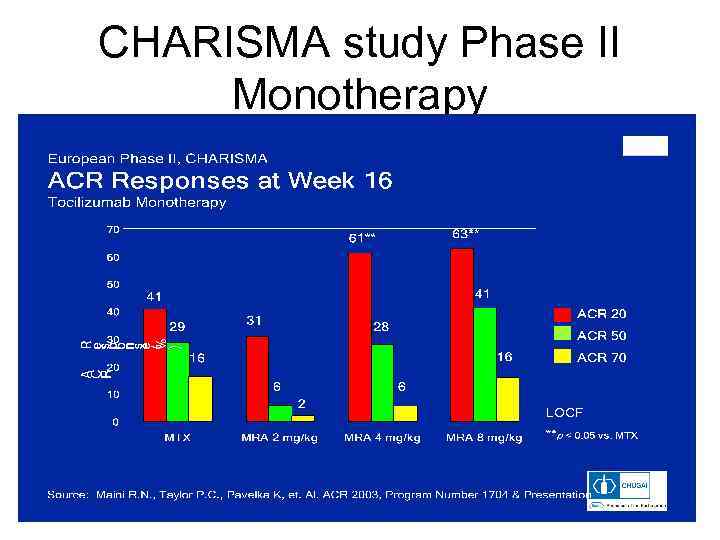
CHARISMA study Phase II Monotherapy

Adverse events Liver enzymes elevation Pneumonitis DVT Lipid levels elevation

Conclusions • • Many new “biological players” Accumulating of experience and evidences Prompt patients selection New studies – humanized anti CD 20+ antibodies, anti CD 19 antibodies, Abatacept (anti-CD 4+ Mab).
biological 2009.ppt