7fdabb52d4c2be09903ac5d5eb6e6741.ppt
- Количество слайдов: 76
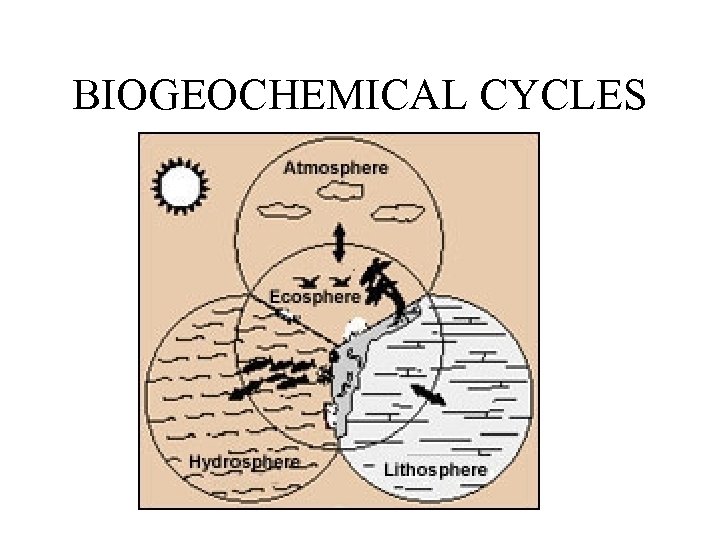
BIOGEOCHEMICAL CYCLES

‘Fundamentals’ of biogeochemical cycles • All matter cycles. . . it is neither created nor destroyed. . . • As the Earth is essentially a closed system with respect to matter, we can say that all matter on Earth cycles. • Biogeochemical cycles: the movement (or cycling) of matter through a system

by matter we mean: elements (carbon, nitrogen, oxygen) or molecules (water) so the movement of matter (for example carbon) between these parts of the system is, practically speaking, a biogeochemical cycle The Cycling Elements: macronutrients : required in relatively large amounts "big six": carbon , hydrogen , oxygen , nitrogen , phosphorous sulfur

other macronutrients: potassium , calcium , iron , magnesium micronutrients : required in very small amounts, (but still necessary) boron (green plants) copper (some enzymes) molybdenum (nitrogen-fixing bacteria)
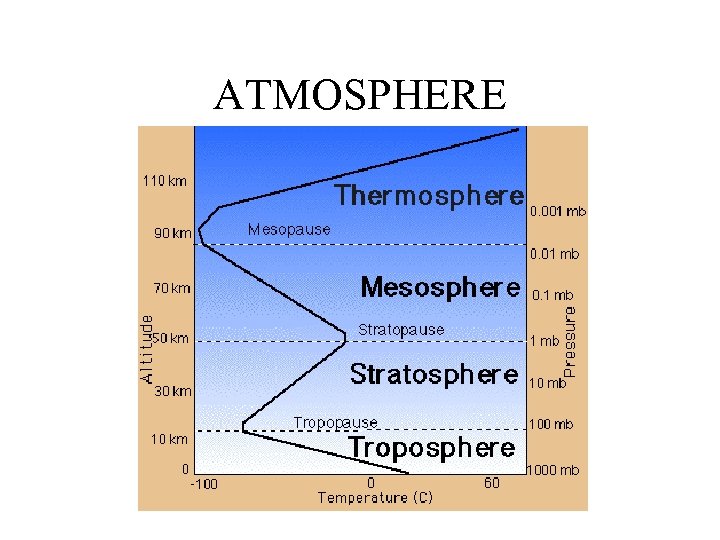
ATMOSPHERE

LITHOSPHERE

HYDROSPHERE

ECOSPHERE

6 of the most important cycles are the water, carbon, nitrogen, sulfur, phosphorus and oxygen.

1. Which part of the atmosphere has the highest altitude? A. Troposphere B. Stratosphere C. Thermosphere D. Mesosphere 2. Which part includes all three of the other parts? Lithosphere B. Ecosphere C. Hydrosphere D. Atmosphere 3. Which one is not a major cycle? A. Hydrogen B. Nitrogen C. Oxygen D. Sulfur E. Water
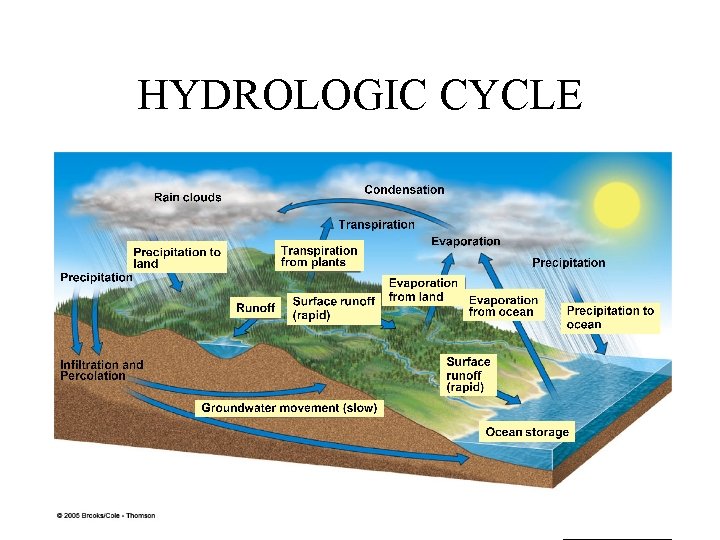
HYDROLOGIC CYCLE
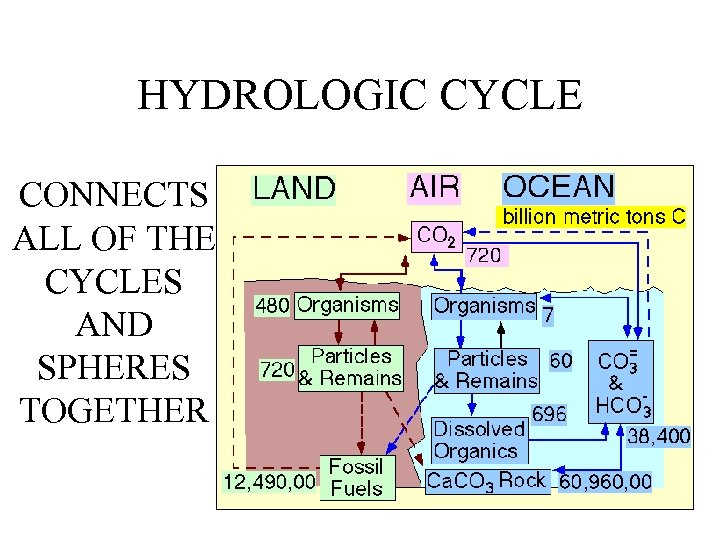
HYDROLOGIC CYCLE CONNECTS ALL OF THE CYCLES AND SPHERES TOGETHER

HUMAN IMPACTS TO WATER CYCLE 1. Water withdrawal from streams, lakes and groundwater. (salt water intrusion and groundwater depletion) 2. Clear vegetation from land for agriculture, mining, road and building construction. (nonpoint source runoff carrying pollutants and reduced recharge of groundwater) 3. Degrade water quality by adding nutrients(NO 2, NO 3, PO 4) and destroying wetlands (natural filters). 4. Degrade water clarity by clearing vegetation and increasing soil erosion.

Water Quality Degradation
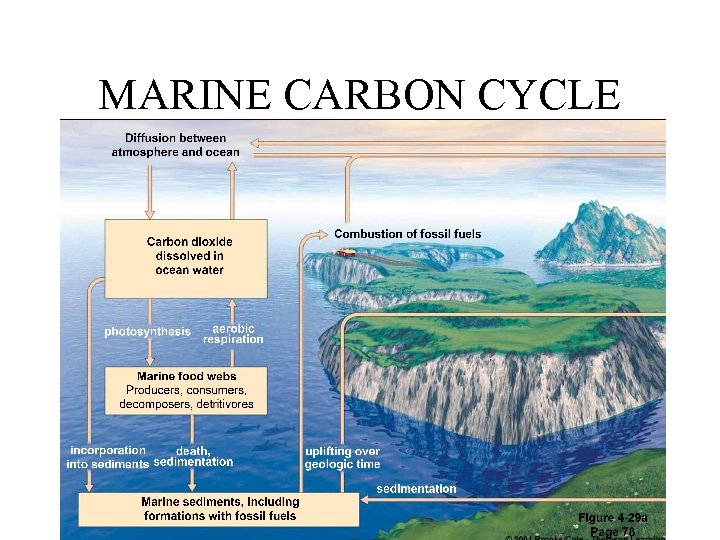
MARINE CARBON CYCLE
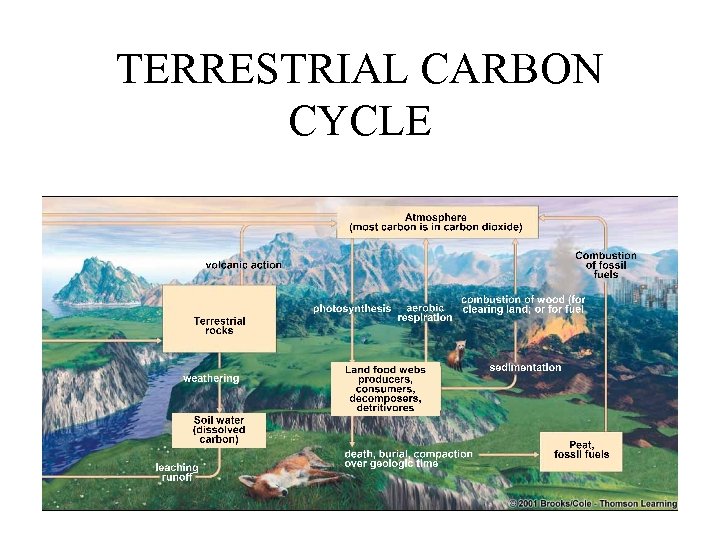
TERRESTRIAL CARBON CYCLE
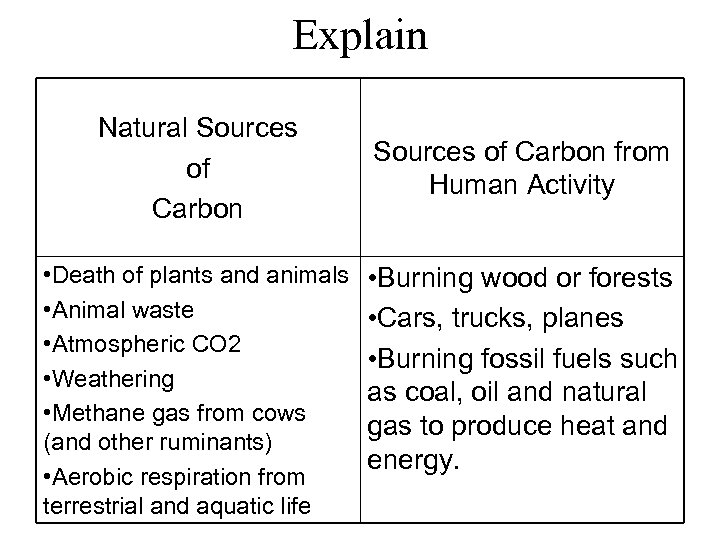
Explain Natural Sources of Carbon • Death of plants and animals • Animal waste • Atmospheric CO 2 • Weathering • Methane gas from cows (and other ruminants) • Aerobic respiration from terrestrial and aquatic life Sources of Carbon from Human Activity • Burning wood or forests • Cars, trucks, planes • Burning fossil fuels such as coal, oil and natural gas to produce heat and energy.

Carbon in Oceans • Additional carbon is stored in the ocean. • Many animals pull carbon from water to use in shells, etc. • Animals die and carbon substances are deposited at the bottom of the ocean. • Oceans contain earth’s largest store of carbon.
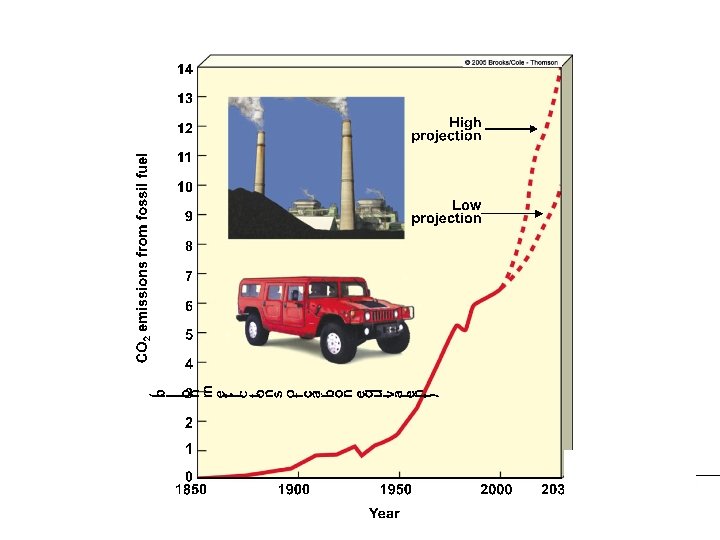

IMPORTANCE OF CARBON CYCLE CARBON IS THE BACKBONE OF LIFE!

1. What is no part of the water cycle? A. Precipitation B. Percolation C. Transpiration D. Surface Runoff E. Boiling 2. Which is not a man made way of adding carbon to the carbon cycle? A. Airplanes B. Natural Fires C. Cars D. Burning fossil fuels 3. What are the predictions for how much carbon will be added from fossil fuels? A. Low B. Medium-Low C. Medium D. High

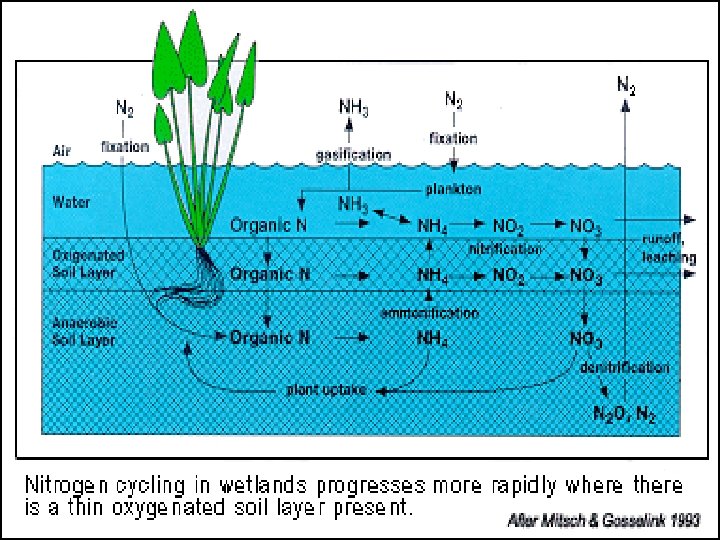
The Nitrogen Cycle

Sources • • • Lightning Inorganic fertilizers Nitrogen Fixation Animal Residues Crop residues Organic fertilizers
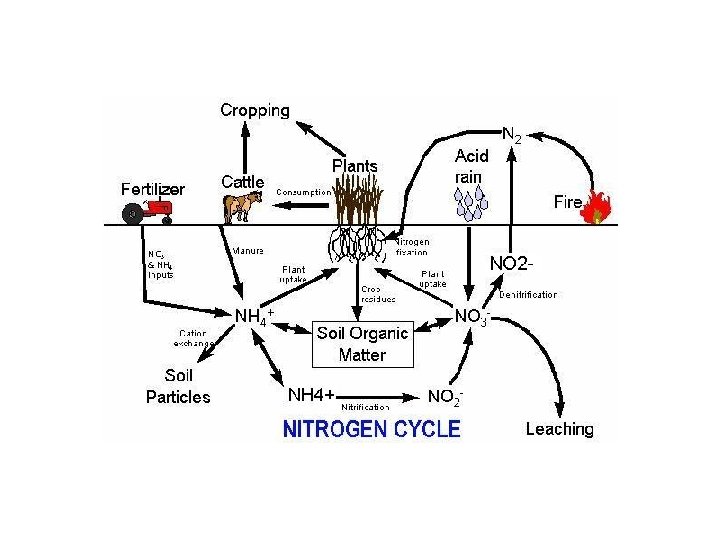

Forms of Nitrogen • • Urea CO(NH 2)2 Ammonia NH 3 (gaseous) Ammonium NH 4 Nitrate NO 3 Nitrite NO 2 Atmospheric Dinitrogen N 2 Organic N
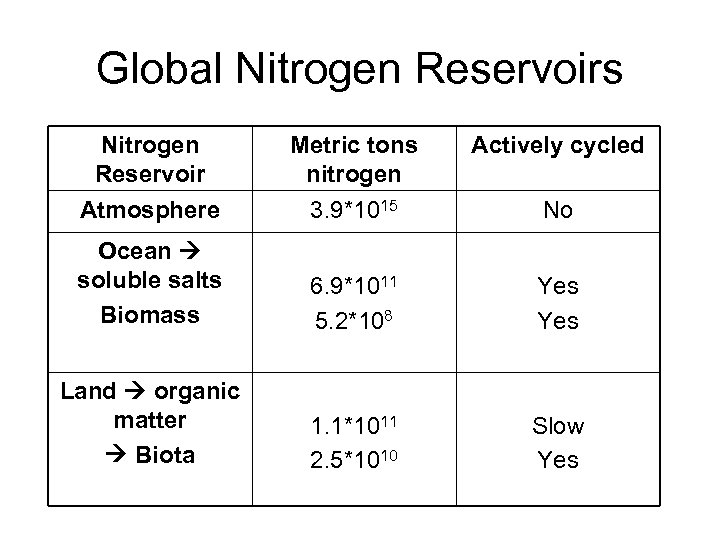
Global Nitrogen Reservoirs Nitrogen Reservoir Atmosphere Metric tons nitrogen 3. 9*1015 Actively cycled Ocean soluble salts Biomass 6. 9*1011 5. 2*108 Yes Land organic matter Biota 1. 1*1011 2. 5*1010 Slow Yes No

Roles of Nitrogen • Plants and bacteria use nitrogen in the form of NH 4+ or NO 3 • It serves as an electron acceptor in anaerobic environment • Nitrogen is often the most limiting nutrient in soil and water.
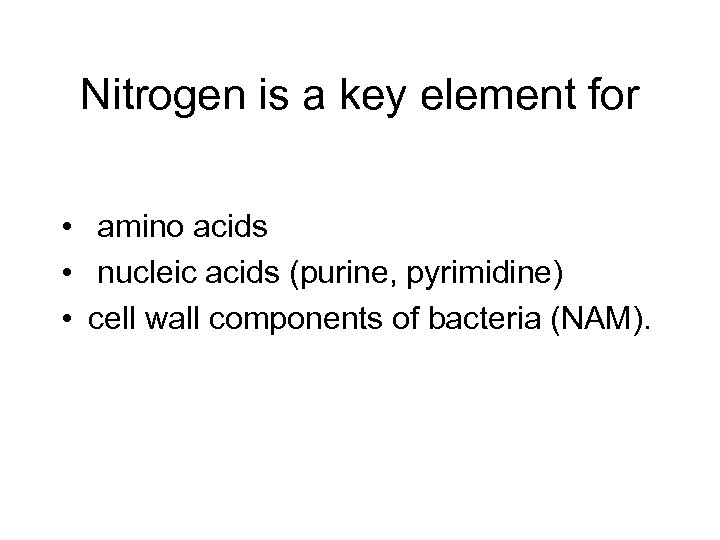
Nitrogen is a key element for • amino acids • nucleic acids (purine, pyrimidine) • cell wall components of bacteria (NAM).

Nitrogen Cycles • • • Ammonification/mineralization Immobilization Nitrogen Fixation Nitrification Denitrification
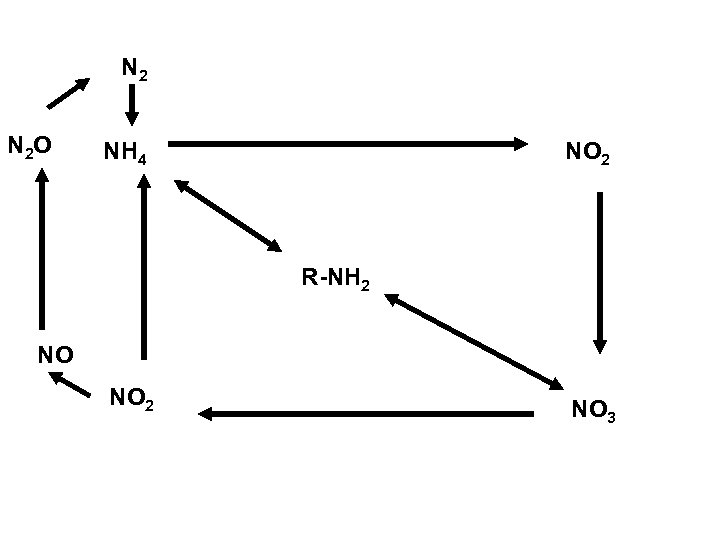
N 2 N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3

Which of the following is not part of the Nitrogen Cycle? A) Ammonification B) Nitrification C) Denitrosation D) Nitrogen Fixation E) Denitrification In what form(s) do plants and bacteria use nitrogen? A) NH 4+ B) NH 3 C) NO 3 D) A and C E) All of the above What is the molecular formula for ammonium? A) NH 4+ B) NH 3 C) NO 3 D) NO 2 E) none of the above
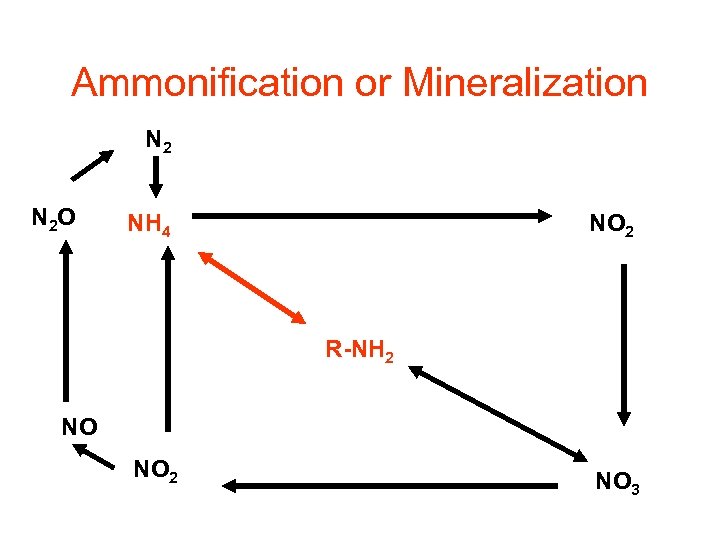
Ammonification or Mineralization N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3

Mineralization or Ammonification • Decomposers: earthworms, termites, slugs, snails, bacteria, and fungi • Uses extracellular enzymes initiate degradation of plant polymers • Microorganisms uses: • Proteases, lysozymes, nucleases to degrade nitrogen containing molecules
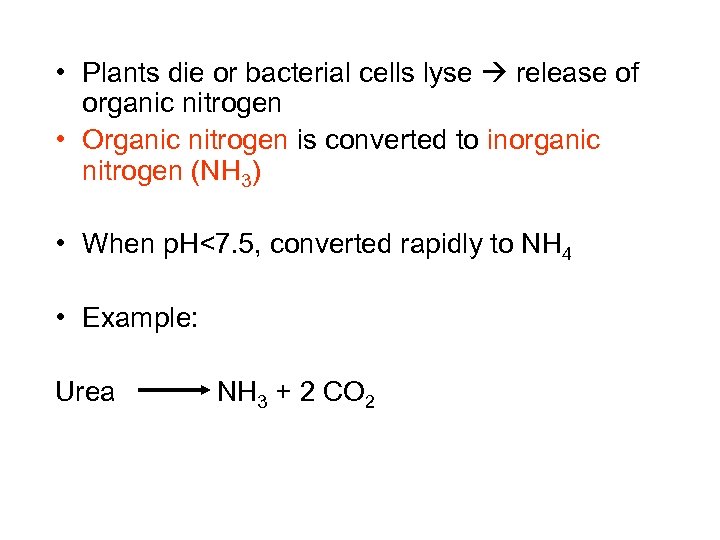
• Plants die or bacterial cells lyse release of organic nitrogen • Organic nitrogen is converted to inorganic nitrogen (NH 3) • When p. H<7. 5, converted rapidly to NH 4 • Example: Urea NH 3 + 2 CO 2
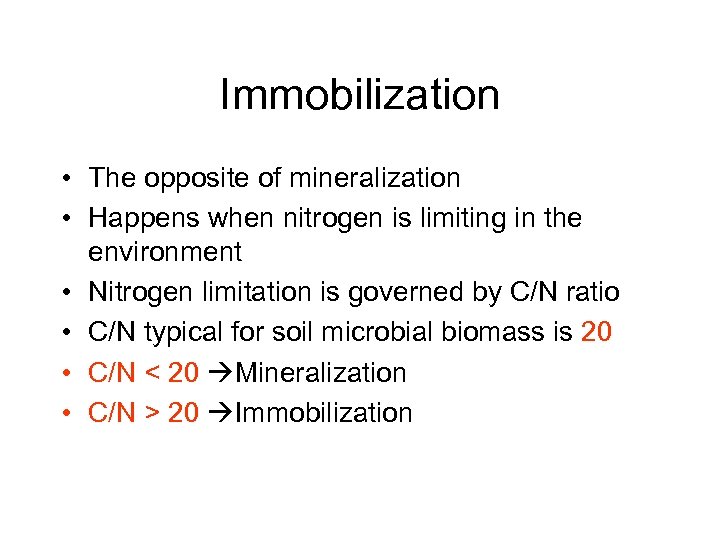
Immobilization • The opposite of mineralization • Happens when nitrogen is limiting in the environment • Nitrogen limitation is governed by C/N ratio • C/N typical for soil microbial biomass is 20 • C/N < 20 Mineralization • C/N > 20 Immobilization
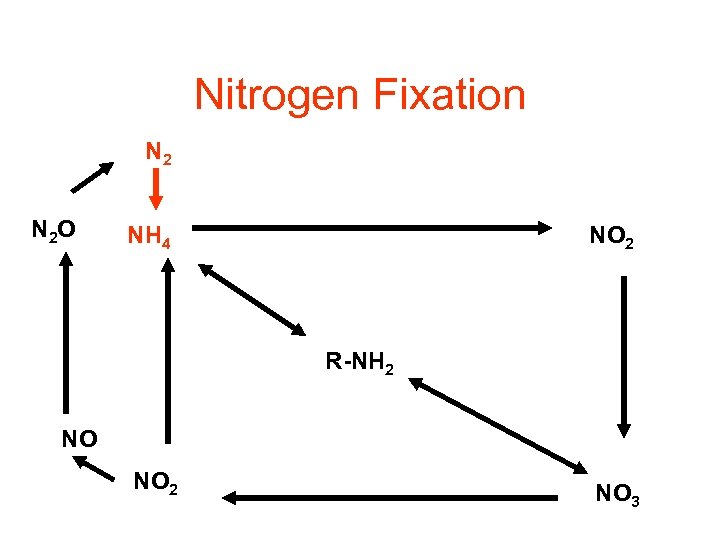
Nitrogen Fixation N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3
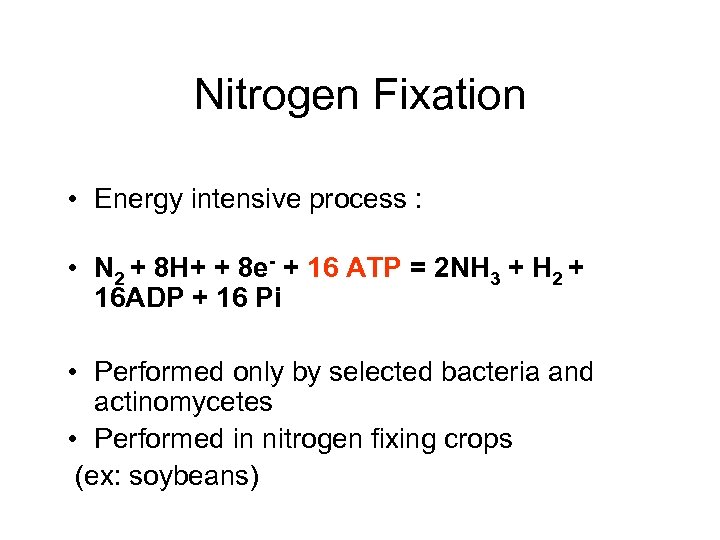
Nitrogen Fixation • Energy intensive process : • N 2 + 8 H+ + 8 e- + 16 ATP = 2 NH 3 + H 2 + 16 ADP + 16 Pi • Performed only by selected bacteria and actinomycetes • Performed in nitrogen fixing crops (ex: soybeans)
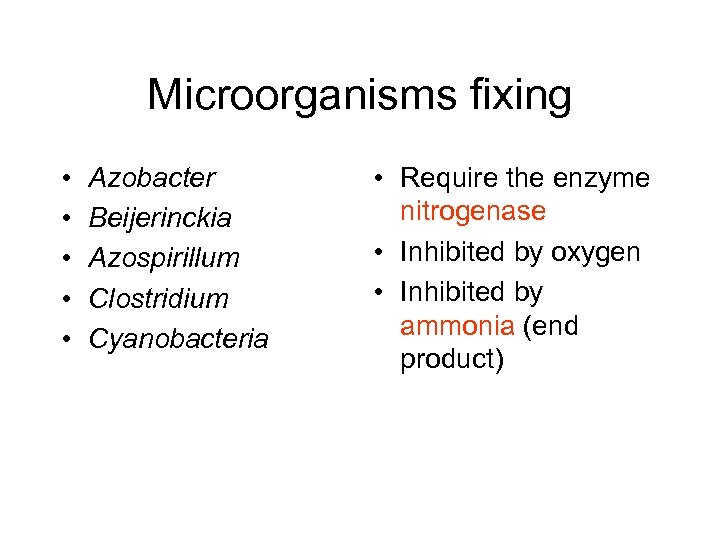
Microorganisms fixing • • • Azobacter Beijerinckia Azospirillum Clostridium Cyanobacteria • Require the enzyme nitrogenase • Inhibited by oxygen • Inhibited by ammonia (end product)
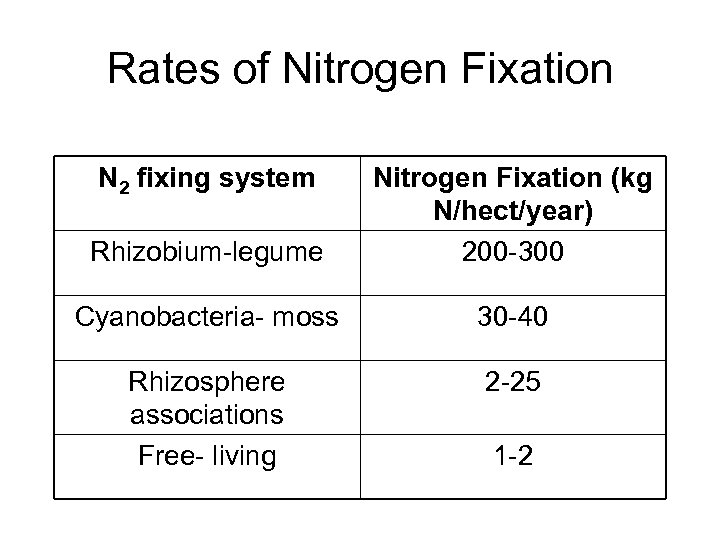
Rates of Nitrogen Fixation N 2 fixing system Rhizobium-legume Nitrogen Fixation (kg N/hect/year) 200 -300 Cyanobacteria- moss 30 -40 Rhizosphere associations Free- living 2 -25 1 -2


Immobilization is the opposite of which process in the cycle? A) Mineralization B) Nitrification C) Immobilization D) Nitrogen Fixation E) Denitrification What process takes place when nitrogen is limiting in the environment? A) Mineralization B) Nitrification C) Immobilization D) Nitrogen Fixation E) Denitrification Which has the highest rate of nitrogen fixation? A) Rhizobium-legume B) Cynaobacteria-moss C) Rhizosphere associations D) Free-living E) Azobacter

Applications to wetlands • • • Occur in overlying waters Aerobic soil Anaerobic soil Oxidized rhizosphere Leaf or stem surfaces of plants

Bacterial Fixation • Occurs mostly in salt marshes • Is absent from low p. H peat of northern bogs • Cyanobacteria found in waterlogged soils
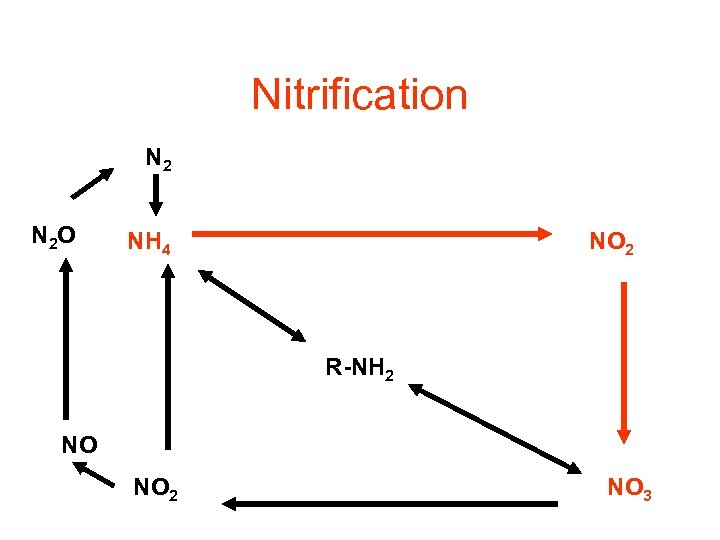
Nitrification N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3
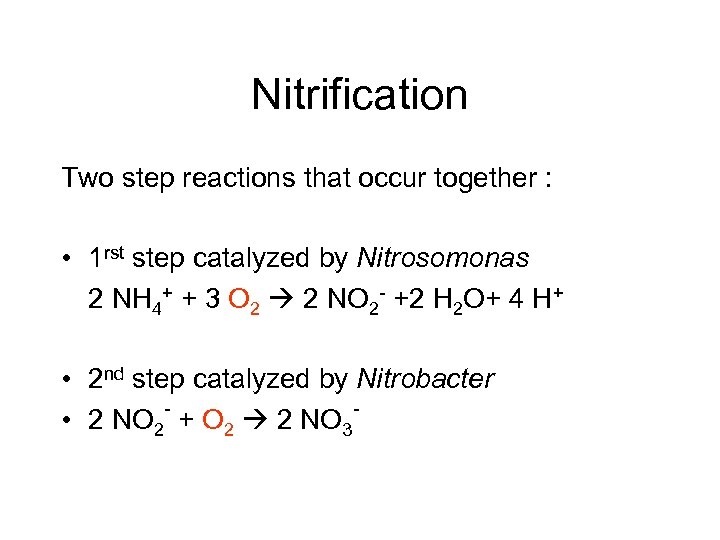
Nitrification Two step reactions that occur together : • 1 rst step catalyzed by Nitrosomonas 2 NH 4+ + 3 O 2 2 NO 2 - +2 H 2 O+ 4 H+ • 2 nd step catalyzed by Nitrobacter • 2 NO 2 + O 2 2 NO 3
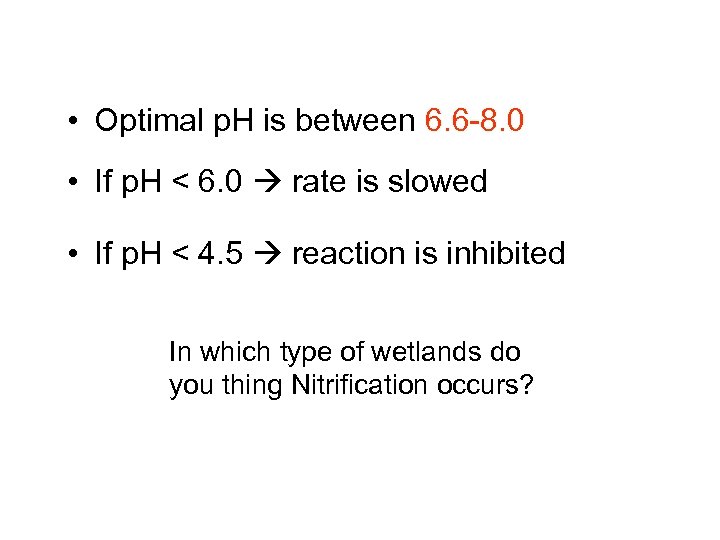
• Optimal p. H is between 6. 6 -8. 0 • If p. H < 6. 0 rate is slowed • If p. H < 4. 5 reaction is inhibited In which type of wetlands do you thing Nitrification occurs?
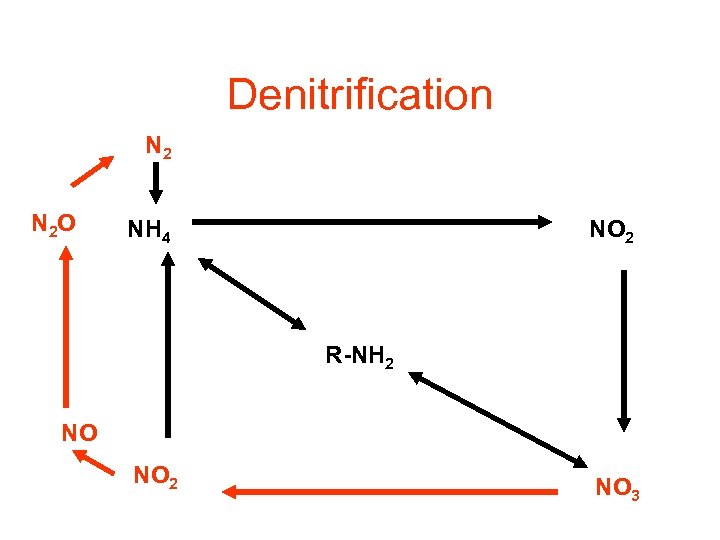
Denitrification N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3
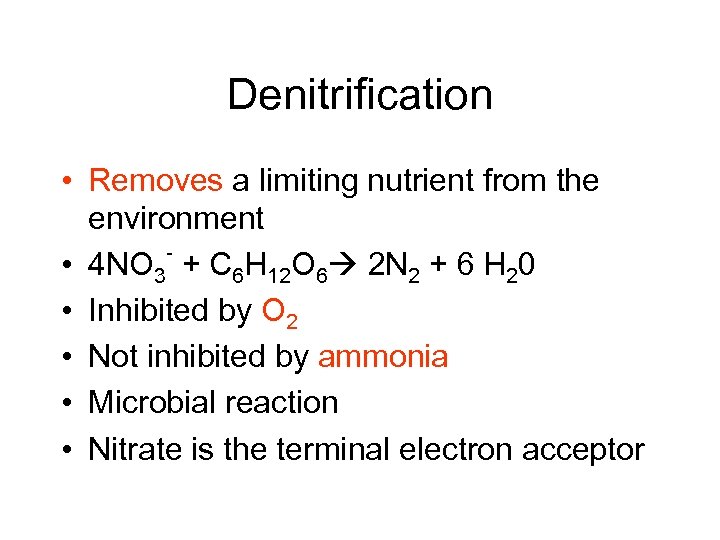
Denitrification • Removes a limiting nutrient from the environment • 4 NO 3 + C 6 H 12 O 6 2 N 2 + 6 H 20 • Inhibited by O 2 • Not inhibited by ammonia • Microbial reaction • Nitrate is the terminal electron acceptor

Looking at the Nitrogen cycle through the eye of NH 4

Denitrication is inhibited by A) NH 3 B) NH 4+ C) NO 2 D) O 2 The second step of Nitrification is catalyzed by A) Nitrosomonas B) Clostridium C) Azobacter D) Nitrobacter E) Beijerinckia Which p. H is within the optimal range for nitrication? A) 1. 5 B) 4. 6 C) 7. 1 D) 8. 7 E) 10. 9
![Surfac e water Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion Surfac e water Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion](https://present5.com/presentation/7fdabb52d4c2be09903ac5d5eb6e6741/image-52.jpg)
Surfac e water Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion [NH 4] HIGH Biodegradati on C/N <20 C/N >20
![Surfac e water nitrificatio n Low [NH 4] Oxidized layer Reduce d soil layer Surfac e water nitrificatio n Low [NH 4] Oxidized layer Reduce d soil layer](https://present5.com/presentation/7fdabb52d4c2be09903ac5d5eb6e6741/image-53.jpg)
Surfac e water nitrificatio n Low [NH 4] Oxidized layer Reduce d soil layer Slow Diffusion [NH 4] HIGH [NO 3] high
![N 2 Surfac e water Oxidized layer Reduce d soil layer [NO 3] high N 2 Surfac e water Oxidized layer Reduce d soil layer [NO 3] high](https://present5.com/presentation/7fdabb52d4c2be09903ac5d5eb6e6741/image-54.jpg)
N 2 Surfac e water Oxidized layer Reduce d soil layer [NO 3] high Leaching [NO 3] Low Denitrification
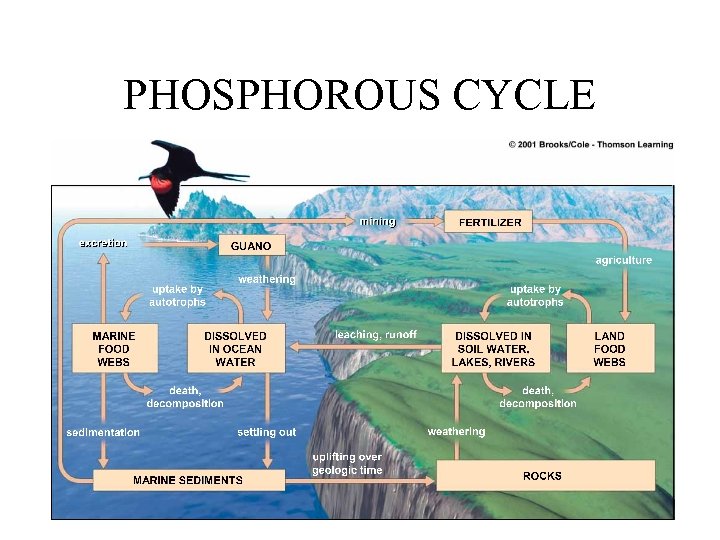
PHOSPHOROUS CYCLE

HUMAN IMPACTS TO PHOSPHOROUS CYCLE 1. Humans mine LARGE quantities of phosphate rock to use in commercial fertilizers and detergents. Phosphorous is NOT found as a gas, only as a solid in the earth’s crust. It takes millions to hundreds of millions of years to replenish. 2. Phosphorous is held in the tissue of the trees and vegetation, not in the soil and as we deforest the land, we remove the ability for phosphorous to replenish globally in ecosystems. 3. Cultural eutrophication – ad excess phosphate to aquatic ecosystems in runoff of animal wastes from livestock feedlots, runoff of commercial phosphate fertilizers fro cropland, and discharge of municipal sewage.
IMPORTANCE OF PHOSPHOROUS CYCLE • 1. Phosphorous is an essential nutrient of both plants and animals. • 2. It is part of DNA molecules which carry genetic information. • 3. It is part of ATP and ADP) that store chemical energy for use by organisms in cellular respiration. • 4. Forms phospholipids in cell membranes of plants and animal cells. • 5. Forms bones, teeth, and shells of animals as calcium phosphate compounds.
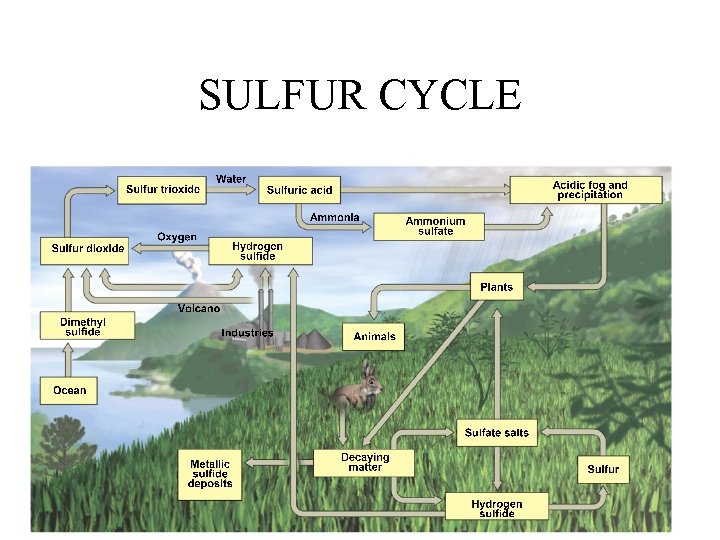
SULFUR CYCLE

HUMAN IMPACTS TO SULFUR CYCLE Approximately 1/3 of all sulfur emitted into atmosphere comes from human activities. • 1. Burning sulfur containing coal and oil to produce electric power (SOx = acid deposition). • 2. Refining petroleum – (SOx emissions) • 3. Smelting to convert sulfur compounds of metallic minerals into free metals (Cu, Pb, Zn) • 4. Industrial processing.

IMPORTANCE OF SULFUR CYCLE 1. Sulfur is a component of most proteins and some vitamins. 2. Sulfate ions (SO 4 2 - ) dissolved in water are common in plant tissue. They are part of sulfur-containing amino acids that are the building blocks for proteins. 3. Sulfur bonds give three dimensional structure of amino acids. 4. Many animals, including humans, depend on plants for sulfur-containing amino acids.
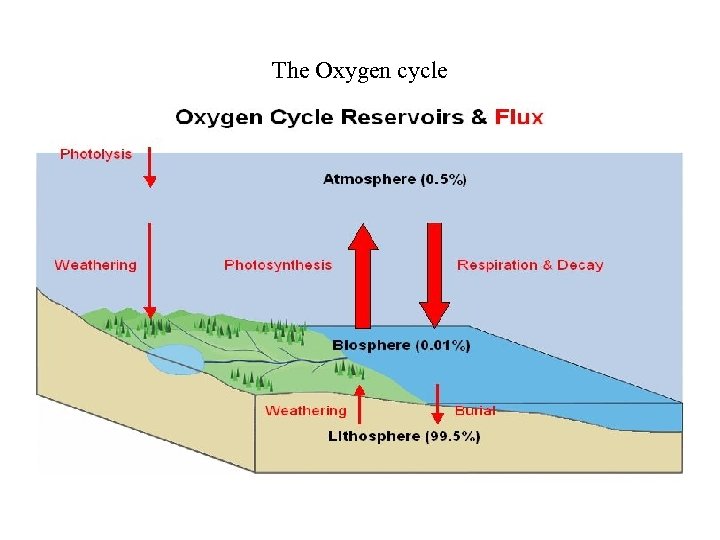
The Oxygen cycle

1. The Phosphorus Cycle takes A. Short time B. 20 years to fully cycle through C. 100 years to cycle through D. Geological Timescal 2. What percentage of sulfur is emmited buy human activity? A. . 01% B. 20% C. 33. 3% D. 66. 7% E. Over 90% 3. The vast majority of oxygen in the ecosphere is in A. Outer space B. Lithosphere C. Atmosphere D. Hydrosphere
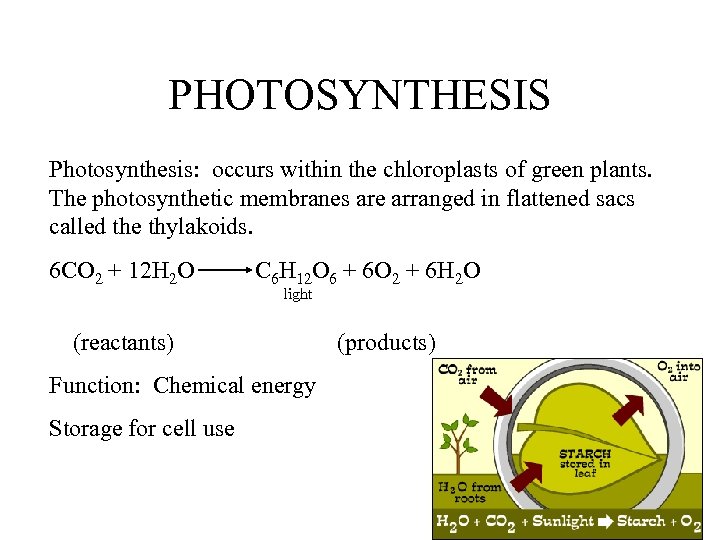
PHOTOSYNTHESIS Photosynthesis: occurs within the chloroplasts of green plants. The photosynthetic membranes are arranged in flattened sacs called the thylakoids. 6 CO 2 + 12 H 2 O C 6 H 12 O 6 + 6 O 2 + 6 H 2 O light (reactants) Function: Chemical energy Storage for cell use (products)
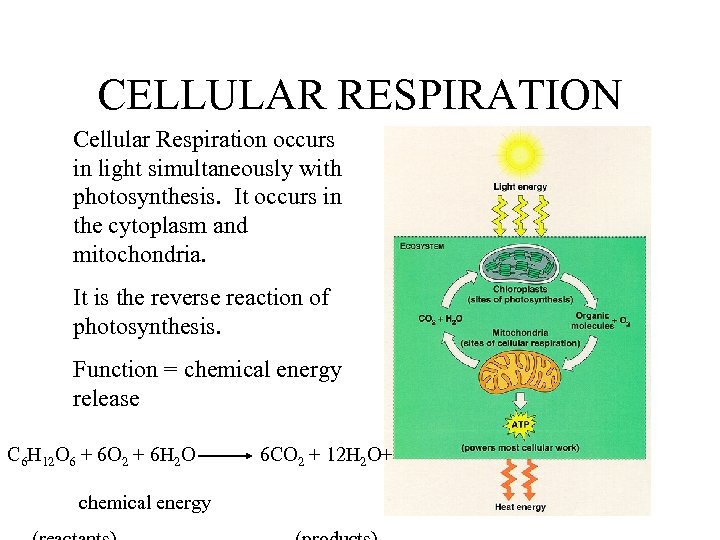
CELLULAR RESPIRATION Cellular Respiration occurs in light simultaneously with photosynthesis. It occurs in the cytoplasm and mitochondria. It is the reverse reaction of photosynthesis. Function = chemical energy release C 6 H 12 O 6 + 6 O 2 + 6 H 2 O chemical energy 6 CO 2 + 12 H 2 O+
Primary Productivity Connection • Gross Primary Productivity (GPP) – the rate at which an ecosystem’s producers capture and store a given amount of chemical energy as biomass in a given period of time. • Net Primary Productivity (NPP) – the rate at which all the plants in an ecosystem produce net useful energy; equal to the difference between energy produced through photosynthesis and energy used for cellular respiration.
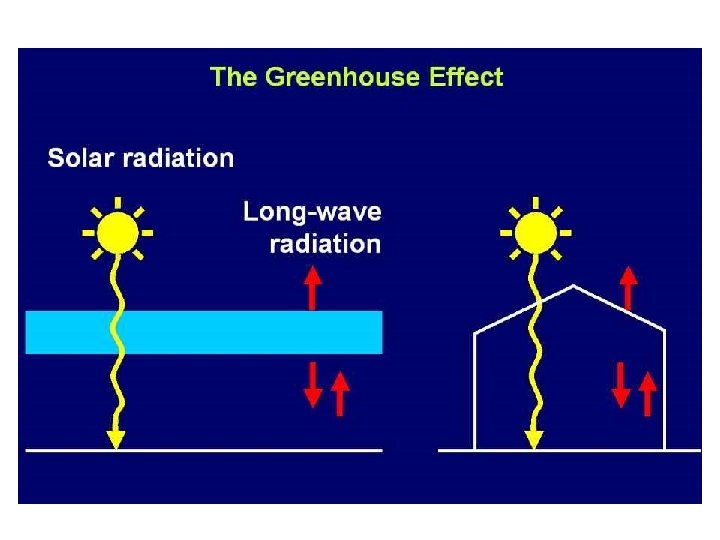
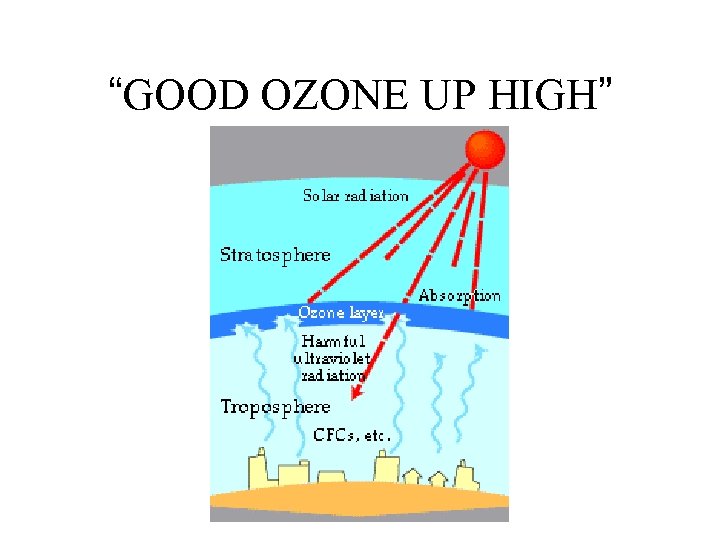
“GOOD OZONE UP HIGH”
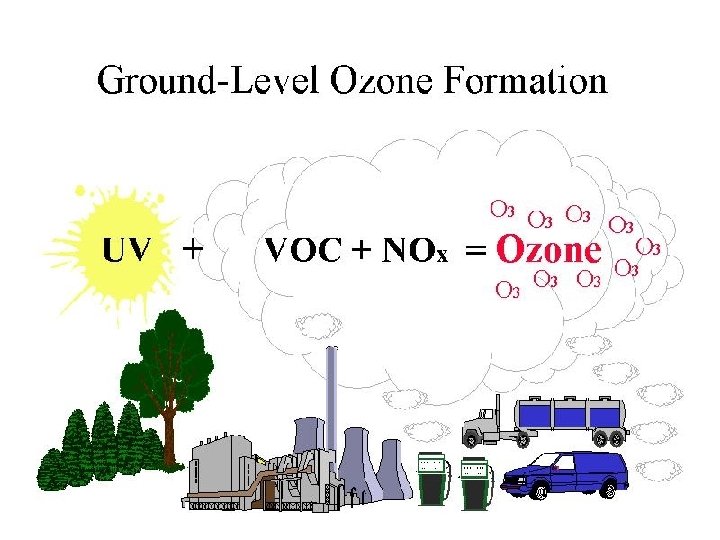
PHOTOCHEMICAL SMOG “BAD OZONE DOWN LOW”
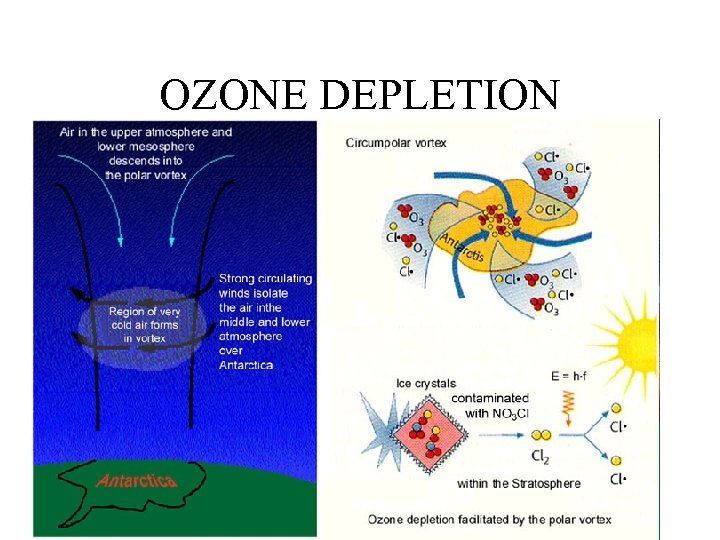
OZONE DEPLETION
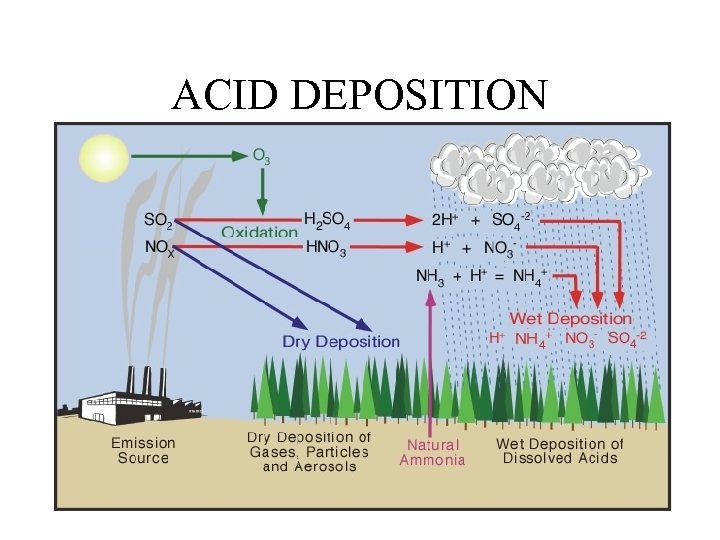
ACID DEPOSITION

CULTURAL EUTROPHICATION

Cultural Eutrophication & Anoxia • Eutrophication: natural process; over 1000’s of years, lakes fill in with sediment, become marshes then dry land • Cultural Eutrophication: same process, but speeded enormously by loading with “limiting nutrients” (typically P, sometimes N)
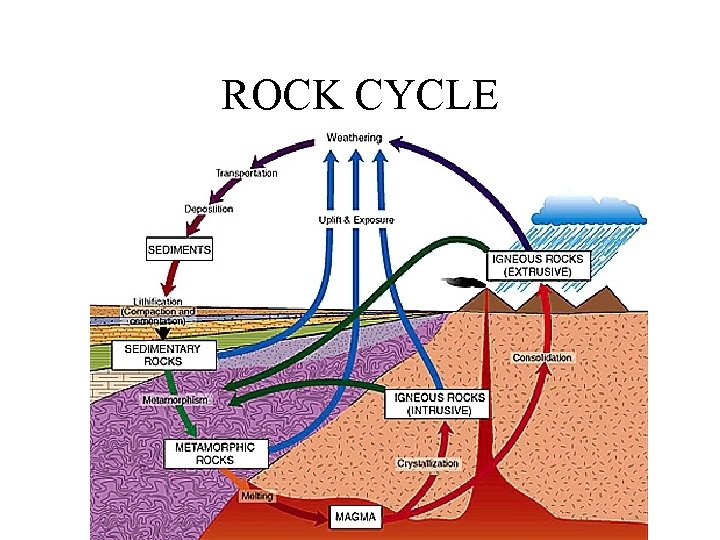
ROCK CYCLE

HUMAN IMPACTS ON THE ROCK CYCLE • 1. Humans are excavating minerals and removing rock material. It takes millions of years for rock to form. • 2. Humans remove sediments for building materials. This removes sediments that may form sedimentary rocks in the future. • 3. Humans are filling in wetlands (peatlands), area that will form future coal beds.

1. Which part of the atmosphere is the ozone layer right above? A. Stratosphere B. Troposphere C. Mesosphere D. Thermosphere 2. How long does it take rock formations to form? A. 1, 000 years B. 10, 000 years C. 100, 000 years D. 1, 000 years E. 10, 000 years 3. What is cultural eutrophication good for? A. Fish B. Dissolved Oxygen in the lake C. algae D. clear lake

Works Cited 1. http: //science. pppst. com/carboncycle. html 2. westernreservepublicmedia. org/earthmotion 3/image s/Carbon_Cycle. ppt 3. clima-dods. ictp. it/d 3/annalisa/ocean_sv/lecture 1. ppt 4. www. geology. wmich. edu/Koretsky/envs 2150/Pcycl e_1. ppt
7fdabb52d4c2be09903ac5d5eb6e6741.ppt