ec8bc2af4030a48a106013bd6ed09e17.ppt
- Количество слайдов: 22

Biochemical Fuels Chapter 11

Fossil Fuels • Today most of our energy needs are met by burning fossil fuels, such as: ▫ Coal ▫ Petroleum ▫ Natural gas • While there are large reserves of coal, petroleum deposits are limited.
Biochemical Fuels • We are making enormous demands on the planet in terms of providing sufficient fuel resources. • We are increasingly aware of the environmental effects of pollution of the atomsphere and global warming. • The latter is caused in part by increased amounts of carbon dioxide produced by the burning of carbon-based fossil fuels • Look at figure 11. 2 on page 168

Biochemical Fuels • The limited nature of crude oil reserves, as well as petroleum reserves and global warming is a huge concern to governments. • Every country in the world is looking for new energy sources that: ▫ are clean (produce less pollution) ▫ are green (sustainable and renewable) ▫ have high efficiency rates
Biochemical fuels (biofuels) • Biofuels are derived from plant material such as grains, sugarcane or vegetable waste. • The two main biofuels are ethanol and biodiesel. • They are used either alone of blended with fossil fuels such as petrol and diesel. • Biofuels are not considered to contribute to an increase in atmospheric carbon dioxide.
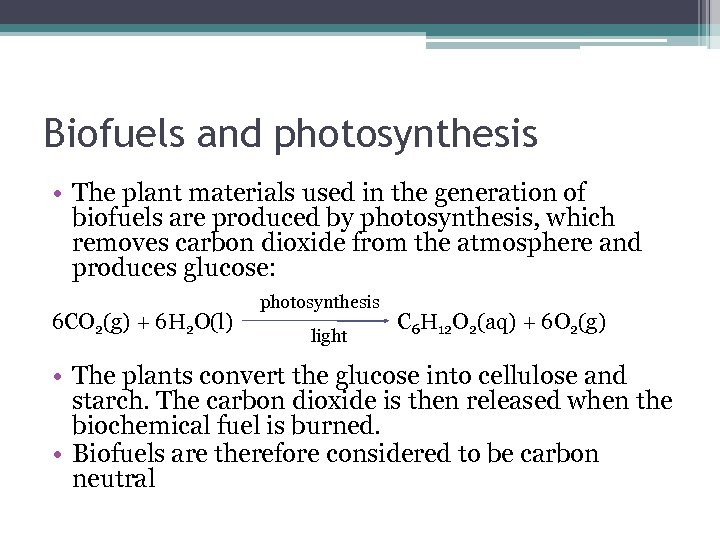
Biofuels and photosynthesis • The plant materials used in the generation of biofuels are produced by photosynthesis, which removes carbon dioxide from the atmosphere and produces glucose: 6 CO 2(g) + 6 H 2 O(l) photosynthesis light C 6 H 12 O 2(aq) + 6 O 2(g) • The plants convert the glucose into cellulose and starch. The carbon dioxide is then released when the biochemical fuel is burned. • Biofuels are therefore considered to be carbon neutral
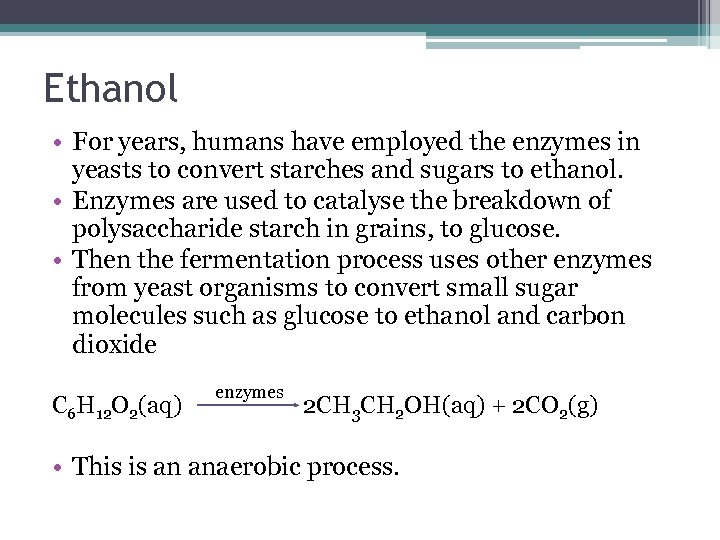
Ethanol • For years, humans have employed the enzymes in yeasts to convert starches and sugars to ethanol. • Enzymes are used to catalyse the breakdown of polysaccharide starch in grains, to glucose. • Then the fermentation process uses other enzymes from yeast organisms to convert small sugar molecules such as glucose to ethanol and carbon dioxide C 6 H 12 O 2(aq) enzymes 2 CH 3 CH 2 OH(aq) + 2 CO 2(g) • This is an anaerobic process.
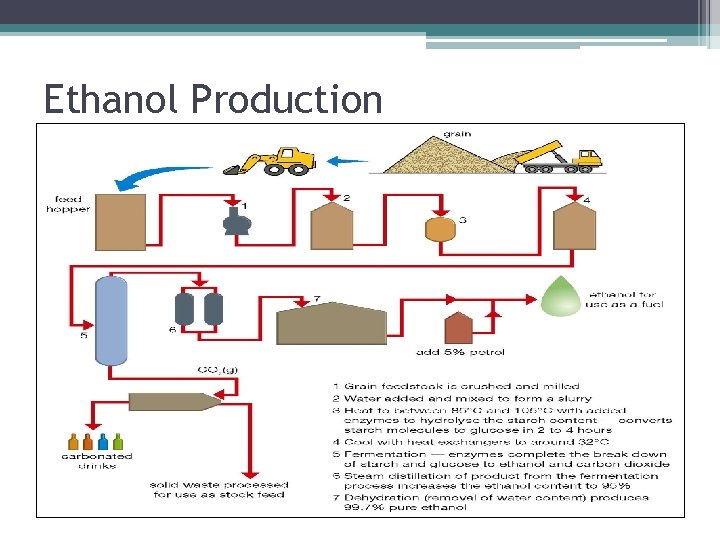
Ethanol Production
Ethanol Production • The fermentation stops when the ethanol content is between 10 and 20%, at which point the yeast and their enzymes can no longer function. • The fermented mixture is pumped to an evaporation plant, where steam is added to cause the ethanol to evaporate off. • When cooled the resulting liquid contains 95% ethanol and 5% water. • This is dehydrated leaving ethanol that is 99. 7% pure. • In Australia this is then poisoned with 5% petrol to make it unsuitable for consumption.

Minimising Waste • The carbon dioxide produced is collected and sold to manufacturers of carbonated drinks. • The waste water and cooling water can be used for irrigating crops. • The protein-rich remains from the fermentation can be sold as animal feed.

Problems • Most older cars won’t take ethanol as a fuel, most will take the E 10 fuel which is only 10% ethanol. • Yeast contains enzymes that will only act on glucose, however fibrous waste material from sugar production has a sugar molecule xylose. • Scientists are trying to come up with a yeast that will act on xylose so we can increase the amount of ethanol produced from sugarcane.

Biodiesel • Biodiesel is a mix of esters produced by a chemical reaction between vegetable oil and an alcohol such as methanol. • The chemical and physical properties of the esters in biodiesel are very similar to diesel. • In europe, biodiesel containing 5% esters has been available since 1995. • It is possible to run diesel engines off 100% ester fuel.

Biodiesel • Fats and oils are triglycerides, have a look at figure 11. 5 on page 170. What do you notice about the structure?
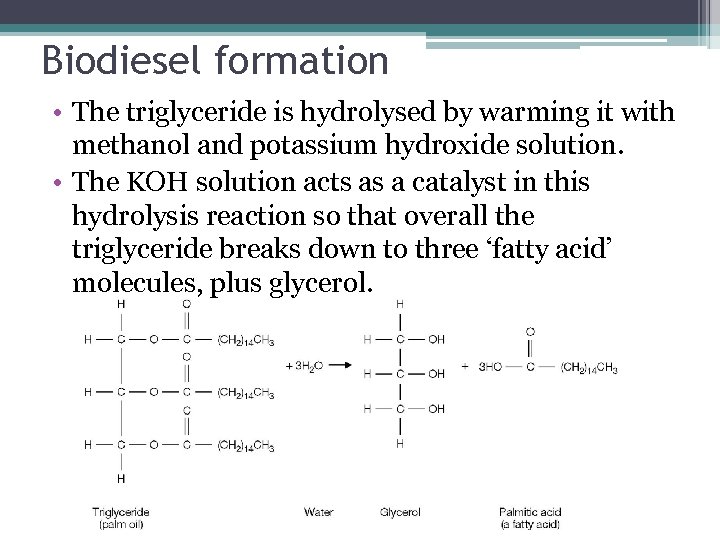
Biodiesel formation • The triglyceride is hydrolysed by warming it with methanol and potassium hydroxide solution. • The KOH solution acts as a catalyst in this hydrolysis reaction so that overall the triglyceride breaks down to three ‘fatty acid’ molecules, plus glycerol.
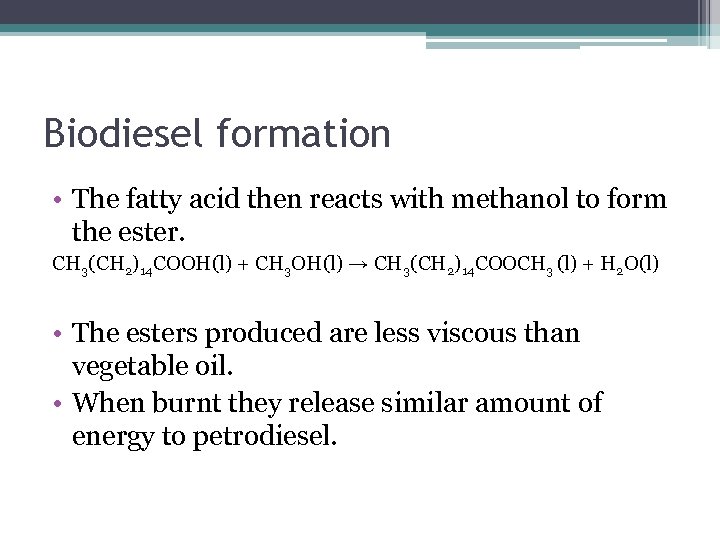
Biodiesel formation • The fatty acid then reacts with methanol to form the ester. CH 3(CH 2)14 COOH(l) + CH 3 OH(l) → CH 3(CH 2)14 COOCH 3 (l) + H 2 O(l) • The esters produced are less viscous than vegetable oil. • When burnt they release similar amount of energy to petrodiesel.

Biodiesel and petrodiesel • Biodiesel is usually used in a mixture with petrodiesel; 5% and 10% mixtures are known as B 5 and B 10. • Biodiesel can act as a better solvent for any contamination that has built up in the engine and hence can cause blockages in filters. • B 100 (100% biodiesel) can be used.

Biodiesel • Biodiesel is: ▫ ▫ Biodegradable Non-toxic Produces fewer pollutants in the vehicle emissions. Does not add to the amount of carbon dioxide in the atmosphere as it is simply recycling the carbon dioxide already present.
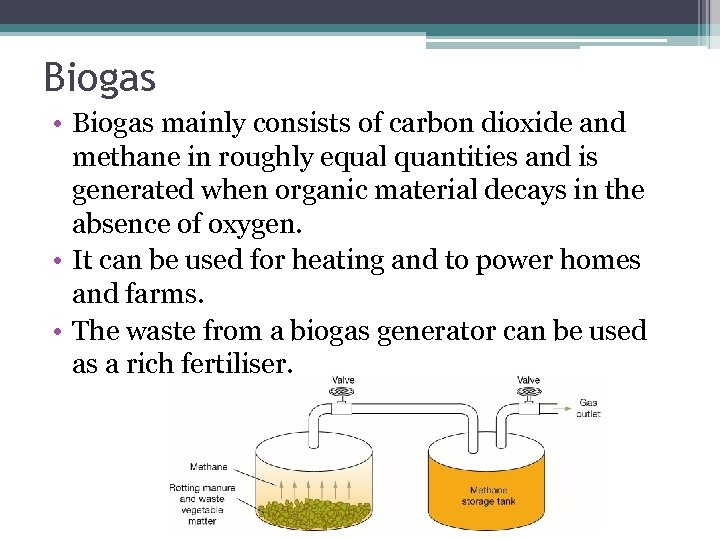
Biogas • Biogas mainly consists of carbon dioxide and methane in roughly equal quantities and is generated when organic material decays in the absence of oxygen. • It can be used for heating and to power homes and farms. • The waste from a biogas generator can be used as a rich fertiliser.
Why develop biochemical fuels • Our current use of fossil fuels is releasing carbon into the environment mainly in the form of atmospheric carbon dioxide. • Gases such as water vapour and carbon dioxide absorb heat energy from the sun and act as sort of a ‘blanket’ to trap this heat in the atmosphere. • Life on earth needs the green house effect but not to this extent. • Although biofuels combustion does produce carbon dioxide, they are produced from plant materials made by photosynthesis which consumes equal amounts of carbon dioxide.
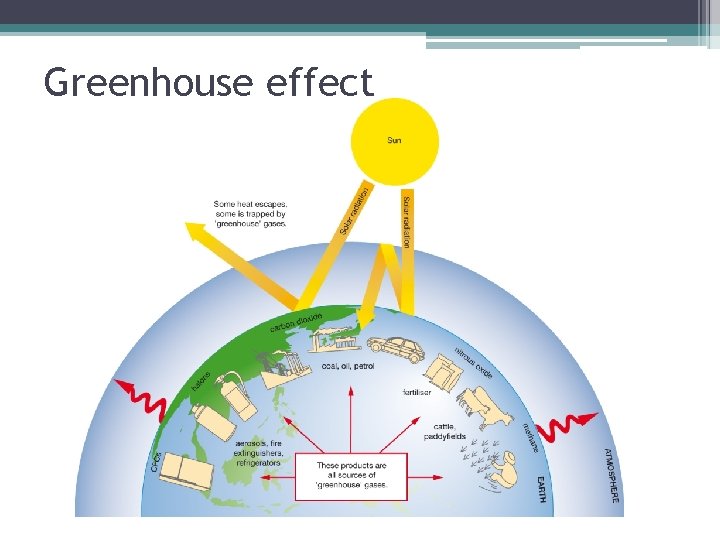
Greenhouse effect
Problems with biofuels • Biofuels are not carbon neutral. • There are non-renewable resources consumed in growing the raw materials and their subsequent processing. • Some energy is used in the processing needed to ferment and distill the industrial ethanol. • How much land is needed to produce crops for the production of biofuels?

Your Turn • Your homework for this chapter • Page 176 • Questions 6 – 11 and 13
ec8bc2af4030a48a106013bd6ed09e17.ppt